INTRODUCTION
Changes in the metabolic pathways involved in homeostasis and growth regulation of hepatic cells, and especially in the mitogen-activated protein kinase (MAPK) system, have been extensively studied in infectious/inflammatory conditions; of those, viral infections, and especially HBV and HCV in relation to hepatic carcinogenesis, have received most attention[1–3]. Very little is known about the capacity of helminth parasites and/or their components/secretions to influence liver cell homeostasis metabolic pathways. Actually, a few helminth parasites do affect the liver[4]. Among them, infection with Echinococcus multilocularis (E. multilocularis) larva (metacestode) affects primarily the liver and causes alveolar echinococcosis (AE) in intermediate hosts. It is an aggressive chronic parasitic infection that is characterized by a multivesicular structure surrounded by an extensive fibro-inflammatory host reaction[5]. In humans, who behave as accidental intermediate hosts, the severity of this life-threatening disease results from both a continuous asexual proliferation of the metacestode and an intense granulomatous infiltration around the parasite; the lesions behave like a slow-growing liver cancer. Invasion of biliary and vascular walls is another hallmark of this severe disease[67]. The ensuing fibrosis protects the patients against parasitic growth, but at the same time distorts the liver parenchyma[8–13]. Hepatomegaly is a usual symptom of AE; it has been ascribed to the liver regeneration which accompanies the pseudo-tumoral process[7]. However, unlike other forms of liver injury, e.g. from neoplasms, viral hepatitis or physical injury in which cell cycle regulatory genes have been extensively investigated[1415], the cellular and molecular consequences of E. multilocularis infection on liver cells have never been studied.
It has been shown that the larval development of E. multilocularis is triggered by cell signaling originating from the intermediate host[1617]. The phosphorylation of EmMPK1, a parasitic orthologue of the extracellular signal-regulated kinase (ERK) MAPK, is specifically induced in in-vitro-cultured E. multilocularis metacestode vesicles, in response to exogenous host serum, hepatic cells and/or human epidermal growth factor (EGF). The E. multilocularis metacestode is thus able to “sense” host factors which results in an activation of the parasite MAPK cascade[18]. The fact that tissue-dwelling E. multilocularis expresses signaling systems with significant homologies to those of the host raises the interesting question whether cross-communication between cytokines and corresponding receptors of host and parasite can occur during an infection, i.e. whether the parasite may also influence signaling mechanisms of host cells through the secretion of various molecules that might bind to host cell surface receptors. Such interactions could contribute to immunomodulatory activities of E. multilocularis or be involved in mechanisms of organotropism and/or in host tissue destruction or regeneration during parasitic development. Only gross changes in carbohydrate metabolism[19] and in protein/albumin secretion by liver cells[20] have been studied in experimental and in vitro models of E. multilocularis growth. To the best of our knowledge, no study has reported on the activation pattern of liver cell MAPK during E. multilocularis host infection. MAPKs are key regulators of cellular signaling systems that mediate responses to a wide variety of extracellular stimuli. MAPK signaling pathways, including c-Jun N-terminal kinase (JNK), p38 MAPK and ERK, play important roles in signal transduction from the cell membrane to the nuclear transcriptional factors; they cross-communicate and regulate the balance between cell survival and cell death in acute and chronic liver injury[2122]. Generally, the JNK and p38 MAPK families appear to be pro-apoptotic, while the ERK pathway appears to be anti-apoptotic in mediating specifically cell growth and survival signals in many cell types[23]. The dynamic balance of their activities appears critical in acute liver injury such as viral hepatitis, drug- or toxin-induced toxicity or acute rejection after liver transplantation as well as in chronic liver injury[124]. For all these reasons we chose them as a first target.
The aim of the present study was thus to explore the influence of E. multilocularis metacestode on the activation of MAPK signaling pathways (ERK1/2, JNK and p38) and on liver cell proliferation. To reach this goal, we first studied the changes induced in the liver of patients with chronic AE, and then, the changes in hepatic cell cultures in contact in vitro with (1) E. multilocularis vesicle fluid (EmF), and (2) E. multilocularis-conditioned medium (EmCM).
MATERIALS AND METHODS
Tissue samples
The diagnosis of AE was established on positive serology with ELISA using crude E. multilocularis and Em2 antigens[25] and characteristic liver lesions observed at ultrasound and CT-scanning, and confirmed by histological examination of the lesions. To demonstrate the influence of E. multilocularis lesions on the surrounding hepatic cells, paired liver specimens (volume: 0.5 cm3 each) were obtained at surgery by an experienced surgeon from AE patients at the Liver Surgery and Transplantation Units of the University Hospital, Besancon, France (one patient), and of 1st Teaching Hospital, Xinjiang Medical University (TH-XMU), Urumqi, China (four patients). In each patient, one specimen was taken close to the parasitic lesions (i.e. 0.5 cm from the macroscopic changes due to the metacestode/granuloma lesion, thus avoiding liver contamination with infiltrating immune cells and parasitic tissue), and one was taken distant from the lesions (i.e. in the non-diseased lobe of the liver whenever possible, or at least at 10 cm from the lesion), according to a previously described procedure[11]. Absence of contamination by the parasitic lesions was checked on all samples by histological examination. The patients gave their informed consent for the use of tissue samples for research, as part of a research project approved by the “Comité Régional de Protection des Personnes en Recherche Biomédicale” de Franche-Comté, according to the French regulation, and by the Ethical Committee of TH-XMU. The liver samples were homogenized in ice-cold lysis buffer as previously described[26] and homogenates were clarified by centrifugation at 10 000 g for 10 min at 4°C. Protein concentration was estimated by the BCA Assay kit (Sigma, Steinheim, Germany). Samples were stored at -80°C until use.
