Published online Apr 21, 2009. doi: 10.3748/wjg.15.1880
Revised: March 3, 2009
Accepted: March 10, 2009
Published online: April 21, 2009
AIM: To review percutaneous transhepatic portal venoplasty and stenting (PTPVS) for portal vein anastomotic stenosis (PVAS) after liver transplantation (LT).
METHODS: From April 2004 to June 2008, 16 of 18 consecutive patients (11 male and 5 female; aged 17-66 years, mean age 40.4 years) underwent PTPVS for PVAS. PVAS occurred 2-10 mo after LT (mean 5.0 mo). Three asymptomatic patients were detected on routine screening color Doppler ultrasonography (CDUS). Fifteen patients who also had typical clinical signs of portal hypertension (PHT) were identified by contrast-enhanced computerized tomography (CT) or magnetic resonance imaging. All procedures were performed under local anesthesia. If there was a PVAS < 75%, the portal pressure was measured. Portal venoplasty was performed with an undersized balloon and slowly inflated. All stents were deployed immediately following the predilation. Follow-ups, including clinical course, stenosis recurrence and stent patency which were evaluated by CDUS and CT, were performed.
RESULTS: Technical success was achieved in all patients. No procedure-related complications occurred. Liver function was normalized gradually and the symptoms of PHT also improved following PTPVS. In 2 of 3 asymptomatic patients, portal venoplasty and stenting were not performed because of pressure gradients < 5 mmHg. They were observed with periodic CDUS or CT. PTPVS was performed in 16 patients. In 2 patients, the mean pressure gradients decreased from 15.5 mmHg to 3.0 mmHg. In the remaining 14 patients, a pressure gradient was not obtained because of > 75% stenosis and typical clinical signs of PHT. In a 51-year-old woman, who suffered from massive ascites and severe bilateral lower limb edema after secondary LT, PVAS complicated hepatic vein stenosis and inferior vena cava (IVC) stenosis. Before PTPVS, a self-expandable and a balloon-expandable metallic stent were deployed in the IVC and right hepatic vein respectively. The ascites and edema resolved gradually after treatment. The portosystemic collateral vessels resulting from PHT were visualized in 14 patients. Gastroesophageal varices became invisible on poststenting portography in 9 patients. In a 28-year-old man with hepatic encephalopathy, a pre-existing meso-caval shunt was detected due to visualization of IVC on portography. After stenting, contrast agents flowed mainly into IVC via the shunt and little flowed into the portal vein. A covered stent was deployed into the superior mesenteric vein to occlude the shunt. Portal hepatopetal flow was restored and the IVC became invisible. The patient recovered from hepatic encephalopathy. A balloon-expandable Palmaz stent was deployed into hepatic artery for anastomotic stenosis before PTPVS. Percutaneous transhepatic internal-external biliary drainage was performed in 2 patients with obstructive jaundice. Portal venous patency was maintained for 3.3-56.6 mo (mean 33.0 mo) and all patients remained asymptomatic.
CONCLUSION: With technical refinements, early detection and prompt treatment of complications, and advances in immunotherapy, excellent results can be achieved in LT.
- Citation: Wei BJ, Zhai RY, Wang JF, Dai DK, Yu P. Percutaneous portal venoplasty and stenting for anastomotic stenosis after liver transplantation. World J Gastroenterol 2009; 15(15): 1880-1885
- URL: https://www.wjgnet.com/1007-9327/full/v15/i15/1880.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1880
Liver transplantation is an important option in the management of end-stage liver disease, severe acute liver failure and some metabolic liver disorders. Postoperative vascular complications have been well documented. The incidence of portal venous complications following liver transplantation is considered to be relatively uncommon in comparison with hepatic arterial complications, yet they can be potentially devastating and lead to graft loss. In the past, portal venous complications were managed with surgical treatments such as thrombectomy, anastomosis revision or retransplantation. However, surgical management of these complications has been limited by technical difficulties due to postsurgical fibrosis and limitations in the length of the involved venous structures[1]. Percutaneous interventional procedures have gained worldwide acceptance for alleviating the symptoms of portal hypertension and preserving the graft, due to their minimal invasiveness as well as low complication and high success rates[2]. In this study, we retrospectively reviewed 16 cases that received percutaneous transhepatic portal venoplasty and stenting (PTPVS).
