Published online May 14, 2009. doi: 10.3748/wjg.15.2252
Revised: April 3, 2009
Accepted: April 10, 2009
Published online: May 14, 2009
AIM: To compare creatinine clearance (Ccr) with estimated glomerular filtration rate (eGFR) in preoperative renal function tests in patients undergoing hepatectomy.
METHODS: The records of 197 patients undergoing hepatectomy between August 2006 and August 2008 were studied, and preoperative Ccr, a three-variable equation for eGFR (eGFR3) and a five-variable equation for eGFR (eGFR5) were calculated. Abnormal values were defined as Ccr < 50 mL/min, eGFR3 and eGFR5 < 60 mL/min per 1.73 m2. The maximum increases in the postoperative serum creatinine (post Cr) level and postoperative rate of increase in the serum Cr level (post Cr rate) were compared.
RESULTS: There were 37 patients (18.8%) with abnormal Ccr, 31 (15.7%) with abnormal eGFR3, and 40 (20.3%) with abnormal eGFR5. Although there were no significant differences in the post Cr rate between patients with normal and abnormal Ccr, eGFR3 and eGFR5 values, the post Cr level was significantly higher in patients with eGFR3 and eGFR5 abnormality than in normal patients (P < 0.0001). Post Cr level tended to be higher in patients with Ccr abnormality (P = 0.0936 and P = 0.0875, respectively).
CONCLUSION: eGFR5 and the simpler eGFR3, rather than Ccr, are recommended as a preoperative renal function test in patients undergoing hepatectomy.
- Citation: Iwasaki Y, Sawada T, Mori S, Iso Y, Katoh M, Rokkaku K, Kita J, Shimoda M, Kubota K. Estimating glomerular filtration rate preoperatively for patients undergoing hepatectomy. World J Gastroenterol 2009; 15(18): 2252-2257
- URL: https://www.wjgnet.com/1007-9327/full/v15/i18/2252.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.2252
The outcome of hepatic resection has improved dramatically during the last 20 years, along with improvements in surgical techniques and perioperative management[1]. Operative mortality is now reportedly less than 1% at most institutions in Japan[2]. However, hepatectomy is associated with intraoperative blood loss, and postoperative complications such as liver failure, infection, bile leakage, ascites, and pleural effusion[3]. Uncontrolled ascites, pleural effusion and intraoperative blood loss disturb blood circulation, leading to dysfunction of not only the liver but also the kidney[4]. Therefore, for appropriate patient selection, it is necessary to evaluate preoperative liver and renal function accurately.
Glomerular filtration rate (GFR) is the most important and comprehensive index of renal function. GFR is measured by inulin clearance, but this takes > 2 h, and requires repeat collection of blood and urine every 15 min[5]. GFR is rarely measured in a clinical setting because of its intricacy. On the other hand, creatinine clearance (Ccr) has been measured clinically by a simple method as a preoperative renal function test[67]. Although Ccr yields an approximate value for GFR, it is usually higher than the GFR as a result of secretion of 10%-15% of the creatinine into urine in the uriniferous tubule[8].
In 1999, Levey et al first reported a prediction equation known as modification of diet in renal disease (MDRD) for estimation of GFR on the basis of age, sex, race, serum creatinine, albumin and blood urea nitrogen (BUN) level for individuals of caucasian and black ethnicity[8]. MDRD has now been accepted as a standard method for evaluation of renal function in North America and Europe. This was a breakthrough for estimation of GFR because of its simplicity and ease of calculation. As a result of differences in physique between caucasian and black individuals, unique variables for estimating GFR in Japanese subjects have been investigated[9]. For this purpose, in 2008, two new equations for estimated GFR (eGFR) were devised on the basis of multiple regression analysis from inulin clearance data of 763 Japanese patients with chronic kidney disease and healthy controls[10]. These were: the three-variable equation for eGFR (mL/min per 1.73 m2) = 194Cr-1.094× Age-0.287 (× 0.739; if the patient is female); the five-variable equation for eGFR (mL/min per 1.73 m2) = 142Cr-0.923× Age-0.185× Alb0.414× BUN-0.233 (× 0.772; if the patient is female).