EmCM and EmF
The EmCM without serum was kindly provided by Klaus Brehm (Institute of Hygiene and Microbiology, University of Würzburg, Germany) and was prepared as described previously[27] and stored at -80°C until used.
EmF was extracted from vesicles in E. multilocularis-infected Cricetulus migratorius maintained at the Experimental Animal Research Laboratory of TH-XMU, according to the international guidelines for the maintenance of experimental animals for medical research. All procedures were carried out in a class II laminar flow cabinet with appropriate protective clothing. The parasite material was removed from the peritoneal cavity under aseptic conditions, and was washed three times in phosphate buffered saline. The membrane was punctured with a 21-gauge needle connected to a 50-mL syringe. Fluid was withdrawn carefully until E. multilocularis vesicles had visibly lost turgidity. The apex was dissected and the remaining fluid removed, ensuring that no protoscoleces were aspirated. EmF was centrifuged (10 000 g, 10 min) to remove debris, filtered through a 0.2-&mgr;m filter and stored at -80°C until use.
Cell isolation, culture of rat hepatocytes and treatment with EmCM or EmF
Rat hepatocytes were prepared as described previously[28] and cultured in William’s E culture medium in a humidified incubator at 37°C and 5% CO2 for 20 h before the start of the experiment, supplemented with 100 U/mL penicillin and 100 mg/mL streptomycin (Invitrogen, Paisley, UK), without the addition of hormones or growth factors. During the attachment period (4 h), 2 mmol/L glutamine, 4 mg/mL bovine insulin, 1 &mgr;mol/L dexamethasone, and 10% fetal calf serum (Life Technologies Ltd) were added to the medium. Hepatocyte viability was always more than 90% and purity more than 95%.
For the experiment, cells were washed and cultured for 20 h in serum-free insulin-free William’s E culture medium, then incubated with either EmF for 15 min, 30 min, 1, 2, 8 and 24 h or EmCM for 15 min, 30 min, 1, 2, 3, 8 and 24 h, respectively.
Western blotting analysis
Western blotting analysis of cell lysates was performed by SDS-PAGE using NuPAGE (Invitrogen, Carlsbad, CA, USA) followed by transfer to nitrocellulose membrane (Invitrogen). Ponceau S (Sigma) staining was used to ensure equal protein loading and electrophoretic transfer. Using the appropriate antibodies, ERK1/2, JNK, p38 and their corresponding phosphoproteins, phosphorylated MAPK/ERK kinase 1/2 (MEK1/2), phosphorylated ribosomal S6 kinase (RSK), phosphorylated transcription factor Elk-1 (Elk-1), [Cell Signaling Technology (Beverly, MA, USA) and β-tubulin (Sigma)] were detected with the WesternBreeze Kit (Invitrogen). The expression levels of p-ERK1/2 /total ERK1/2 (signal at 44 kDa), p-p38/total p38 and p-JNK/total JNK (signal at 54 kDa) proteins (in “relative units”) in control cultures and cultures treated with EmCM or EmF were quantified using Quantity One software (Bio-Rad, Hercules, CA, USA).
Assay for cytotoxicity of EmCM or EmF
Primary cultures of rat hepatocytes were plated in 96-multiwell plates. After attachment, they were treated with EmF (diluted by half in William’s E culture medium) or pure EmCM for 24 h and cell viability was assessed[29]. No toxic effect was found.
Detection of proliferating cell nuclear antigen (PCNA) in liver sections
Formalin-fixed, paraffin-embedded sections of the five AE patients’ livers were stained for the presence of PCNA using a biotinylated anti-PCNA antibody (Boshide Inc., Wuhan, China) according to the manufacturer’s instructions. PCNA-positive hepatocytes were counted in three random visual fields of 0.95 mm2 each, at initial magnification × 20, for each sample, and the number expressed as the percentage of PCNA-positive cells to the total number of cells counted.
Statistics analysis
Data were presented as the mean ± SD and analyzed using SPSS version 11.0 software (SPSS, Chicago, IL, USA). Statistical significance was tested using the Student t test; a P value of less than 0.05 was considered significant.
