Published online Mar 28, 2009. doi: 10.3748/wjg.15.1431
Revised: October 15, 2008
Accepted: October 22, 2008
Published online: March 28, 2009
AIM: To investigate the effects of transforming growth factor β1 (TGF-β1) on the differentiation of colonic lamina propria fibroblasts (CLPF) into myofibroblasts in vitro.
METHODS: Primary CLPF cultures were incubated with TGF-β1 and analyzed for production of α-smooth muscle actin (α-SMA), fibronectin (FN) and FN isoforms. Migration assays were performed in a modified 48-well Boyden chamber. Levels of total and phosphorylated focal adhesion kinase (FAK) in CLPF were analyzed after induction of migration.
RESULTS: Incubation of CLPF with TGF-β1 for 2 d did not change α-SMA levels, while TGF-β1 treatment for 6 d significantly increased α-SMA production. Short term incubation (6 h) with TGF-β1 enhanced CLPF migration, while long term treatment (6 d) of CLPF with TGF-β1 reduced migration to 15%-37% compared to untreated cells. FN and FN isoform mRNA expression were increased after short term incubation with TGF-β1 (2 d) in contrast to long term incubation with TGF-β1 for 6 d. After induction of migration, TGF-β1-preincubated CLPF showed higher amounts of FN and its isoforms and lower levels of total and phosphorylated FAK than untreated cells.
CONCLUSION: Long term incubation of CLPF with TGF-β1 induced differentiation into myofibroblasts with enhanced α-SMA, reduced migratory potential and FAK phosphorylation, and increased FN production. In contrast, short term contact (6 h) of fibroblasts with TGF-β1 induced a dose-dependent increase of cell migration and FAK phosphorylation without induction of α-SMA production.
- Citation: Brenmoehl J, Miller SN, Hofmann C, Vogl D, Falk W, Schölmerich J, Rogler G. Transforming growth factor-β1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol 2009; 15(12): 1431-1442
- URL: https://www.wjgnet.com/1007-9327/full/v15/i12/1431.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1431
Transforming growth factor-β (TGF-β) is involved in multiple fundamental biological processes such as cell proliferation[1–3], cell migration[4–8], cell differentiation[9], extracellular matrix deposition[671011], and immune responses[12]. TGF-β is secreted by a variety of cells including platelets, monocytes, macrophages, and lymphocytes. It is thought to play a critical role in embryogenesis, host response to tumors, and the repair response that follows damage to tissues by immune and nonimmune reactions[8].
TGF-β not only affects remodeling during normal wound healing after tissue injury, but also enhances fibrogenesis. Therefore, it is an important mediator in multiple fibrotic diseases, including pulmonary fibrosis, liver fibrosis, chronic pancreatitis[12], and in stricture formation in Crohn’s disease (CD)[13]. Increased expression of TGF-β, especially of the isoform TGF-β1, has been demonstrated in fibrotic tissues and regions of increased extracellular matrix deposition[12]. TGF-β changes the balance between extracellular matrix synthesis and degradation, inducing an increase in the synthesis of matrix components and a parallel decrease in the matrix-proteolytic activity[1112].
TGF-β is synthesized as a pro-form which can be activated by multiple mechanisms. After activation of latent TGF-β and binding to its receptors, the activated receptors phosphorylate and assemble cytoplasmic Smad proteins. Subsequently, Smad complexes move to the nucleus as transcriptional regulators[1214].
TGF-β is also a potent chemoattractant for human dermal fibroblasts. Intact disulfide bonds and perhaps the dimeric structure of TGF-β are essential for its ability to stimulate migration of fibroblasts, since reduction of TGF-β results in a marked loss of its chemoattractant potency[8].