From April 2004 to June 2008, 16 of 18 consecutive patients (11 male and 5 female; aged 17-66 years, mean age 40.4 years) underwent PTPVS for portal vein anastomotic stenosis (PVAS) after liver transplantation (LT). Nine patients were from other hospitals. One patient had a living donor LT; another patient received a second graft. The right branch of the portal vein of the donor was anastomosed to the main portal vein of the recipient by end-to-end anastomosis in the living donor LT; all other reconstructions of the portal vein were performed by standard end-to-end anastomosis of the recipient and donor portal vein. PVAS occurred 2-10 mo after LT (mean 5.0 mo).
Three of 18 patients were asymptomatic and were detected on routine screening color Doppler ultrasonography (CDUS). Seven patients presented with increased liver function tests; two patients complicated with obstructive jaundice. Fifteen patients presented the typical clinical signs of portal hypertension (PHT), which included variceal bleeding (n = 7), ascites (n = 5), and splenomegaly (n = 4) with or without thrombocytopenia. The 15 symptomatic patients also were identified by other noninvasive imaging examinations.
The initial diagnosis of PVAS was based on CDUS in all 18 patients, and 15 of 18 patients also underwent contrast-enhanced computerized tomography (CT) or magnetic resonance imaging (MRI) to confirm the stenosis. The criteria of CDUS for the detection of PVAS were as follows: > 50% narrowing of the stenotic segment compared with the main portal venous diameter in adults or to a diameter < 2.5 mm in children on gray-scale imaging; the presence of an acceleration of flow at the stenosis or a poststenotic jet flow or scarcity flow of the intrahepatic portal vein on Doppler US[134]. The criterion for PVAS on CT or MRI was > 50% narrowing of the main portal venous diameter with or without poststenotic dilatation[5].
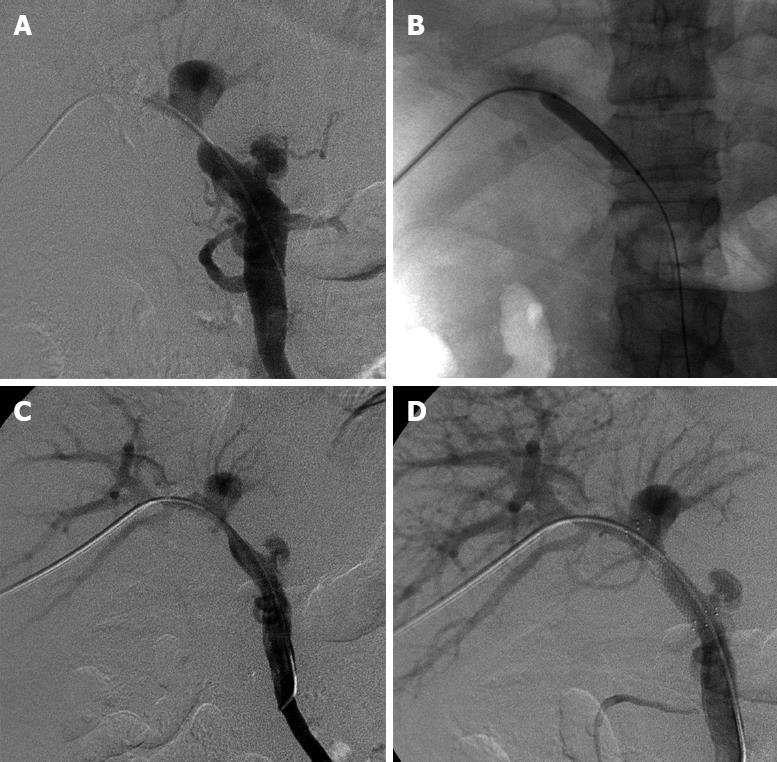
Informed consent was obtained from each patient. Our institutional review committee did not have to give approval for this retrospective study. All procedures were performed via a right-sided intercostal approach under local anesthesia. The transplanted liver was punctured with a 22-gauge Chiba needle (Neff Percutaneous Access Set, NPAS-100-RH-NT; COOK Co., USA) under fluoroscopic guidance, and the needle was targeted to the peripheral branch of the portal vein. After confirming puncture of the intrahepatic portal vein with a test dose injection of contrast media, a 0.018-inch nitinol guidewire (NPAS; COOK, USA) was advanced into the main portal vein. The needle was exchanged for a 4.0-French coaxial dilator and 6.0-French sheath (NPAS; COOK, USA) combination included in the introducer system over the guidewire, then the 0.018-inch guidewire was exchanged for a 0.035-inch hydrophilic coating guidewire (Terumo Co., Japan), and then a 7.0-French vascular sheath (Terumo, Japan) was inserted over the guidewire into the portal vein. Initial portography was obtained with a 5.0-French catheter (KMP; COOK, USA or Cobra; Terumo, Japan). If there was an anastomotic stenosis < 75%, the portal pressure was measured at the postanastomotic main portal vein or at the level of the hepatic hilar portal bifurcation. A disposable pressure transducer system (Utah Medical Products, USA) was used for measuring portal pressure. The catheter was guided by the 0.035-inch guidewire through the stenotic segment. Portography including the splenic vein and superior mesenteric vein was obtained (Figure 1A) and the preanastomotic portal pressure was also measured. The criteria for definite diagnosis of PVAS were as follows: stenosis > 50% of the main portal venous diameter[6] and a pressure gradient across the stenosis > 5 mmHg[378].