RESULTS
ERK1/2 and p38 activation in AE patients
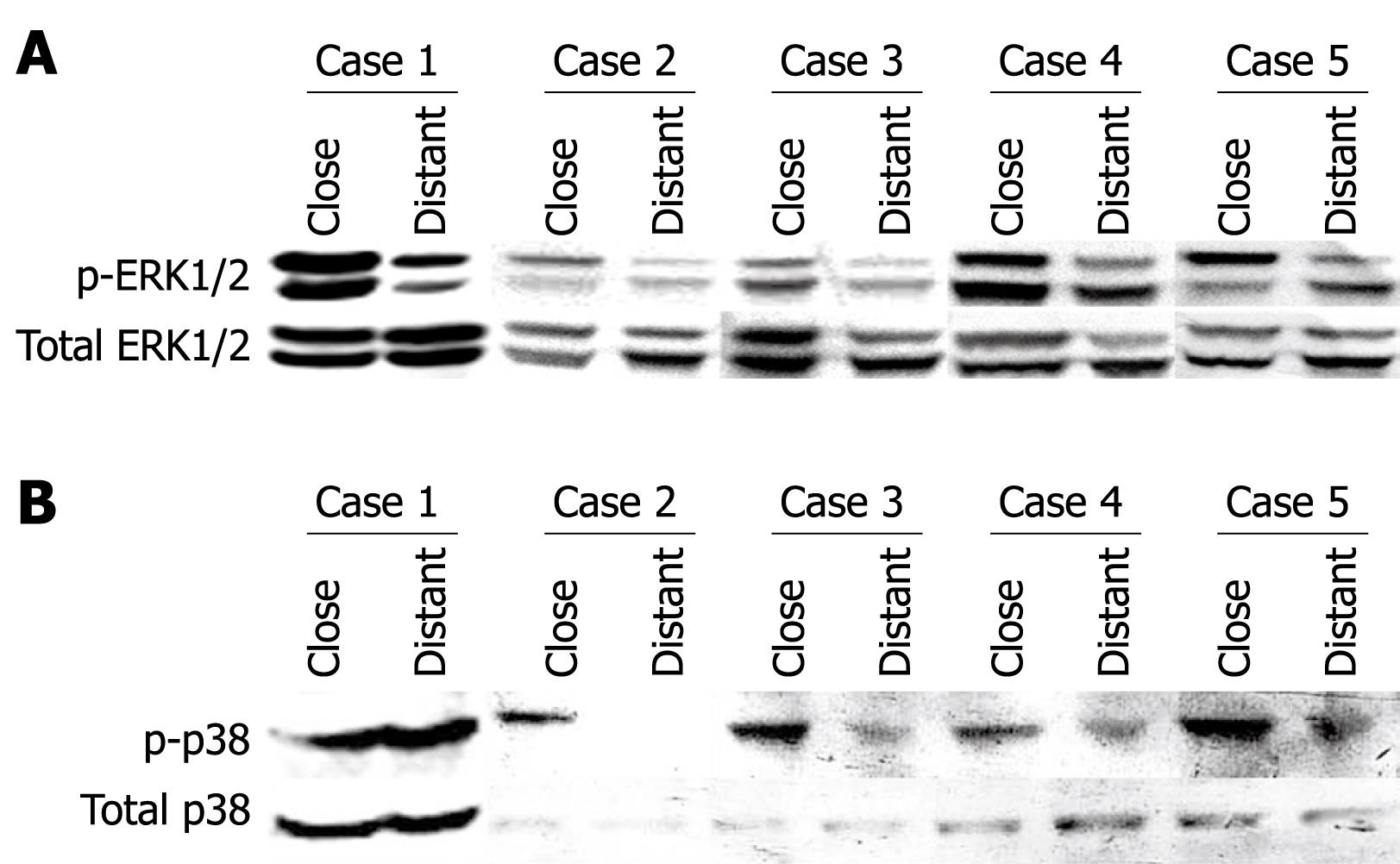
ERK1/2 phosphorylation was assessed in liver samples taken close to and distant from the parasitic lesions in five AE patients. As shown in Figure 1A, ERK1/2 phosphorylation was 1.58-fold to 4.26-fold higher in the liver close to the parasitic lesion than in the distant liver. p38 phosphorylation was found in the liver of all AE patients; it was more prominent in the liver close to the parasitic lesion than in liver distant from the lesion (1.70 to 3.40-fold), except in one patient (0.55-fold) (Figure 1B).
Figure 1 ERK1/2 (A) and p38 (B) activation in liver samples from five AE patients.
Western immunoblot analyses were performed on lysates with antibodies that recognize phosphorylated and total ERK1/2 respectively (A), and phosphorylated- and total p38, respectively (B). Close: Liver samples close to the parasitic lesions in AE patients; Distant: Liver samples distant from the parasitic lesions in AE patients.
Expression of PCNA in AE patients
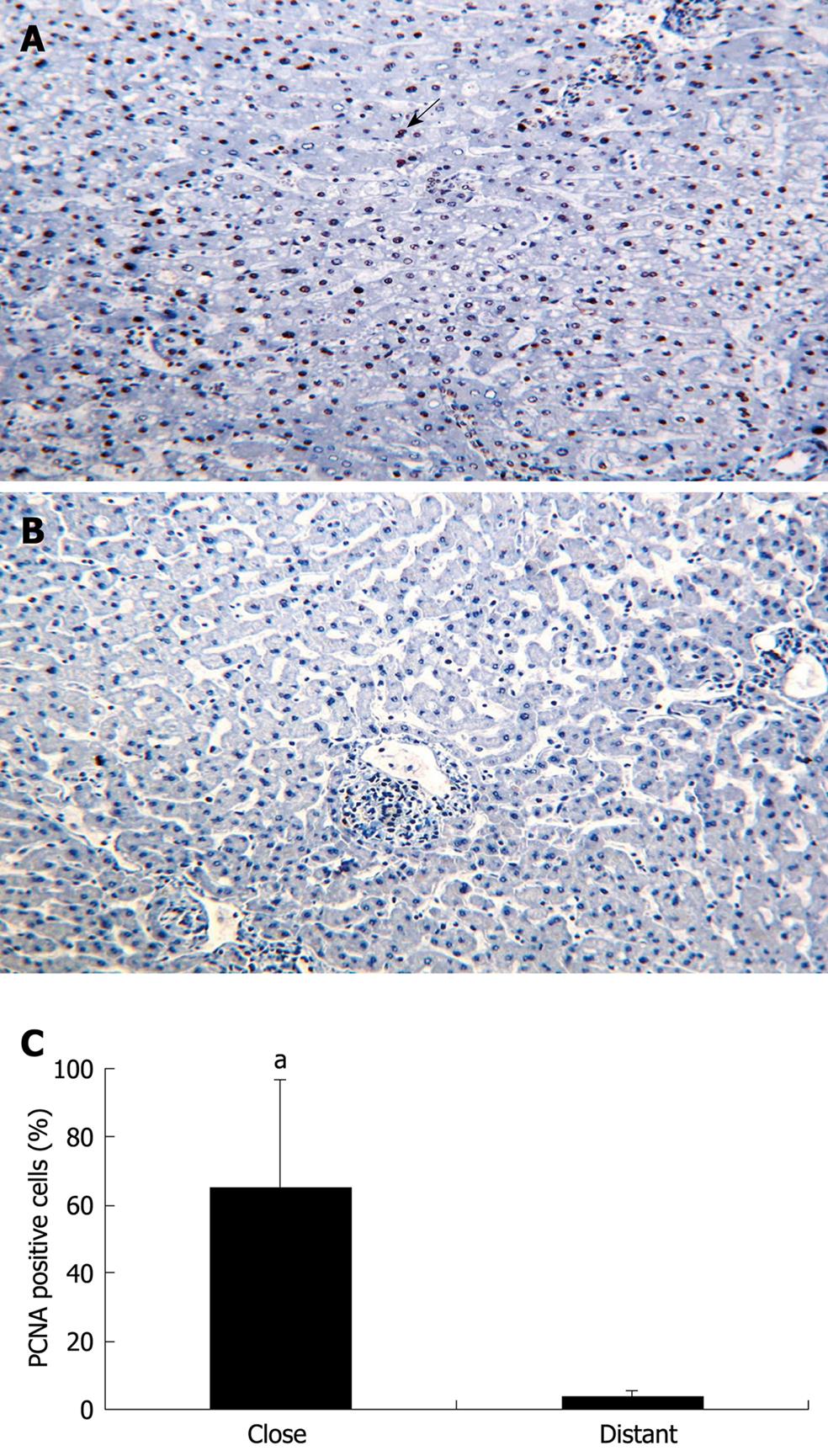
The expression of PCNA, an important growth marker and DNA replication regulator, was assessed in the liver close to and distant from the parasitic lesions in five AE patients. As shown in Figure 2A, an increased expression of PCNA was observed in the liver close to the parasitic lesions compared to the liver distant from the parasitic lesion (Figure 2B). Although a faint expression of PCNA could be detected in the distant liver in one case, there was a significant difference between PCNA expression in the hepatocytes close to and distant from the parasitic lesion (P < 0.05, Figure 2C).