Fibroblast migration plays an important role in tissue formation and wound healing. Following injury, tissue repair takes place involving inflammation, new tissue formation and scar constitution[15]. Migration of fibroblasts into and through the extracellular matrix during the initial phase of wound healing appears to be a fundamental component of wound contraction. In recent studies, we found that colonic lamina propria fibroblasts (CLPF) conditioned media induce migration of primary human CLPF in the modified Boyden chamber[16]. Furthermore, we demonstrated that fibronectin (FN) was mainly responsible for the autocrine induction of CLPF migration and was an essential requirement for the induction of CLPF migration, since different growth factors enhanced CLPF migration only in the presence of conditioned medium or recombinant FN[16]. Reduced and enhanced migratory potential of CLPF correlated with decreased and increased amounts of FN or FN isoforms, respectively[17]. FNs occur in up to 20 different isoforms as a result of alternative splicing of the primary transcript in the two homologous type III domains named ED-A and ED-B and the one non-homologous repeat termed IIICS[18–22]. The ED-A and ED-B segments are either entirely included or excluded. The type III repeat may be included, excluded, or partially included in FN[23–25].
The alternatively spliced FN isoforms show distinct functional differences. The expression of FN containing ED-A and ED-B domains is significantly increased during physiological wound healing and pathological tissue fibrosis[26]. Changes in the migratory potential of CLPF from patients with CD were associated with changes in FN isoform level, while the expression of integrin α5β1, the main FN receptor on the surface of CLPF, was unchanged[17].
The differentiation or activation of fibroblasts into myofibroblasts is an important step in tissue repair. Transient appearance of myofibroblasts is a feature of normal wound healing, but the persistence of these activated cells is associated with excessive collagen deposition and fibrosis[9]. Their prolonged presence and over-representation are hallmarks in the pathophysiology of tissue fibrosis[27]. TGF-β1 potently stimulates the production of α-smooth muscle actin (α-SMA) and stress fiber formation in fibroblasts and therefore their differentiation into myofibroblasts[9].
Since the regulation of migration and differentiation of intestinal fibroblasts is an important mechanism during intestinal wound healing and fibrosis, the effect of TGF-β1 on these processes and on FN and FN isoform production was investigated in this study.
Primary CLPF cultures were obtained from endoscopic biopsies or surgical specimens taken from healthy areas of the mucosa of patients undergoing surveillance colonoscopy or surgery for colorectal carcinoma. The study was approved by the Ethics Committee of the University of Regensburg.
Human CLPF were isolated and cultured as described earlier[28]. Briefly, mucosa from surgical specimens was cut into 1 mm pieces while the biopsies were used directly for the isolation of CLPF. Epithelial cells were removed in Hank’s Balanced Salt Solution without Ca2+ and Mg2+ (PAA, Cölbe, Germany) with 2 mmol/L EDTA (SIGMA, Deisenhofen, Germany). The remaining tissue was rinsed and then digested for 30 min at 37°C with 1 mg/mL collagenase 1 (SIGMA), 0.3 mg/mL DNase I (Boehringer, Mannheim, Germany) and 2 mg/mL hyaluronidase (SIGMA) in PBS (Gibco, Karlsruhe, Germany). The isolated cells were cultured in 25 cm2 culture flasks (Costar, Bodenheim, Germany) with DMEM containing 10% FCS, penicillin (100 IE/mL), streptomycin (100 &mgr;g/mL), ciprofloxacin (8 &mgr;g/mL), gentamycin (50 &mgr;g/mL) and amphotericin B (1 &mgr;g/mL). Non-adherent cells were removed by subsequent changes of medium. The remaining cells were used between passage 3 and 8.
CLPF were incubated for two days or six days with 0, 0.1, 1, 10 and 50 ng/mL of TGF-β1 (Biozol, Germany) in serum-free medium. The culture medium of 6 d treated cells was changed after 3 d and new TGF-β1 was added for a further three days. Subsequently, cells were used for investigation of α-SMA production, cell migration, quantitative mRNA analysis, FN, and FAK production.
CLPF were incubated for 6 d with or without 10 ng/mL of TGF-β1 (Biozol, Germany) in serum-free medium. The medium was changed after three days and new TGF-β1 was added for a further three days. The cells cultured in 5 cm dishes were wounded with a comb, washed and incubated for a further 4 h with different concentrations of platelet derived growth factor (PDGF)-AB (R&D Systems, Minneapolis, MN, USA; 0, 5, 10, and 20 ng/mL). Cells were harvested and used for quantitative analysis of FN and FN splicing form mRNA and for Western blot analysis of FN and FN-splicing form protein as well as levels of total and phosphorylated FAK.