However, no studies have evaluated the usefulness of eGFR as a preoperative renal function test parameter. If eGFR is superior to Ccr as a preoperative renal function test, then eGFR should replace Ccr because of its simplicity of measurement. In this study, we retrospectively calculated the preoperative three-variable and five-variable equations for eGFR, and compared the results with Ccr, to clarify their superiority as a preoperative renal function test in patients undergoing hepatectomy.
At Dokkyo Medical University, a total of 211 hepatic resections were performed for hepatobiliary disease between August 2006 and August 2008. Of these patients, 14 who were on hemodialysis, or for whom the results of preoperative Ccr were not available, were excluded. A total of 197 patients who underwent hepatectomy alone or hepatectomy plus combined surgery such as splenectomy, Hassab’s operation, gastrectomy and colectomy for hepatocellular carcinoma (HCC), metastatic liver tumor, biliary malignancy and other benign disease were included in this study. There were 147 men and 50 women, with a mean age of 65.0 ± 10.0 years.
Preoperative Ccr was measured by the 24-h method in all patients. The indocyanine green retention rate at 15 min (ICGR15) was also performed before hepatectomy. Serum creatinine, BUN and albumin levels were examined before hepatectomy, and 1, 2, 3, 5, 7, 14, 21 and 28 d after hepatectomy. The preoperative three-variable equation for eGFR (eGFR3) and the five-variable equation for eGFR (eGFR5) were calculated using the new formulas for Japanese patients[10]. The maximum serum creatinine and BUN levels after hepatectomy (post Cr, post BUN) were determined, and the postoperative rate of increase in the serum creatinine level (post Cr rate) was calculated by the following formula using the preoperative serum creatinine level (pre Cr) and post Cr. Post Cr rate (%) = (post Cr - preCr) × 100/post Cr.
Abnormal Ccr was defined as < 50 mL/min according to the New York Heart Association criteria[1112], and groups with abnormal eGFR3 and eGFR5 were defined as < 60 mL/min per 1.73 m2 according to the stage of chronic kidney disease[13].
Indications for hepatectomy were determined using the criteria of Makuuchi[14]. Portal embolization (PE) before hepatectomy was indicated in patients undergoing major hepatectomy when the estimated liver volume after hepatectomy was not sufficient to tolerate surgery (remnant liver volume < 40%)[1516]. Transcatheter arterial embolization (TAE) was performed preoperatively in patients with massive HCC in order to occlude arterio-portal shunts. Preoperative biliary drainage was carried out in patients with obstructive jaundice. Liver resection was performed when the serum total bilirubin level was < 2.0 mg/dL. Simultaneous hepatectomy plus splenectomy, or Hassab’s operation, were indicated for control of portal hypertension, esophageal and gastric varices, and thrombocytopenia (platelet count < 5.0 × 104/mm3) in patients with HCC and liver cirrhosis[17]. The liver parenchyma was transected by the crush method using a Pean forceps or Cavitron Ultrasonic Aspirator while employing the intermittent Pringle maneuver. After resection of the liver tumors and subsequent hemostasis, the cut liver surface was coated with fibrin glue. The abdomen was then closed after placing drains around the cut liver surface. Major hepatectomy was classified as removal of one Couinaud segment or more, and minor hepatectomy as removal of less than one Couinaud segment.
Data were expressed as median (range). Non-parametric data were evaluated by χ2 test and Kruskal-Wallis test between groups showing normal and abnormal values of Ccr, eGFR3 and eGFR5. Parametric data including post Cr and post Cr rate were compared among groups with normal and abnormal Ccr, eGFR3 and eGFR5 values using the Mann-Whitney U test. Correlations between Ccr or post Cr and eGFR3 and eGFR5 were analyzed using Pearson’s correlation coefficient. Differences at P < 0.05 were considered to be significant.