Figure 2 PCNA expression by hepatic cells in the liver from five patients with AE (immunohistochemical analysis).
A: Hepatic cells close to the parasitic lesions were strongly labeled by the anti-PCNA antibody; all cells with a dark-brown/black nucleus are positive cells; some of them indicated by an arrow (initial magnification: × 20); B: Hepatic cells distant from the parasitic lesions did not express PCNA (initial magnification: × 20); C: Quantitative expression of PCNA was significantly higher in the liver cells close to the parasitic lesions than in those distant to them (aP < 0.05).
MAPKs (ERK1/2, JNK and p38) activation by exposure of primary hepatocytes to EmF or EmCM
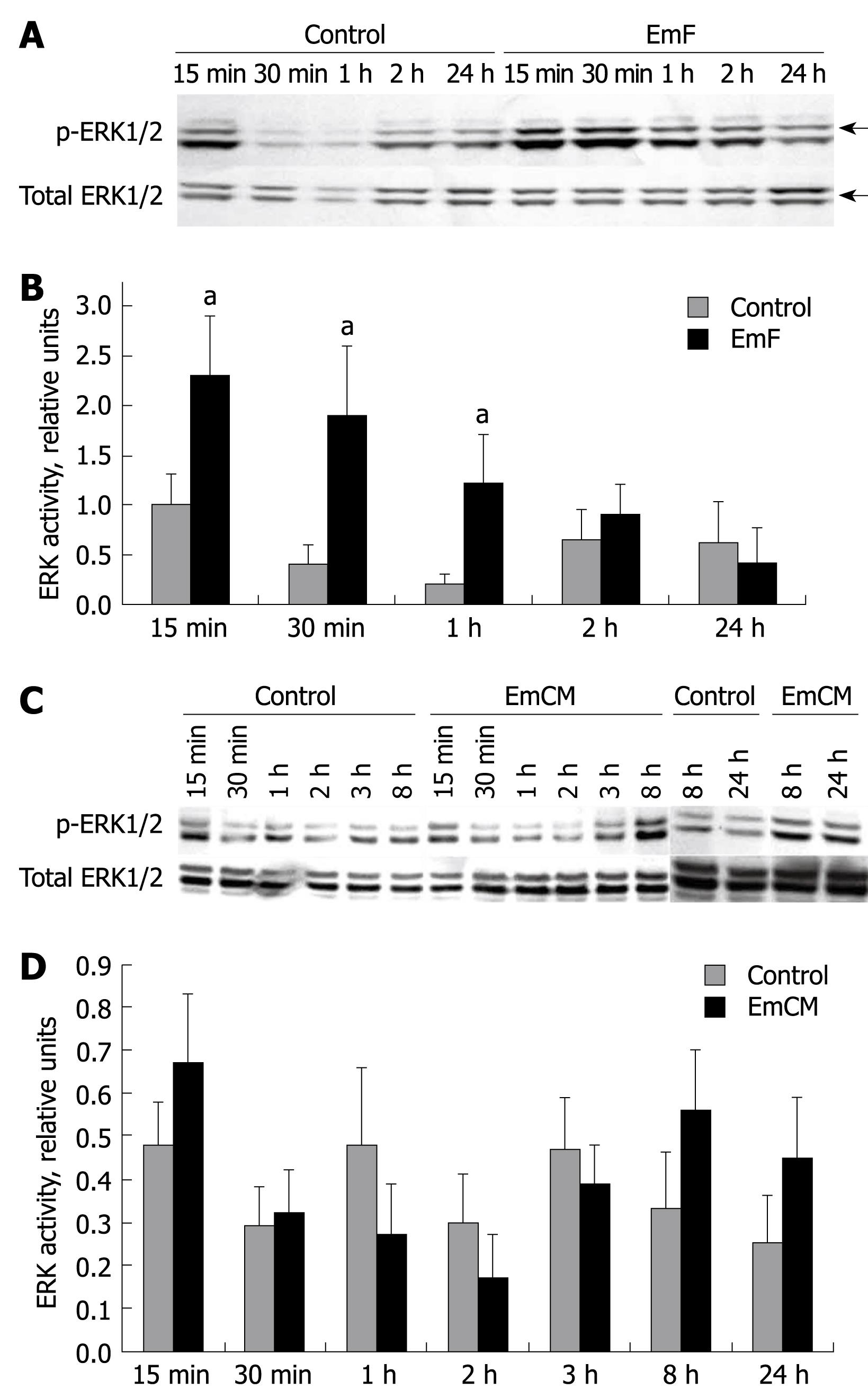
To investigate whether the MAPKs were also activated in primary cultured hepatocytes in contact with EmF or EmCM, we measured phosphorylated and total ERK1/2, JNK and p38. As shown in Figure 3A, increased ERK1/2 phosphorylation was observed from 15 min to 2 h and peaked at 1 h after incubation with EmF. EmF increased the phosphorylation of ERK1/2 (threonine-202, tyrosine-204) from approximate 2.50-fold at 15 min to 6.50-fold at 1 h (Figure 3B). There was a significant difference between non-treated and EmF-treated liver cell cultures at the 15 min, 30 min and 1 h time-points (P < 0.05). In contrast, EmCM only weakly stimulated ERK activity from approximately 1.37-fold at 15 min and approximately 1.84-fold at 8 h to approximately 2.42-fold at 24 h (Figure 3C and D).