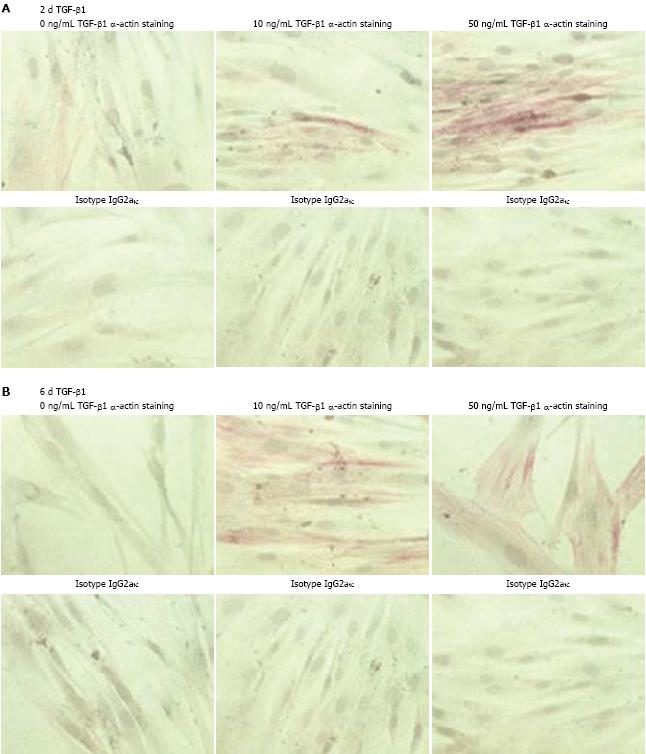
For immunocytochemical staining of α-SMA, 20 000 cells were seeded onto LabTek® Chamber slides (Nunc, Wiesbaden, Germany) and fixed with ice-cold acetone. Immunocytochemical stainings were performed according to the manufacturer’s APAAP protocol (Dako, Hamburg, Germany). The anti α-SMA antibody (1:25, clone 1A4, Dako) was used to investigate the differentiation of CLPF into myofibroblasts. IgG2a (MOPC-141, Sigma) was used as an isotype control.
Western blot analysis was performed as described earlier[1729]. Protein detection was carried out with a specific antibody to α-SMA (clone 1A4, Dako, Hamburg, Germany) and incubation with peroxidase-conjugated secondary antibody. Protein bands were visualized using a commercial chemoluminescence detection kit (ECL Plus; Amersham) according to the manufacturer’s protocol. Loading was checked by Ponceau S staining.
For FN and FAK protein immunoblots specific antibodies to FN (1:1000; 35041A, Pharmingen), FN ED-A (1:400; clone IST-9, Abcam), FN ED-B (1:200; PhiloMab-2, Philogen), FAK (1:666; Chemicon), phosphoFAK (1:1000; Biosource) and β-actin (1:10 000; Chemicon) were used. After washing of the membranes horseradish peroxidase-conjugated secondary antibodies (Santa Cruz, CA) were added and incubated with chemiluminescent substrate (ECL Plus; Amersham Biosciences, Arlington Heights, IL) for 5 min before a film (Amersham Biosciences) was exposed.
After exposure, the antibody was stripped from the membrane with Re-Blot Plus-Strong (Chemicon, Hofheim, Germany) for 15 min and the blot was blocked again with 5% milk in 0.1% Tween 20-TBS before using it for the next antibody.