Liver resections were performed for malignant disease in 180 patients and for benign disease in 17 patients. Malignant disease included 117 HCCs, 40 metastatic liver tumors, 16 biliary malignancies, three cholangiocarcinomas and four other malignancies. Benign lesions included three giant hepatic cysts, three biliary cyst adenomas, three donors of living-related liver transplantation, two cases of hepatolithiasis, two of massive hemangioma, two of cholecystitis, and two cases of regenerative nodules. There were no significant differences in diseases between the groups with normal and abnormal Ccr, eGFR3 and eGFR5 values (Table 1).
| Overall | Abnormal Ccr group | Abnormal eGFR3 group | Abnormal eGFR5 group | |
| Malignant tumors | ||||
| HCC | 117 | 23 | 15 | 23 |
| Cholangiocarcinoma | 3 | 1 | 0 | 0 |
| Metastatic liver tumor | 40 | 7 | 7 | 8 |
| Hilar BDC | 12 | 2 | 0 | 1 |
| Gall bladder carcinoma | 4 | 1 | 2 | 2 |
| Combined HCC | 3 | 1 | 1 | 1 |
| GIST | 1 | 1 | 1 | 1 |
| Total | 180 | 36 | 26 | 36 |
| Benign diseases | ||||
| Hepatolithiasis | 2 | 0 | 0 | 0 |
| Hepatic cyst | 3 | 0 | 2 | 2 |
| Hemagioma | 2 | 0 | 1 | 1 |
| Biliary cyst adenoma | 3 | 1 | 1 | 1 |
| Cholecystitis | 2 | 0 | 1 | 0 |
| Regenerative nodule | 2 | 0 | 0 | 0 |
| Donor | 3 | 0 | 0 | 0 |
| Total | 17 | 1 | 5 | 4 |
| P = 0.9065 | P = 0.4630 | P = 0.3889 |
Clinical background characteristics of the Ccr, eGFR3 and eGFR5 groups are shown in Tables 2, 3, 4, respectively. The median ages of patients with abnormal Ccr and eGFR5 values were significantly greater than those of patients with normal values. There were no significant differences in sex, height, weight, viral infection, ICGR15, or frequency of preoperative treatment between the groups with normal and abnormal Ccr, eGFR3 and eGFR5 values.
| Normal Ccr group (n = 160) | Abnormal Ccr group (n = 37) | P | |
| Age (yr) | 66 (30-82) | 71 (46-85) | 0.0344 |