Portal vein anastomotic venoplasty was performed with a percutaneous transluminal angioplasty balloon dilatation catheter (Powerflex P3; Cordis, Johnson & Johnson Co., USA or Synergy; Boston Scientific Co., USA). The balloon had a smaller diameter than that of the allograft portal vein. Balloon pressure was slowly and gradually elevated using an inflation syringe (Basix Compak; Merit Medical Systems Co., USA) within a pressure limit of 10-atm until the balloon’s waist was effaced (Figure 1B). Postvenoplasty portography was repeated to assess the results (Figure 1C). A self-expandable metallic stent (SMART Control; Cordis, USA) was deployed to cover the stenosis with minimal angulation between the portal vein and the stent. The stent had the same or 1-2 mm larger diameter than that of the allograft portal vein. Poststenting portography (Figure 1D) and the pressure gradient were obtained repeatedly to assess the results. If the deployed stent showed an hourglass deformity of > 50% of its normal diameter, balloon postdilatation was performed. If a satisfactory result had been achieved, the catheter was removed and the puncture tract was embolized with compressed gelfoam bars through the cut vascular sheath.
No intravenous or systemic heparinization was used. Poststenting anticoagulation was achieved by oral administration of aspirin enteric-coated tablets (Bayaspirin, Bayer S.p.A., Italy) 100 mg/d for at least 6 mo.
Technical success, complications, clinical signs and symptoms, laboratory values including the liver function test and the imaging surveillance results after PTPVS were documented. Technical success of the procedure was defined as < 30% residual stenosis being observed on portography with the absence of varices or collateral circulation[3]. Follow-up, including clinical course, stenosis recurrence and stent patency which were evaluated by CDUS and CT, were performed.
Technical success of PTPVS was achieved in all 16 patients. No procedure-related complications occurred. In these patients, liver function was normalized gradually and clinical manifestations related to PHT were improved following PTPVS.
In three asymptomatic patients, a Yashiro type catheter (Terumo, Japan) was introduced into the splenic artery or superior mesenteric artery via a right femoral artery approach and indirect portography was obtained to confirm the diagnosis of PVAS. More than 50% stenosis of portal vein anastomosis was further demonstrated using percutaneous transhepatic portography, but portal venoplasty and stenting were not performed in two patients because pressure gradients across the stenosis were < 5 mmHg. They were observed with periodic CDUS or CT. Portal venoplasty (using 8-10 mm diameter and 40 mm length balloons) and stenting (using 10-12 mm diameter and 40-60 mm length stents) was performed in 16 patients. In 2 patients, the mean initial pressure gradient across the stenosis was 15.5 mmHg and then it decreased to 3.0 mmHg after PTPVS. In the remaining 14 patients, a pressure gradient was not obtained because of > 75% stenosis and typical clinical signs of PHT.