Migration assays were performed in a modified 48-well Boyden chamber as described earlier[28]: A polycarbonate filter (8 &mgr;m pore size, polyvinylpyrrolidone-free, Gerbu Biotechnik, Gaiberg, Germany) divided the chamber into an upper and a lower compartment. Each test substance was placed in the wells of the lower compartment in replicates of three. Conditioned media with or without PDGF-AB, IGF-I (R&D Systems), EGF (Biosource, Germany) or TGF-β1 (Biozol) were tested. DMEM high glucose medium with or without 1% bovine serum albumin (BSA, SIGMA) served as negative controls. A total of 20 000 CLPF/well in DMEM high glucose medium with 1% BSA were seeded into the wells of the upper compartment of the Boyden chamber. The Boyden chamber was incubated at 37°C in 10% CO2 atmosphere for 6 h. The filter was removed from the chamber and the non-migrated cells on the upper side of the filter were scraped off with a rubber policeman. The migrated cells on the lower side of the filter were fixed and stained with Hemacolor staining kit (Merck, Darmstadt, Germany) and counted in 4 microscopic high power fields of view (hpf) at a 400-fold magnification. Unless otherwise indicated, each experiment was repeated at least three times (n = 3).
The culture medium was removed from a confluent CLPF monolayer. The cells were washed twice with PBS and subsequently cultured with DMEM lacking FCS for 24 h. The conditioned medium was centrifuged to remove all cell debris and stored at -20°C for no more than 3 mo.
For the isolation of total RNA stimulated and unstimulated primary human CLPF cultures were washed twice with PBS. CLPF were scraped off, centrifuged for 5 min, and resuspended with lysis buffer from the RNeasy® kit (Qiagen, Hilden, Germany). Total RNA was prepared from these CLPF according to the manufacturer’s protocol and stored at -80°C. The isolated RNA was reverse transcribed using the Promega Reverse Transcription System (Promega, Madison, WI, USA).
Amounts of FN, ED-A and ED-B mRNA were quantified by real-time PCR as previously described[17].
Released reporter dye fluorescence during 40 cycles of amplification was monitored using Sequence Detector software (SDS version 2.0, PE Applied Biosystems). Reporter dye fluorescence versus PCR cycles was plotted. A threshold was set in the exponential phase of the fluorescence curves. The threshold cycle numbers (Ct) were calculated.
Ct values of GAPDH were subtracted from those of FN isoforms: dCt = Ct (FN isoforms) - Ct (GAPDH).The mean value of dCt values was calculated. The values of cDNA from stimulated CLPF were subtracted from those of untreated cDNA of the same CLPF: ddCt = dCt (stimulated) - dCt (unstimulated). The relative start amount of cDNA was calculated in consideration of the exponential amplification: x = 2-ddCt.
All data are given as mean ± SE. The Student’s t-test was used for analysis of parametric data, while the Mann-Whitney Rank Sum Test was used for evaluation of non-parametric data. Real time PCR data were pictured as boxplots. P < 0.05 were considered to be statistically significant.
To investigate the effect of TGF-β1 on α-SMA production of CLPF, CLPF were incubated with different concentrations of TGF-β1 in serum-free medium. Subsequently, APAAP-staining of α-SMA or its isotype control IgG2aκ was performed. Since there was no remarkable change in α-SMA production after treatment for one day or two days with 0.1, 1, and 10 ng/mL TGF-β1 (data not shown), we compared α-SMA production of CLPF incubated for two days (Figure 1A) or six days (Figure 1B) with 0, 10 and 50 ng/mL TGF-β1. A time- and dose-dependent increase of α-SMA was observed: short-term treatment with 50 ng/mL TGF-β1 resulted in enhanced α-SMA production, however, after long-term incubation α-SMA was detected in almost all cells.
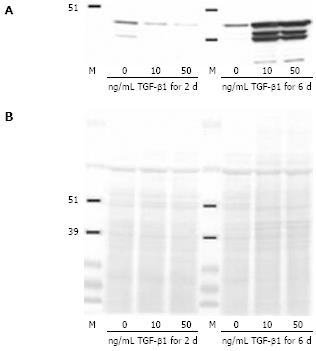
In order to confirm these results, Western blot analysis was performed (Figure 2A). CLPF treated for two days or six days with 10 ng/mL or 50 ng/mL TGF-β1 were lysed and levels of α-SMA were detected. In each lane 30 &mgr;g/mL cell lysate protein were loaded and the loading was checked by Ponceau S staining (Figure 2B). Western blot data confirmed the induction of α-SMA production by long-term incubation of CLPF with TGF-β1 in contrast to short-term incubation, where no α-SMA was detected.