Figure 3 Time course of EmF- or EmCM- induced phosphorylation of ERK1/2 kinase.
Primary cultures of rat hepatocytes were stimulated with EmF (A, B) or EmCM (C, D) and harvested at the indicated time points (15 min to 24 h). Western immunoblot analyses were performed on lysates with antibodies that recognize phosphorylated (p-) and total ERK1/2 (A, C), respectively. Relative amount of phosphorylated to total ERK1/2 and ERK1/2 was calculated from semi-quantitative analysis of the Western blotting using densitometry (B, D). aP < 0.05, EmF or EmCM-induced versus control hepatocytes. All experiments were performed three times independently with similar results.
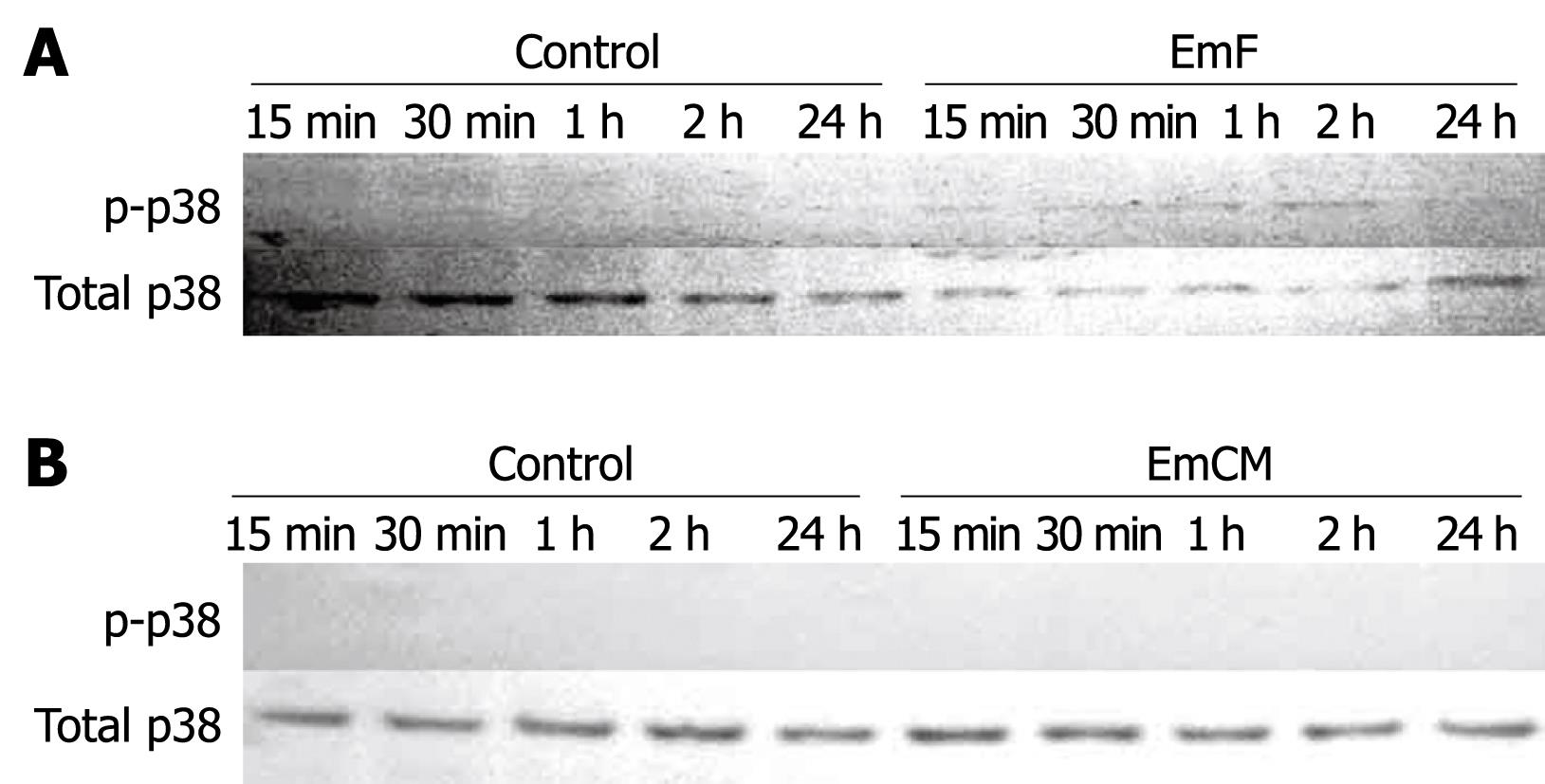
EmF slightly activated p38 at 1, 2 and 24 h (Figure 4A). No activation of p38 MAPK could be detected in EmCM-stimulated hepatocytes (Figure 4B).