In a 51-year-old woman, who suffered from massive ascites and severe bilateral lower limb edema after secondary LT, CDUS and CT detected PVAS complicated by hepatic vein stenosis and inferior vena cava stenosis. These venous stenoses were identified by indirect portography, percutaneous transhepatic hepatic venography and inferior vena cavography. First, a self-expandable metallic stent (COOK-Z, GZV-30-75; COOK, USA) was placed in the inferior vena cava via a right femoral vein approach. Second, a balloon-expandable metallic stent (IntraStent, SPM16-26-08-B; ev3 Co., USA) was deployed in the right hepatic vein via an intercostal transhepatic route. Last, PTPVS was performed. The ascites and edema resolved gradually after treatment.
The portosystemic collateral vessels resulting from PHT were visualized by initial portography in 14 patients. Gastroesophageal varices became invisible on poststenting portography in 9 patients. To avoid variceal bleeding, the residual gastroesophageal flow was obstructed with platinum embolization coils (Tornado, MWCE-35-8/4-5-Tornado; COOK, USA) in 2 remaining patients. No further procedure was performed for anorectal varices and pre-existing splenorenal shunt because the abnormal flow became reduced after stenting.
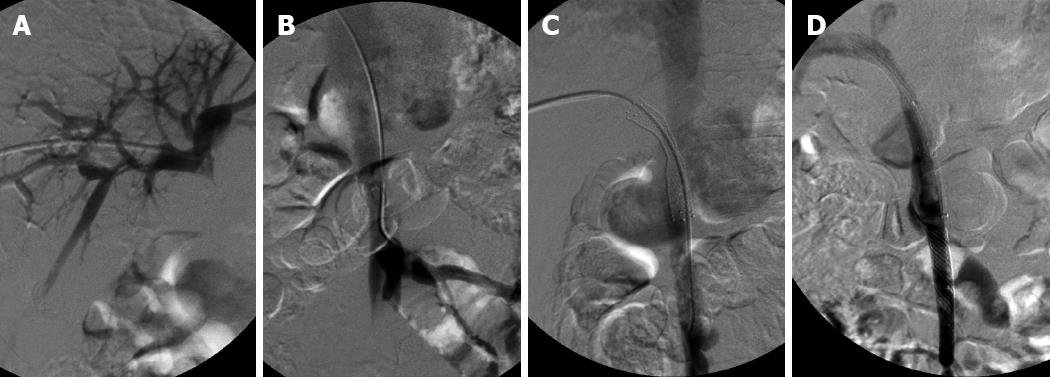
In a 28-year-old man with hepatic encephalopathy, a pre-existing meso-caval shunt was detected due to visualization of the IVC on initial portography (Figure 2A and B). A meso-caval shunt was performed due to refractory massive ascites and recurrent variceal bleeding before LT. After stenting, it was seen that the contrast agents flowed mainly into the IVC via the shunt and little flowed into the portal vein (Figure 2C). To maintain adequate hepatoportal perfusion pressure and to avoid thrombosis in the portal stent or liver failure, a covered stent (Wallgraft Endoprosthesis; Boston Scientific, USA) with a 10 mm diameter and a 50 mm length was deployed into the superior mesenteric vein to occlude the shunt. Once more portography revealed that portal hepatopetal flow was restored and the IVC became invisible (Figure 2D). The patient felt abdominal pain after the procedure, but the symptom subsided a week later. The patient recovered from hepatic encephalopathy.
A balloon-expandable Palmaz stent (Genesis PG1840PMW; Cordis, USA) was deployed into the hepatic artery via a right femoral artery route for anastomotic stenosis 2 mo before PTPVS. Percutaneous transhepatic internal-external biliary drainages were performed with a biliary drainage catheter (Ultrathane MAC-LOC, ULT8.5-38-40-P-32S-CLB-RH; COOK, USA) in 2 patients complicated with obstructive jaundice.
The follow-up results of CDUS in all 16 patients and CT scan in 6 patients revealed portal venous patency was maintained for 3.3-56.6 mo (mean 33.0 mo). These patients remained asymptomatic at the time this manuscript was completed.