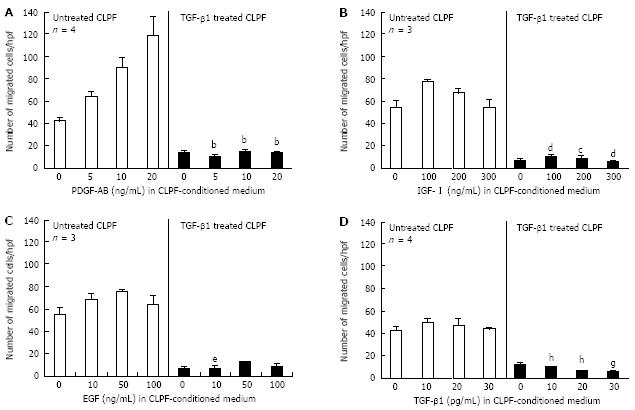
Since TGF-β1 potently stimulated the production of α-SMA in CLPF and as a consequence their differentiation into myofibroblasts, we investigated the effect of long term TGF-β1 incubation on the migration of CLPF. Because 10 ng/mL TGF-β1 induced similar α-SMA levels as 50 ng/mL TGF-β1 (Figure 2), we treated CLPF for six days with 10 ng/mL TGF-β1 and performed migration assays in the presence of PDGF-AB, IGF-I, EGF and TGF-β1 diluted in 24 h CLPF-conditioned medium in a modified Boyden chamber (Figure 3). The Boyden chamber was incubated at 37°C in 10% CO2 atmosphere for 6 h. We reported earlier that growth factors like PDGF-AB, IGF-I, EGF and TGF-β1 diluted in conditioned media enhance CLPF migration[28]. However, pre-incubation of CLPF with TGF-β1 for six days significantly decreased CLPF migration. This decreased migratory potential of myofibroblast-like CLPF could not be re-enhanced by addition of growth factors (Figure 3).
Migration assays with different dilutions of PDGF-AB in conditioned medium showed that short-term incubation (6 h) in the modified Boyden chamber enhanced migration of TGF-β1-untreated CLPF in a dose-dependent manner (Figure 3A). Addition of 5 ng/mL PDGF-AB to CLPF conditioned medium increased migration up to 60% (64 ± 5 cells/hpf) when compared to conditioned medium without additional PDGF-AB (control, 44 ± 4 cells/hpf). Addition of 20 ng/mL PDGF-AB to CLPF conditioned medium induced a more than two-fold migration rate (121 ± 19 cells/hpf) when compared to control. Pre-incubation of CLPF for six days with 10 ng/ml TGF-β1 reduced migration to 37% (16 ± 2 cells/hpf) when compared to untreated CLPF (44 ± 4 cells/hpf) and could not be re-enhanced by PDGF-AB (P < 0.0001; Figure 3A).
Similar results were obtained in migration assays using different concentrations of IGF-I, EGF or TGF-β1 in conditioned medium (Figure 3B-D). Addition of the growth factors to the conditioned medium enhanced the migration of TGF-β1-untreated CLPF, although the effect was not as strong as with PDGF-AB. Again, pre-incubation of CLPF for six days with TGF-β1 led to a reduced migratory potential of the cells and could not be re-enhanced by the growth factors IGF-I (Figure 3B: 100 ng/mL, P < 0.005; 200 ng/mL, P < 0.05; 300 ng/mL, P < 0.0001), EGF (Figure 3C: 10 ng/mL, P < 0.05) or TGF-β1 (Figure 3D: 10 pg/mL, P < 0.005; 20 pg/mL, P < 0.0001; 30 pg/mL, P < 0.05).