| Sex (male:female) | 116:44 | 31:6 | 0.1552 |
| Height (cm) | 160.6 (134.5-179.6) | 162.4 (133.9-182.7) | 0.4109 |
| Weight (kg) | 59.2 (33.0-95.6) | 61.6 (44.5-93.0) | 0.3543 |
| Hepatitis virus (-:+) | 76:84 | 15:22 | 0.4441 |
| ICGR15 (%) | 13 (1-74) | 14 (4-49) | 0.9085 |
| Preoperative treatment (-:+) | 134:26 | 32:5 | 0.6804 |
| Normal eGFR3 group (n = 160) | Abnormal eGFR3 group (n = 37) | P | |
| Age (yr) | 66 (30- 82) | 69 (48-85) | 0.0887 |
| Gender (male:female) | 125:41 | 22:9 | 0.6108 |
| Height (cm) | 161.0 (133.9-182.7) | 160.4 (143.2-173.0) | 0.0511 |
| Weight (kg) | 59.2 (33.0-93.4) | 60.1 (44.6-95.6) | 0.1656 |
| Hepatitis virus (-:+) | 77:89 | 14:17 | 0.9001 |
| ICGR15 (%) | 13 (3-74) | 13 (1-31) | 0.9085 |
| Preoperative treatment (-:+) | 138:28 | 28:3 | 0.3129 |
| Normal eGFR5 group (n = 157) | Abnormal eGFR5 group (n = 40) | P | |
| Age (yr) | 65 (30-82) | 71 (55-85) | 0.0003 |
| Sex (male:female) | 119:38 | 28:12 | 0.4521 |
| Height (cm) | 161.5 (133.9-182.7) | 158.5 (138.0-173.0) | 0.0751 |
| Weight (kg) | 59.7 (33.0-95.6) | 58.4 (44.0-93.0) | 0.5478 |
| Hepatitis virus (-:+) | 75:82 | 16:24 | 0.3788 |
| ICGR15 (%) | 13 (3-74) | 14 (1-31) | 0.6363 |
| Preoperative treatment (-:+) | 130:27 | 36:4 | 0.2644 |
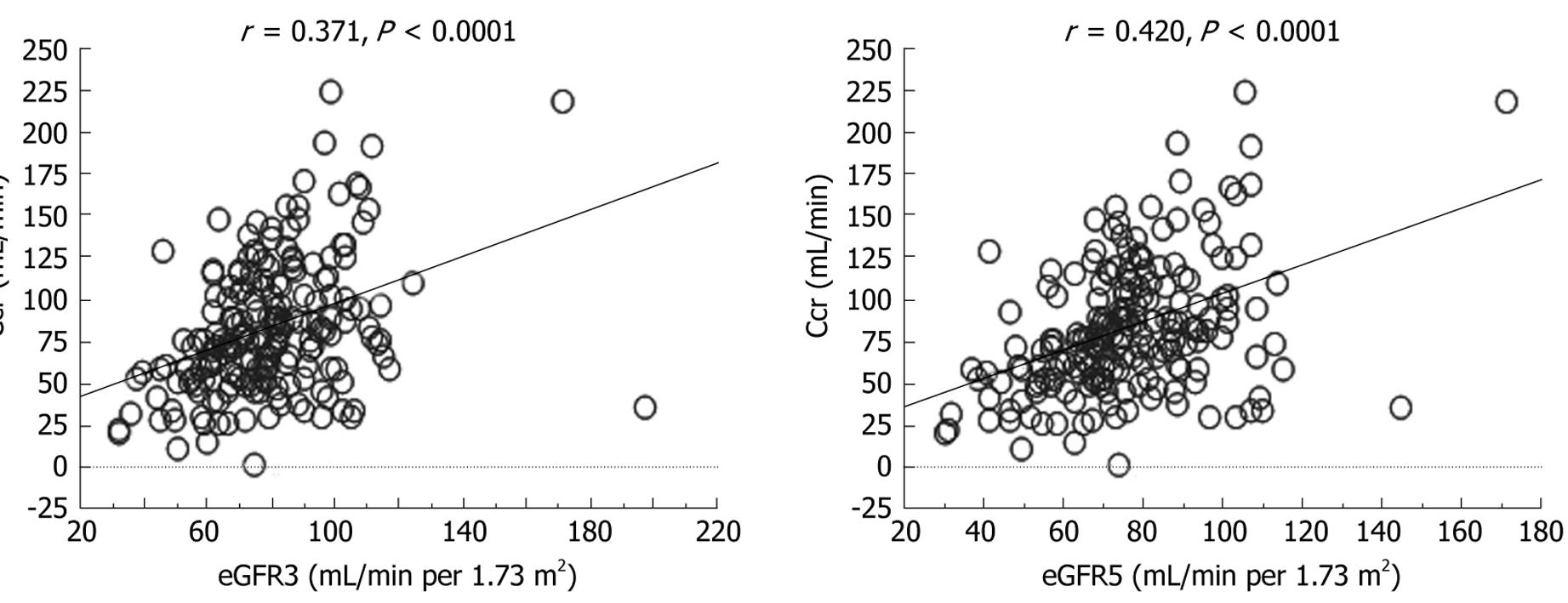
Thirty-seven patients (18.8%) had abnormal Ccr, 31 (15.7%) had abnormal eGFR3, and 40 (20.3%) had abnormal eGFR5 values. Preoperative serum Cr and BUN levels, Ccr, eGFR3 and eGFR5 in all the patients with abnormal parameters were significantly worse than those in all the normal patients. Although there were no differences in serum albumin levels between the groups that had normal and abnormal Ccr and eGFR3, the serum albumin level was significantly decreased only in the group with eGFR5 abnormality (Table 5). The correlation between Ccr and eGFR5 was stronger than that between Ccr and eGFR3 (Figure 1).