The rate of portal venous complications after LT, which include primary portal vein anastomotic stenosis or portal vein thrombosis, has been reported to be below 3%[9–11]. However, in children with reduced-size LT and living donor LT, the incidence of portal venous stenosis or thrombosis is higher than in adults with deceased donor transplantation, because the donor portal segment is relatively short, and due to interposition grafts and size mismatch of the portal vein diameter between donors and recipients. Factors that increased the risk of portal venous complications were pre-existing vein thrombosis or hypoplasia and large portocaval collaterals[12].
Since the first report of portal venous angioplasty and stent placement through a transhepatic approach after LT by Olcott et al[13] in 1990, percutaneous transhepatic interventional procedures have gained worldwide acceptance for treatment of these complications following LT, due to their minimal invasiveness as well as low complication and high success rates[214]. Nonetheless, the reported recurrence rate has been relatively high, i.e. 28.6%-36.8%, following balloon angioplasty alone[367].
Stents have usually been used to treat recurrent and elastic portal venous stenoses following balloon angioplasty, as this procedure has several potential complications[367]. Nevertheless, Ko et al[4] preferred to perform primary stent placement rather than balloon angioplasty in the early posttransplantation period (less than 1 mo). In our study, all stents were deployed immediately following balloon angioplasty for two reasons. First, balloon predilation can reduce the incidence of stent displacement (most jump forward) during stent deployment, especially when the stenosis is severe. Second, direct venoplasty and stenting can recover the normal portal flow once and for all, because repeat percutaneous transhepatic portal venoplasty may lead to puncture injuries in the transplanted liver and increase the incidence of procedure-related complications, such as intrahepatic pseudoaneurysm, arteriovenous fistulas, subcapsular hematoma, bleeding through the puncture tract of the graft, or portal venous thrombosis[2–467].
The luminal area is proportional to the square of the radius; flow is proportional to the fourth power of the radius. Thus, any small improvement is magnified. Intimal damage can lead to platelet aggregation that can in turn lead to short-term occlusion or long-term restenosis. Therefore, any intimal tears should be avoided at all costs, even to the extent of accepting a less visually appealing angiographic result. Slow and gradual inflation of the balloon during angioplasty results in fewer large intimal tears, flaps, and dissections than the more commonly used method of “blow it up, let it down”[15]. In our study, portal venoplasty was performed with an undersized balloon and the balloon was slowly and gradually inflated. Although portal vein thrombosis or stent-edge stenosis may occur, the follow-up results revealed portal venous patency was maintained for a mean 33.0 mo.
Although the etiology of anastomotic stenosis was unclear in our patients, it suggested fibrosis or intimal hyperplasia, which retained an hourglass deformity on the deployed stent.
It is important to detect vascular complications after liver transplantation, because most stenoses or thromboses are frequently treatable with interventional procedures; but, if left untreated, many vascular complications may progress to graft failure. However, compared with biliary obstructions, early portal venous stenosis is difficult to detect from clinical signs and symptoms alone. Furthermore, sometimes the portal venous anastomotic site cannot be seen with ultrasonography because of intestinal artifacts. In such a case, other noninvasive cross-sectional imaging modalities, CT and MRI, may be valuable.
Ultrasonography is often used to screen for vascular abnormalities, including hepatic arterial stenosis and thrombosis, and the less common stenoses or thromboses of the portal vein, hepatic veins, and inferior vena cava. Precise anatomy of the vascular abnormalities is often better determined on CT or MRI, especially when a focal stenosis occurs in the distal IVC, or in the hepatic artery proximal to the porta hepatis where it is difficult to image directly by ultrasonography. CT and MRI give detailed imaging, while ultrasonography tends to give more physiologic data. CT and MRI can provide a more comprehensive evaluation of the transplanted liver; reveal abnormalities of vascular structure; and depict bile ducts, liver parenchyma, and extrahepatic tissues. Moreover, CT angiography and MR angiography can be used to evaluate the extent and degree of the portosystemic collateral vessels resulting from PHT. Magnetic resonance cholangiopancreatography can be valuable to detect focal biliary abnormalities; however, percutaneous transhepatic cholangiography remains the gold standard for biliary complications[16].