Figure 4 Time course of EmF- or EmCM-induced phosphorylation of p38 kinase.
Primary cultures of rat hepatocytes were stimulated with EmF (A) or EmCM (B) and harvested at the indicated time points (15 min to 24 h). Western immunoblot analyses were performed on lysates with antibodies that recognize phosphorylated (p-) and total p38. All experiments were performed three times independently with similar results.
EmF increased the phosphorylation of JNK (threonine-183, tyrosine-185) from 2.63-fold at 15 min to 2.23-fold at 30 min, respectively (Figure 5A and B). Similar results were found in the EmCM-treated liver cells, as shown in Figure 5C and D: increased JNK phosphorylation was observed from 3.26-fold at 15 min to 1.94-fold at 30 min, respectively, and then there was a decrease to the baseline.
Figure 5 Time course of EmF- or EmCM-induced phosphorylation of JNK.
Primary cultures of rat hepatocytes were stimulated with EmF (A, B) or EmCM (C, D) and harvested at the indicated time points (15 min to 24 h). Western immunoblot analyses were performed on lysates with antibodies that recognize phosphorylated (p-) and total JNK respectively (A, C). Relative amount of phosphorylated to total JNK was calculated from semi-quantitative analysis of the Western blots using densitometry (B, D). aP < 0.05, EmF or EmCM-induced versus control hepatocytes. All experiments were performed three times independently with similar results.
Taken together, these results clearly show that EmF stimulated all 3 classes of MAPKs, but EmCM only induced ERK1/2 and JNK activation in primary hepatocytes.
ERK1/2 pathway activation by exposure of primary hepatocytes to EmF or EmCM
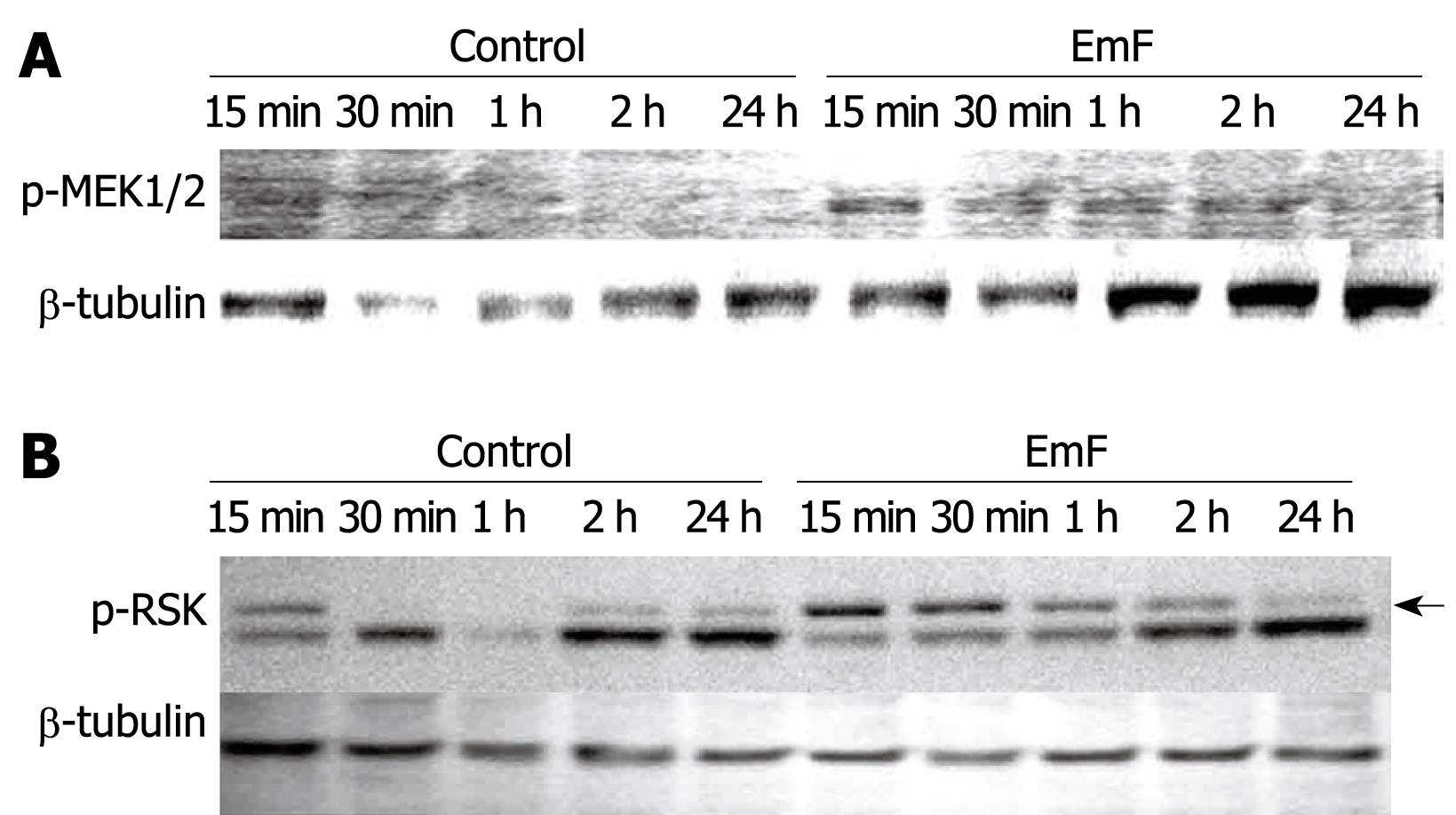
To further explore the effect of EmF and EmCM on the ERK activation pathway, we first studied the activation of MEK1/2, the physiological activator of ERK[2130]. We did indeed observe an activation of MEK1/2 from 15 min to 2 h of EmF exposure (Figure 6A). In contrast, MEK1/2 activation was not detectable at any time points during EmCM exposure (data not shown). Then, we studied the phosphorylation of RSK and Elk-1, cytoplasmic substrates of ERK1/2 and mediators of cell survival[233132]. As shown in Figure 6B, RSK phosphorylation was observed after exposure to EmF and maximal RSK activation was observed at 30 min. No phosphorylation of Elk-1 could be detected neither after EmF nor EmCM incubation (data not shown).