| sCr (mg/dL) | BUN (mg/dL) | sAlb (g/dL) | Ccr (mL/min) | eGFR3 | eGFR5 | |
| Overall (n = 197) | 0.73 (0.33-1.74) | 13 (5-31) | 3.3 (2.1-4.4) | 76.8 (1.3-226.1) | 77.6 (30.7-196.8) | 73.9 (29.8-171.0) |
| Normal Ccr (n = 160) | 0.73 (0.33-1.51) | 13 (5-30) | 3.3 (2.1-4.4) | 86.3 (50.0-226.1) | 78.3 (36.7-171.0) | 75.1 (36.4-171.0) |
| P < 0.05 | P < 0.05 | NS | P < 0.05 | P < 0.05 | P < 0.05 | |
| Abnormal Ccr (n = 37) | 0.81 (0.35-1.74) | 15 (6-31) | 3.3 (2.5-4.0) | 34.1 (1.3-49.9) | 66.6 (30.7-196.8) | 64.5 (29.8-144.6) |
| Normal eGFR3 (n = 166) | 0.71 (0.33-1.04) | 13 (5-31) | 3.3 (2.1-4.4) | 83.6 (1.3-226.1) | 80.7 (60.2-196.8) | 77.0 (45.9-171.0) |
| P < 0.05 | P < 0.05 | NS | P < 0.05 | P < 0.05 | P < 0.05 | |
| Abnormal eGFR3 (n = 31) | 1.05 (0.75-1.74) | 17 (9-26) | 3.2 (2.1-4.1) | 52.4 (11.8-129.1) | 52.4 (30.7-59.9) | 48.7 (29.8-68.7) |
| Normal eGFR5 (n = 157) | 0.70 (0.33-1.07) | 12 (5-22) | 3.4 (2.1-4.4) | 84.2 (1.3-226.1) | 81.0 (58.0-196.8) | 78.3 (62.1-171.0) |
| P < 0.05 | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.05 | P < 0.05 | |
| Abnormal eGFR5 (n = 40) | 0.97 (0.70-1.74) | 18 (10-31) | 3.1 (2.1-4.1) | 53.6 (11.8-129.1) | 55.4 (30.7-68.3) | 52.6 (29.8-59.9) |
Surgical details of the patients are shown in Table 6. Seventy-three patients underwent extensive hepatectomy. Among these patients, 28 (14.2%) underwent extended lobectomy, and 19 (9.6%) underwent lobectomy. According to the Couinaud classification, 26 patients (13.2%) underwent bisegmentectomy, and 37 (18.8%) underwent segmentectomy. Eighty-seven patients (44.2%) underwent partial hepatectomy. Although intraoperative blood loss in the patients with eGFR5 abnormality was significantly greater than that of normal patients, there were no significant differences in other surgical background factors, such as operation time, Pringle time, and type of surgical treatment between patients who were normal and abnormal for Ccr, eGFR3 and eGFR5.
| Operative times (min) | Blood loss (mL) | Pringle time (min) | Hepatectomy (minor:major) | Hepatectomy (alone:plus) | |
| Overall (n = 197) | 320 (117-806) | 590 (0-12762) | 42 (5-176) | 124:73 | 160:37 |
| Normal Ccr (n = 160) | 320 (117-721) | 573 (0-7240) | 43 (5-176) | 99:61 | 132:28 |
| NS | NS | NS | NS | NS | |
| Abnormal Ccr (n = 37) | 321 (180-806) | 618 (114-12762) | 35 (9-98) | 25:12 | 28:9 |
| Normal eGFR3 (n = 166) | 323 (117-721) | 553 (0-7240) | 42 (5-176) | 103:63 | 136:30 |
| NS | NS | NS | NS | NS | |
| Abnormal eGFR3 (n = 31) | 297 (168-806) | 680 (126-12762) | 46 (12-87) | 21:10 | 24:7 |
| Normal eGFR5 (n = 157) | 325 (117-721) | 551 (0-7240) | 42 (5-176) | 97:60 | 128:29 |
| NS | P < 0.05 | NS | NS | NS | |
| Abnormal eGFR5 (n = 40) | 296 (142-806) | 694 (126-12762) | 44 (11-98) | 27:13 | 32:8 |
Although neither operative nor hospital deaths were recorded, three patients (1.52%) required hemodialysis after hepatectomy because of multiple organ failure (two cases) and enterocolitis. Hepatic failure occurred in two patients. Postoperative results are shown in Table 7. Post Cr and post BUN of patients with eGFR3 and eGFR5 abnormalities were significantly higher than in normal patients, but post Cr and post BUN in patients with Ccr abnormality were not significantly higher than those in in normal patients.