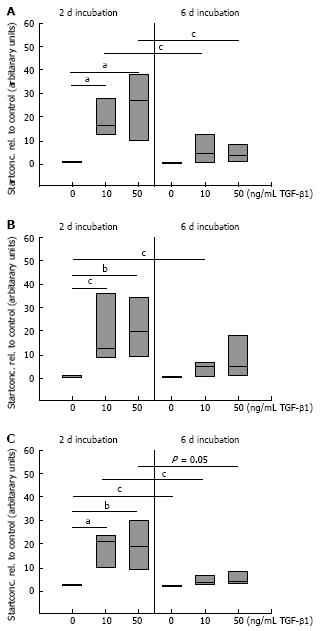
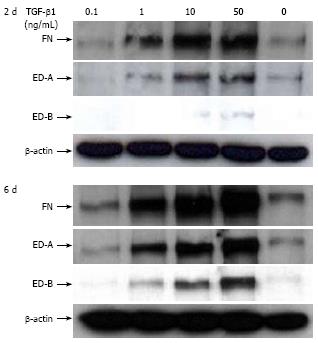
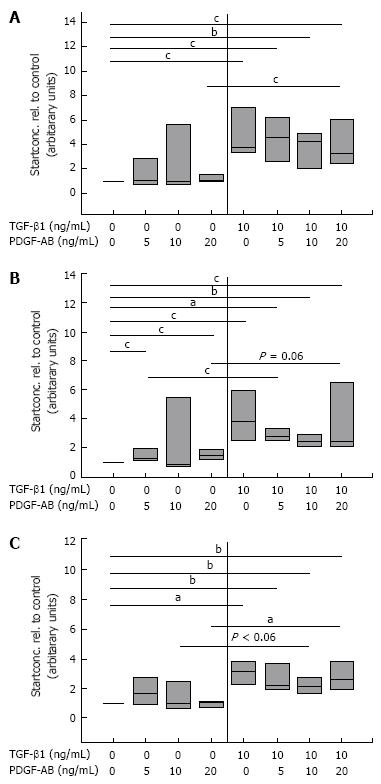
It is known that TGF-β1 promotes the insertion of the ED-A domain in FN[3031] and we have recently shown that changes in the migratory potential of CLPF were correlated with changes in FN isoform production[17]. For this reason, the effect of TGF-β1 on splicing of FN mRNA in CLPF was assessed. CLPF were incubated with different TGF-β1 concentrations for two days or six days and the amounts of FN, FN ED-A-, and FN ED-B were determined. Therefore the cells were treated with 10 ng/mL and 50 ng/mL TGF-β1 and untreated cells were used as controls. For the comparison of mRNA expression quantified by real time PCR untreated controls were set as 1. The results of 7 independent experiments were evaluated statistically (Figure 4).
After two days incubation with 10 ng/mL TGF-β1 total FN mRNA expression was increased 20-fold and 25-fold with 50 ng/mL (both P < 0.005, paired t-test). When CLPF were incubated for six days, FN mRNA was increased 6-fold with 10 ng/mL TGF-β1 and 10-fold with 50 ng/mL TGF-β1 compared to control (Figure 4A).
mRNA amounts of FN ED-A and FN ED-B were increased 20-fold (P < 0.05) and 13-fold (P < 0.005), respectively, with 10 ng/mL TGF-β1 after two days, while after six days 5-fold (FN ED-A, P < 0.05) or 3-fold (FN ED-B, P < 0.05) increased mRNA levels compared to controls were found (Figure 4B and C). Treatment with 50 ng/mL TGF-β1 for 2 d caused a 23-fold (P < 0.01) increase of FN ED-A mRNA which was reduced to 6-fold after a further four days. mRNA amounts of FN ED-B was 15-fold (P < 0.01) after two days and reduced to 4-fold (P < 0.05) after six days (Figure 4B and C).
Together these data indicate that TGF-β1 causes a strong increase in mRNA expression of FN and migration inducing FN isoforms. However, this effect was not maintained but was clearly reduced after six days. Values obtained after six days of incubation were not statistically different to unstimulated controls despite the presence of TGF-β1. On average, FN mRNA amounts were 70% lower after pre-incubation with 10 ng/mL TGF-β1 for six days than after pre-incubation for two days (P < 0.05), while FN ED-A and FN ED-B (P < 0.05) mRNA levels were reduced by 80%. Similar data were obtained for incubation with 50 ng/mL TGF-β1 for six days versus stimulation for two days (total FN 55% lower (P < 0.05) after six days compared to two days, FN ED-A and FN ED-B 75% lower (P = 0.05).