Figure 6 Time course of EmF-induced phosphorylation of MEK1/2 (A) and RSK (B).
Primary cultures of rat hepatocytes were stimulated with EmF and harvested at the indicated time points (15 min to 24 h). Western immunoblot analyses were performed on lysates with antibodies that recognize phosphorylated (p-) MEK1/2 and p-RSK. Protein loading control was performed using β-tubulin. MEK: MAPK/ERK kinase.
Thus, EmF exposure, but not EmCM exposure, induced RSK activation in hepatocyte cultures; none of them activated Elk-1.
DISCUSSION
In this study we found a significant influence of E. multilocularis metacestode on the activation of MAPK signaling pathways. In vivo, in the liver of AE patients, increased proliferation of hepatocytes was observed and ERK1/2 and p38 were phosphorylated, both being higher in the vicinity of the parasitic lesions. In vitro, in primary cultures of rat hepatocytes, three MAPKs (p38, JNK and ERK1/2) were activated upon exposure to E. multilocularis parasitic fluid, while p38 was undetectable and only JNK was up-regulated after incubation with supernatants of E. multilocularis axenic cultures.
The liver has the unique ability to regenerate after injury or loss of tissue. Liver regeneration is controlled by a wide array of signaling factors and plays a key role in recovery after acute and chronic liver injury[33]. Hepatic cell proliferation represents a central and unique feature of tissue repair after liver injury. ERK1/2 is considered to be an important inducer of the pro-mitogenic pathway and ERK1/2 activation is correlated with hepatocyte DNA replication in vivo and hepatocyte proliferation in vitro[3334]. In E. multilocularis infection, parasitic influence on liver cell proliferation might be crucial to ensure metacestode survival within the liver. Our data indicate that E. multilocularis infection of the liver actually activates ERK1/2 and induces cell proliferation. The major extent of size increase of the normal liver lobes has often been stressed in AE patients[7].
Specific stimulation of hepatocyte proliferation by metacestode-derived substances may add to the regeneration process that normally occurs following liver injury and explain this clinical observation. Such influence may be due either to a direct effect of substances of parasitic origin or to an indirect effect, through a response to host cytokines which are secreted by the macrophages and lymphocytes surrounding the parasitic lesions. A variety of host cytokines are actually present in the periparasitic environment of E. multilocularis infection[13]. They include both pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin-6 (IL-6) and IL-1β[1335] and anti-inflammatory cytokines such as IL-10[3637] and transforming growth factor-β (TGF-β)[38], and might be responsible for the observed changes in the MAPK system. As in vivo studies in infected patients did not allow us to determine precisely the mechanism of activation and the pathways involved, we used in vitro cultures of hepatocytes directly in contact with substances of parasitic origin to further analyze the origin of the activation processes.
MAPK activation occurred in rat hepatocyte cultures incubated with fluids of parasitic origin, in the absence of inflammatory cells. We may anticipate that at least part of the activation was related to direct interactions between E. multilocularis metacestode-derived components and the liver cells. Cross-functioning between parasite-derived molecules and host liver was described for parasite-derived enzymes: for instance, E. multilocularis-derived transglutaminase was shown to efficiently catalyze human liver-derived osteonectin cross-linking[8]. The significant changes observed using EmCM, which is totally free of host components, demonstrated that parasitic components specifically activated JNK and were actually acting on hepatocyte metabolic pathways. The most consistent data, however, were obtained by the incubation of rat hepatic cells with EmF. Upon exposure of hepatic cells to EmF, the expression of phosphorylated ERK1/2 paralleled that of phosphorylated JNKs. EmF exposure also induced the activation of MEK1/2 and RSK in hepatocytes. The differences between both stimuli might result from differences in the concentration of potential activators, EmF being more concentrated than EmCM. Alternatively, they might be due to the simultaneous presence of activating and inhibiting factors after 40 h of metacestode culture, while EmF collected in intermediate hosts infected with E. multilocularis for several weeks could be more concentrated in activating factors. Involvement of host factors stored in EmF could also explain the differences. In fact, in addition to proteins secreted by the germinal layer of Echinococcus sp. metacestodes, the vesicle fluid (often called hydatid fluid) may contain host proteins that are transported across the laminated layer and the germinal layer of the parasite. Albumin and globulins[39], inhibitors of the complement cascade[40] and, recently, host-derived active matrix metalloproteinase 9[41], were found in Echinococcus granulosus hydatid fluid or bound to the cyst wall. Heat shock proteins hsp70 and hsp20, which can interfere with MAPKs, especially p38, were also found in E. granulosus hydatid fluid[39]. It is highly likely that hydatid fluid from both species, E. granulosus and E. multilocularis, may also contain cytokines and growth factors of host origin and serve as storage for continuous release of factors both to the parasite and to the host through the laminated layer which appears critical at the host-parasite interface[42]. Dual interactions could thus ensure growth and survival of the parasite while interfering with host liver cells.