| Post Cr (mg/dL) | Post BUN (mg/dL) | Post Cr rate (%) | |
| Overall (n = 197) | 0.87 (0.43-8.43) | 18 (7-97) | 11.3 (0-88.1) |
| Normal Ccr (n = 160) | 0.85 (0.43-5.71) | 17 (7-81) | 11.0 (0-88.1) |
| P = 0.0936 | P = 0.0875 | P = 0.8253 | |
| Abnormal Ccr (n = 37) | 0.95 (0.50-8.43) | 20 (10-97) | 12.3 (0-79.4) |
| Normal eGFR3 (n = 166) | 0.83 (0.43-5.71) | 17 (7-81) | 11.4 (0-88.1) |
| P < 0.0001 | P = 0.0033 | P = 0.4575 | |
| Abnormal eGFR3 (n = 31) | 1.17 (0.70-8.43) | 21 (11-97) | 9.0 (0-79.4) |
| Normal eGFR5 (n = 157) | 0.81 (0.43-5.71) | 16 (7-81) | 11.3 (0-88.1) |
| P < 0.0001 | P < 0.0001 | P = 0.8950 | |
| Abnormal eGFR5 (n = 40) | 1.14 (0.70-8.43) | 23 (11-97) | 11.5 (0-79.4) |
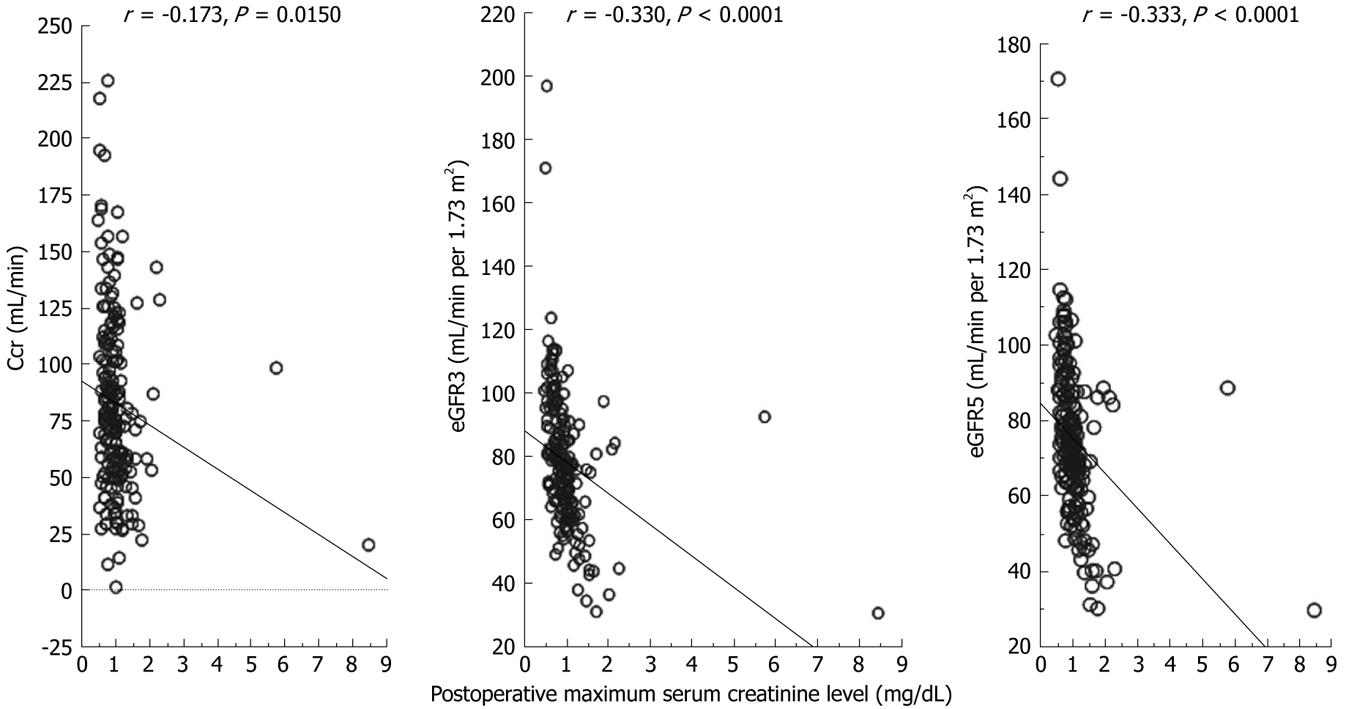
Figure 2 shows the correlation between Ccr, eGFR3 and eGFR5 and post Cr. Although a weak correlation between post Cr and Ccr was observed, there were significant correlations between post Cr and eGFR5, and the correlation between post Cr and eGFR5 was higher than that between post Cr and eGFR3. Post Cr rates of patients with Ccr, eGFR3 and eGFR5 abnormality were not significant (Table 7).