In addition, TGF-β1-mediated induction of FN and FN splice variant production was confirmed by Western blot analyses. A time- and dose-dependent increase of total FN, FN ED-A and FN ED-B was detected after TGF-β1 treatment (Figure 5). In contrast to the findings for mRNA expression after six days TGF treatment higher levels of FN, ED-A and ED-B protein compared to two days were observed. In the absence of TGF-β1, levels of FN and FN isoforms were low and did not differ between two- and six- day cultures. A concentration of 0.1 ng/mL TGF-β1 did not show a significant effect while 1 ng/mL TGF-β1 induced an increase in FN and FN ED-A production after two days and six days compared to untreated cells. After two days FN ED-B protein was only found after incubation with TGF-β1 concentrations of 10 ng/mL and 50 ng/mL, while this isoform could be detected after six days with 1, 10, and 50 ng/mL TGF-β1. The most prominent effect on FN, FN ED-A, and FN ED-B production was observed after six days incubation with 50 ng/mL TGF-β1.
Because of the observed reduced migratory potential of CLPF incubated long-term with TGF-β1 but increased protein levels of migration inducing factor FN and FN splice variants, we analyzed migrating cells in a wounding assay in more detail. CLPF were pre-incubated with and without 10 ng/mL TGF-β1 in serum free medium (six days). The CLPF monolayer was wounded with a comb, migration was induced by incubation with different concentrations of PDGF-AB (0, 5, 10, 20 ng/mL) in conditioned medium for 4 further hours and mRNA amounts of FN isoforms were assessed (Figure 6).
While CLPF without TGF-β1 pre-treatment displayed a slight PDGF-dependent increase in the expression of FN, FN ED-A, and a slight decrease in FN ED-B mRNA in this wounding assay, TGF-β1 pre-treated cells displayed a stronger enhancement of FN, FN ED-A, and FN ED-B mRNA-expression.
After stimulation with 5, 10, and 20 ng/mL PDGF-AB in conditioned medium FN mRNA-expression was 4.5- (Paired t-test, P < 0.05), 4- (P < 0.01), and 4.5-fold (P < 0.05) increased in TGF-β1 pre-treated and 2-, 3.5-, and 1.5-fold enhanced in TGF-β1 untreated CLPF as compared to the control, respectively (Figure 6A). PDGF-AB stimulation of pre-incubated CLPF led to 3- (5 ng/mL PDGF, P < 0.005), 2.5- (10 ng/mL PDGF, P < 0.01), and 4-fold increases (20 ng/mL PDGF, P < 0.05) in FN ED-A mRNA expression, whereas TGF-β1 unstimulated cells showed a 2- (P < 0.05), 3.5-, and 1.5-fold enhancement, respectively (Figure 6B). PDGF caused a 3- (5 ng/mL, P < 0.01), 2.5- (10 ng/mL, P < 0.01), and 3-fold increase (20 ng/mL, P < 0.01) in FN ED-B mRNA in TGF-β1-treated CLPF and a 1.5-, 2.5- (5 and 10 ng/ mL, P < 0.01), and 1.5-fold (20 ng/mL) enhancement in TGF-β1 untreated cells (Figure 6C).
In summary, pre-treatment of CLPF with TGF-β1 (six days) and subsequent treatment with PDGF-AB (4 h) after wounding causes a much stronger induction of FN mRNA expression than without TGF-β1 pre-treatment.