When a patient is asymptomatic, indirect portography is a recommended option to identify a portal venous stenosis, as this procedure has a relatively lower incidence of procedure-related complications and no puncture injuries to the graft. When there is a requirement for measurement of portal pressure gradients across a stenosis or further interventional procedures, percutaneous transhepatic portography is necessary.
Portal venography with measurement of pressure gradient across the stenosis remains the most reliable examination[16]; but, the procedure is an invasive one. Although some reports have considered a transstenotic pressure gradient of > 5 mmHg as abnormal[7817], no standard guidelines for a significant pressure gradient have yet been established. Park et al[3] believed that the pressure gradient is not directly correlated with the clinical results, and mentioned that portal venoplasty might not be so helpful for patients whose clinical symptoms are possibly related with graft dysfunction and not with the stenosis. The treatment is valuable if patients have symptoms related to portal venous inflow abnormality or PHT even though the pressure gradient is not significant[4]. In patients who do not have evidence of PHT, and have normal hepatic function, stenoses may be observed for progression with periodic ultrasound. Moreover, in patients with PHT, the potential contribution of underlying hepatic parenchymal disease (rejection or recurrent hepatitis) must be considered. However, if portal venous stenosis is suspected as being a significant contributor to PHT, therapeutic intervention is necessary.
Negative findings on serial CDUS and the absence of clinical symptoms during the follow-up period might prompt us to regard these patients as not having any hemodynamically significant vascular abnormalities.
In conclusion, percutaneous transhepatic portal venoplasty and stenting for anastomotic stenosis after liver transplantation is a safe and effective procedure for alleviating the signs and symptoms of portal hypertension and preserving the graft. With technical refinements, early detection and prompt treatment of complications, and advances in immunotherapy, excellent results can be achieved in liver transplantation.
Portal vein anastomotic stenosis after liver transplantation is an uncommon vascular complication that may result in graft loss if not promptly treated. In the past, portal venous complications were managed with surgical treatments such as thrombectomy, anastomosis revision or retransplantation. However, surgical management of these complications has been limited by technical difficulties due to postsurgical fibrosis and limitations in the length of the involved venous structures. Percutaneous interventional procedures have gained worldwide acceptance for alleviating the symptoms of portal hypertension and preserving the graft, due to their minimal invasiveness as well as low complication and high success rates.
The reported recurrence rate has been relatively high, i.e. 28.6%-36.8%, following balloon angioplasty alone. Stents have usually been used to treat recurrent and elastic portal venous stenoses following balloon angioplasty, as this procedure has several potential complications. Nevertheless, Ko et al preferred to perform primary stent placement rather than balloon angioplasty in the early posttransplantation period (< 1 mo).
In this study, all stents were deployed immediately following balloon angioplasty for two reasons. First, balloon predilation can reduce the incidence of stent displacement during its deployment. Second, direct venoplasty and stenting can recover normal portal flow once and for all, because repeat percutaneous transhepatic portal venoplasty may lead to puncture injuries to the transplanted liver and increase the incidence of procedure-related complications. Intimal damage can lead to platelet aggregation that can in turn lead to short-term occlusion or long-term restenosis. Slow and gradual inflation of the balloon during angioplasty results in fewer large intimal tears, flaps, and dissections than the more commonly used method of “blow it up, let it down.” In this study, portal venoplasty was performed with an undersized balloon and the balloon was slowly and gradually inflated. Although portal vein thrombosis or stent-edge stenosis may occur, the follow-up results revealed portal venous patency was maintained for a mean of 33.0 mo. The treatment is valuable if patients have symptoms related to portal venous inflow abnormality or portal hypertension even though the pressure gradient is not significant.
Percutaneous transhepatic portal venoplasty and stenting (PTPVS) could be used for portal anastomotic stenosis after liver transplantation to alleviate the signs and symptoms of portal hypertension and preserve the graft.