As a preoperative renal function test, it is ideal to measure GFR by inulin clearance, but this is not practical in a clinical setting. Although the easiest renal function test to perform is measurement of serum creatinine level, use of this parameter alone is not recommended because it is affected by various factors such as muscle mass, sex, age, diet, and renal tubule function[913]. Therefore, Ccr has been measured routinely in patients undergoing major surgery for a long time. Determination of Ccr requires timed urine collection and blood sampling. Twenty-four-hour urine collection is especially inconvenient for patients with neurogenic bladder or the elderly. On the other hand, eGFR3 and eGFR5 require only a single blood sample, and can be estimated on the basis of age, sex, serum creatinine, BUN and albumin without the need for urine collection. If eGFR3 and eGFR5 are superior to Ccr for preoperative renal function testing, there would be certain advantages in terms of clinical effort and cost.
The results of this study demonstrated that 24-h urine collection for measurement of Ccr no longer appears necessary on a routine basis for estimation of preoperative renal function. In fact, there were no significant differences in the post Cr or BUN level after hepatectomy between patients who had normal and abnormal preoperative Ccr values (Table 7). Post Cr and BUN levels in patients with eGFR3 and eGFR5 abnormalities were significantly higher than those in normal patients. In addition, the correlations between post Cr and eGFR3, and eGFR5 were significant, but that between post Cr and Ccr was not significant (Figure 2). These results indicate that eGFR3 and eGFR5 are superior to Ccr for predicting post renal dysfunction.
In this study, the post Cr rate after hepatectomy was also evaluated in patients who had normal and abnormal Ccr, eGFR3 and eGFR5 values, and no significant differences were evident (Table 7). We have already reported that hepatectomy can be performed safely without rapid and progressive deterioration of renal function in patients with non-uremic renal failure (Ccr of > 20 but < 50 mL/min)[6]. The factors affecting the post Cr rate after hepatectomy are preoperative liver function (ICGR15 > 20%), intraoperative blood loss and operation time, and not preoperative renal dysfunction (data not shown).
In this study, eGFR5 differentiated 40 patients with preoperative renal dysfunction among 197 patients more sensitively than Ccr or eGFR3. There was a stronger positive correlation between Ccr and eGFR5 than between Ccr and eGFR3 (Figure 1). Although the equation for eGFR5 is a little complex, eGFR5 is more suitable than eGFR3 for patients undergoing hepatectomy. Since serum albumin level is one of the factors that reflects liver preservation, patients with HCC and liver cirrhosis frequently have lower levels of serum albumin. In fact, in this study, preoperative serum albumin levels ranged from 2.1 to 4.4 g/dL, with a median value of 3.3 g/dL. Thus, eGFR5 appears to be a more acceptable parameter for accurate preoperative evaluation of renal function in hepatectomy patients presenting a wide range of serum albumin levels.
To the best of our knowledge, this is the first retrospective study to have compared Ccr and eGFR as a preoperative renal function test in patients undergoing hepatectomy. Since equations for eGFR in individuals of caucasian, black and Japanese ethnicity have been established, eGFR is now almost universally available. We suggest that eGFR3 and eGFR5 are useful as preoperative renal function parameters in patients undergoing hepatectomy worldwide.