Several lines of evidence suggest that E. multilocularis differentiation is dependent on the receipt of appropriate host signals through surface receptors and their transduction through functional MAPK signaling pathways in the parasite[16184344]. Our data show that the reverse, i.e. parasite-derived signals efficiently acting on MAPK signaling pathways in host liver cells, is actually operating. Although the precise nature of these signals cannot be inferred from the present study, insulin and EGF, which have been identified as candidates for MAPK activation from the host to the parasite[1844] are possible candidates for MAPK activation from the parasite to the host. This has to be studied by additional experiments in vitro. In addition, other candidates cannot be ruled out; among them, TGF-β, which is present in the serum of infected experimental intermediate hosts[45] and in the periparasitic environment of E. multilocularis in the human liver[38]. TGF-β does not activate the MAPKs directly but may exert an indirect influence through the activation of Smads. E. multilocularis metacestode is sensitive to TGF-β signaling[4647] and the metacestode ERK-like kinase, EmMPK1, phosphorylates EmSmadD, a metacestode analogue of the Co-Smads of the TGF-β signaling cascade[46]. TGF-β is involved in immune suppression/tolerance[48], liver cell proliferation[49] and liver fibrosis, where it plays a major role in the activation and progression processes[50], where all three effects are essential to the pathogenesis of AE. This does not preclude, however, the importance of other cytokines or stress molecules.
In summary, three MAPKs, p38, JNK and ERK1/2, and the upstream (MEK1/2) and downstream (RSK) components of the ERK1/2 signaling pathways, are activated in primary cultures of rat hepatocytes by parasite- and/or host-derived substances. JNK activation by host-free supernatant of E. multilocularis cultures suggests that liver cell signaling pathways are actually activated by parasitic components. Hepatic proliferation in AE could thus be induced through a direct influence of the parasite and not only linked to the usual reaction of hepatic cells to the occupying process that takes place in the liver. The current investigation is the first which addresses the possible influence of E. multilocularis-related molecules on liver cells and demonstrates changes that are consistent with liver cell signaling through these molecules. Attempts to elucidate the nature and origin of the parasite-derived factors which influence intracellular signaling pathways in host cells may especially clarify the mechanism used by E. multilocularis to increase cell proliferation but also concomitant events, including parasite survival, immune suppression and induction of liver fibrosis.
COMMENTS
Background
Changes in the metabolic pathways involved in homeostasis and growth regulation of hepatic cells, and especially in the mitogen-activated protein kinase (MAPK) system, have been extensively studied in infectious/inflammatory conditions. Very little is known, however, on the capacity of helminth parasites and/or their components/secretions to influence liver cell homeostasis metabolic pathways and no study has reported on the activation pattern of liver cell MAPK during Echinococcus multilocularis (E. multilocularis) infection. Helminths developing in the liver may influence hepatic cell proliferation through the activation of MAPKs. The authors thus explored the effect of E. multilocularis on the activation of MAPKs signaling pathways and on liver cell proliferation.
Research frontiers
MAPKs play important roles in signal transduction from the cell membrane to the nuclear transcriptional factors; they cross-communicate and regulate the balance between cell survival and cell death in acute and chronic liver injury. The dynamic balance of their activities appears critical in acute liver injury such as viral hepatitis, drug- or toxin-induced toxicity or acute rejection after liver transplantation, as well as in chronic liver injury. Thus, exploring this system is the best way to study the interactions between the parasite and the host, relating to proliferation processes.
Innovations and breakthroughs
It is the first in vivo demonstration that a helminth parasite influences the proliferation/regeneration of hepatic cells and the concomitant activation of the MAPK metabolic pathway. Previous studies have demonstrated an influence of the host liver on the MAPK cascade in E. multilocularis metacestode; the data suggest that the reverse, i.e. parasite-derived signals efficiently acting on MAPK signaling pathways in host liver cells, is actually operating.
Applications
The observed changes could be involved in the development of the massive hepatomegaly often observed as a presenting symptom in alveolar echinococcosis in humans, and which makes major hepatic resections a therapeutic option for this disease. It could also be involved in other aspects of the host-parasite relationship, including parasite survival, immune suppression and induction of liver fibrosis. This opens new avenues of research to understand parasite-host interactions in the liver.
Terminology
MAPKs are cell signaling pathways that include c-Jun N-terminal kinase (JNK), p38 MAPK and extracellular signal-regulated kinase (ERK). Generally, the JNK and p38 MAPK families appear to be pro-apoptotic, while the ERK pathway appears to be anti-apoptotic in specifically mediating cell growth and survival signals in many cell types. The metacestode of E. multilocularis is the larval form of this cestode, which develops in rodent intermediate hosts and is responsible for the hepatic disease alveolar echinococcosis in humans.
Peer review
The manuscript describes an investigation on cell signaling events in the liver induced by infection with E. multilocularis. Experiments were performed on samples of infected human liver specimens or using conditioned media or vesicle fluid from infected animals to induce activation of the MAPK pathway in cultured hepatocytes. Whilst the in vitro hepatocyte data are supported by evidence of global MAPK activation in whole liver lysates, it would be interesting to complete the study by immunostaining with phospho-specific monoclonal antibodies for ERK and p38 in liver tissue, to identify which cell types are being modulated by the presence of parasite and the precise location of these cells. The study is well conceived and on the whole the experiments have been well thought out.