PTPVS for anastomotic stenosis after liver transplantation is a safe and effective procedure for alleviating the signs and symptoms of portal hypertension and preserving the graft. The results are encouraging and suggest that the method of undersized balloon and slow and gradual inflation can reduce intimal damage and keep the portal venous patency for a mean of 33.0 mo after stenting.
| 1. | Woo DH, Laberge JM, Gordon RL, Wilson MW, Kerlan RK Jr. Management of portal venous complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:233-239. |
| 2. | Vignali C, Cioni R, Petruzzi P, Cicorelli A, Bargellini I, Perri M, Urbani L, Filipponi F, Bartolozzi C. Role of interventional radiology in the management of vascular complications after liver transplantation. Transplant Proc. 2004;36:552-554. |
| 3. | Park KB, Choo SW, Do YS, Shin SW, Cho SG, Choo IW. Percutaneous angioplasty of portal vein stenosis that complicates liver transplantation: the mid-term therapeutic results. Korean J Radiol. 2005;6:161-166. |
| 4. | Ko GY, Sung KB, Yoon HK, Lee S. Early posttransplantation portal vein stenosis following living donor liver transplantation: percutaneous transhepatic primary stent placement. Liver Transpl. 2007;13:530-536. |
| 5. | Kim BS, Kim TK, Jung DJ, Kim JH, Bae IY, Sung KB, Kim PN, Ha HK, Lee SG, Lee MG. Vascular complications after living related liver transplantation: evaluation with gadolinium-enhanced three-dimensional MR angiography. AJR Am J Roentgenol. 2003;181:467-474. |
| 6. | Shibata T, Itoh K, Kubo T, Maetani Y, Shibata T, Togashi K, Tanaka K. Percutaneous transhepatic balloon dilation of portal venous stenosis in patients with living donor liver transplantation. Radiology. 2005;235:1078-1083. |
| 7. | Funaki B, Rosenblum JD, Leef JA, Zaleski GX, Farrell T, Lorenz J, Brady L. Percutaneous treatment of portal venous stenosis in children and adolescents with segmental hepatic transplants: long-term results. Radiology. 2000;215:147-151. |
| 8. | Raby N, Karani J, Thomas S, O'Grady J, Williams R. Stenoses of vascular anastomoses after hepatic transplantation: treatment with balloon angioplasty. AJR Am J Roentgenol. 1991;157:167-171. |
| 9. | Settmacher U, Nüssler NC, Glanemann M, Haase R, Heise M, Bechstein WO, Neuhaus P. Venous complications after orthotopic liver transplantation. Clin Transplant. 2000;14:235-241. |
| 10. | Langnas AN, Marujo W, Stratta RJ, Wood RP, Shaw BW Jr. Vascular complications after orthotopic liver transplantation. Am J Surg. 1991;161:76-82; discussion 82-83. |
| 11. | Cavallari A, Vivarelli M, Bellusci R, Jovine E, Mazziotti A, Rossi C. Treatment of vascular complications following liver transplantation: multidisciplinary approach. Hepatogastroenterology. 2001;48:179-183. |
| 12. | Lerut J, Tzakis AG, Bron K, Gordon RD, Iwatsuki S, Esquivel CO, Makowka L, Todo S, Starzl TE. Complications of venous reconstruction in human orthotopic liver transplantation. Ann Surg. 1987;205:404-414. |
| 13. | Olcott EW, Ring EJ, Roberts JP, Ascher NL, Lake JR, Gordon RL. Percutaneous transhepatic portal vein angioplasty and stent placement after liver transplantation: early experience. J Vasc Interv Radiol. 1990;1:17-22. |
| 14. | Wang JF, Zhai RY, Wei BJ, Li JJ, Jin WH, Dai DK, Yu P. Percutaneous intravascular stents for treatment of portal venous stenosis after liver transplantation: midterm results. Transplant Proc. 2006;38:1461-1462. |
| 15. | Connors JJ 3rd, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg. 1999;91:415-423. |
| 16. | Saad WE, Lin E, Ormanoski M, Darcy MD, Rubens DJ. Noninvasive imaging of liver transplant complications. Tech Vasc Interv Radiol. 2007;10:191-206. |
| 17. | Funaki B, Rosenblum JD, Leef JA, Hackworth CA, Szymski GX, Alonso EM, Piper JB, Whitington PF. Portal vein stenosis in children with segmental liver transplants: treatment with percutaneous transhepatic venoplasty. AJR Am J Roentgenol. 1995;165:161-165. |