In conclusion, we recommend eGFR5 using serum albumin level as a preoperative renal function test in patients undergoing hepatectomy. Ccr is no longer recommended as a first-choice preoperative renal function test.
Although creatinine clearance (Ccr) has been measured clinically by a simple method as a preoperative renal function test, Ccr is not strictly equal to glomerular filtration rate (GFR). Recently, an equation for estimated GFR (eGFR) for Japanese individuals has been postulated. It has been accepted that eGFR is equal to measured GFR in chronic kidney disease. However, there have been no previous studies regarding the reliability of eGFR as a preoperative renal function test.
If eGFR is superior to, or equal to Ccr as a preoperative renal function test, eGFR should replace Ccr because of its simplicity of measurement. The authors retrospectively compared Ccr and eGFR as a preoperative renal function test in patients undergoing hepatectomy.
eGFR is useful as preoperative renal function parameters in patients undergoing hepatectomy. Ccr is no longer recommended as a first-choice preoperative renal function test.
Although Ccr has been used as preoperative renal function test, eGFR should replace Ccr as a routine preoperative renal function test in various surgical fields.
eGFR is estimated GFR which is calculated from age, sex, serum creatinine value (eGFR3), or adding serum albumin concentration and BUN value (eGFR5).
This is a well-written paper on normal and sensitive parameters of renal function, i.e. eGFR3 and eGFR5 as predictors of renal function after hepatectomy. These parameters seem easy to determine, accurate and well-associated with the stage of kidney disease. The study seems well-designed and performed, original in concept and statistically valid.
| 1. | Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, van Heerden JA, Adson MA. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493-504; discussion 504-505. |
| 2. | Miyazaki M, Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S, Furukawa K, Takeuchi D. Surgical treatment for liver cancer. Current issues. Dig Surg. 2007;24:120-125. |
| 3. | Buell JF, Koffron A, Yoshida A, Hanaway M, Lo A, Layman R, Cronin DC, Posner MC, Millis JM. Is any method of vascular control superior in hepatic resection of metastatic cancers? Longmire clamping, pringle maneuver, and total vascular isolation. Arch Surg. 2001;136:569-575. |
| 4. | Vauthey JN, Klimstra D, Franceschi D, Tao Y, Fortner J, Blumgart L, Brennan M. Factors affecting long-term outcome after hepatic resection for hepatocellular carcinoma. Am J Surg. 1995;169:28-34; discussion 34-35. |
| 5. | Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Hirakata H, Watanabe T. Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol. 2007;11:41-50. |
| 6. | Sawada T, Kita J, Rokkaku K, Kato M, Shimoda M, Kubota K. Hepatectomy in patients with nonuremic minimal renal failure. J Gastrointest Surg. 2006;10:740-745. |
| 7. | Mori S, Sawada T, Hamada K, Kita J, Shimoda M, Tagaya N, Kubota K. Gastrectomy for patients with gastric cancer and non-uremic renal failure. World J Gastroenterol. 2007;13:4589-4592. |
| 8. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. |
| 9. | Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S. Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis. 2007;50:927-937. |
| 10. | Imai E, Horio M, Iseki K, Yamagata K, Watanabe T, Hara S, Ura N, Kiyohara Y, Hirakata H, Moriyama T. Prevalence of chronic kidney disease (CKD) in the Japanese general population predicted by the MDRD equation modified by a Japanese coefficient. Clin Exp Nephrol. 2007;11:156-163. |
| 11. | Oken DE. Criteria for the evaluation of the severity of established renal disease. Nephron. 1970;7:385-388. |
| 12. | Winearls CG. Clinical evaluation and manifestations of chronic renal failure. Comprehensive Clinical Nephrology. 1st edition. Mosby: London 2003; 68.1. |
| 13. | Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473-2483. |
| 14. | Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589-594. |
| 15. | Kinoshita H, Sakai K, Hirohashi K, Igawa S, Yamasaki O, Kubo S. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. 1986;10:803-808. |
| 16. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. |
| 17. | Sugawara Y, Yamamoto J, Shimada K, Yamasaki S, Kosuge T, Takayama T, Makuuchi M. Splenectomy in patients with hepatocellular carcinoma and hypersplenism. J Am Coll Surg. 2000;190:446-450. |