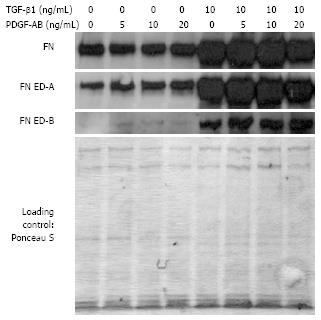
After TGF-β1-incubation, wounding, and incubation without PDGF-AB mRNA levels of total FN, FN ED-A, and FN ED-B increased significantly by 4-fold when compared to TGF-β1- and PDGF-AB-untreated controls (P < 0.05 for FN and FN ED-A; FN ED-B P < 0.005). With 5 ng/mL PDGF-AB mRNA amounts of FN splice forms doubled by TGF-β1-stimulation [FN ED-A (P < 0.05)]. After TGF-β1 pre-stimulation expression of FN and FN ED-B rose by 20% compared to untreated cells, while FN ED-A expression was even decreased by 30%. With 10 ng/mL TGF-β1 and 20 ng/mL PDGF-AB, after cell wounding total FN expression showed a 3-fold (P < 0.05), FN ED-A expression a 2.5-fold (P = 0.06), and FN ED-B expression a 2-fold (P < 0.005) increase.
Western blot analyses of protein production confirmed these findings on mRNA expression (Figure 7). Protein amounts of FN, FN ED-A, and FN ED-B were strongly increased by TGF-β1 incubation. In comparison to TGF-β1 untreated controls the levels of FNs were enhanced after wounding and further enhanced by addition of PDGF-AB in conditioned medium. No significant differences were observed between protein amounts in TGF-β1 pre- and not incubated CLPF by PDGF-treatment, respectively. FN ED-B protein levels of CLPF without pre-treatment were barely detectable.
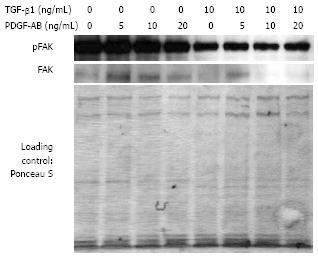
We have demonstrated in previous studies that enhanced cell migration of CLPF was associated with higher FAK phosphorylation and total FAK protein production[29]. Therefore, we determined TGF-β1-modulated FAK production and phosphorylation by Western blot (Figure 8) in CLPF that were induced to migrate by wounding and stimulation with PDGF-AB in conditioned medium. Pre-incubation of CLPF with 10 ng/mL TGF-β1 for six days decreased FAK phosphorylation as compared to untreated controls. In addition, levels of FAK protein were reduced by TGF-β1 stimulation. CLPF that were not pre-treated with TGF-β1 contained enhanced levels of FAK with a constant higher FAK phosphorylation that was highest with an addition of 5 ng/mL PDGF-AB when compared to CLPF not treated with TGF-β1.
In this study we demonstrate that TGF-β1 potently stimulates the production of α-SMA in CLPF and therefore their differentiation into myofibroblasts as a result of long term contact. This differentiation into myofibroblasts is accompanied by a reduced migratory potential, reduced FAK phosphorylation and increased synthesis of FN as a component of extracellular matrix (ECM).
Myofibroblast differentiation and activation by TGF-β1 is a critical event in tissue repair and the pathogenesis of human fibrotic diseases. Myofibroblasts and TGF-β1 are key elements for the generation of contractile force associated with wound contraction and pathological contractures as in the development of tissue fibrosis. Myofibroblasts are characterized by the presence of α-SMA-containing stress fibers, vinculin-containing fibronexus adhesion complexes, and FN fibrils containing the ED-A splice variant[32]. TGF-β1 promotes contraction of collagen gels by fibroblasts through their differentiation into myofibroblasts[33]. In addition, TGF-β1 induces a dose-dependent increase in the generation of contractile force and a concomitant increase in the production of α-SMA[32].
In the current study much higher TGF-β1 doses were required to induce α-SMA production than in the studies by Simmons et al[9] and Vaughan et al[32]. This may be a cell-type-specific effect. Simmons et al. used CCD-18Co fibroblasts isolated from the colon of a 2.5 mo old child, while in this study intestinal fibroblasts of adults have been used. Vaughan et al[32] used myofibroblasts obtained as explant cultures or by collagenase digestion of palmar aponeurosis from patients with Dupuytren’s disease. Therefore, it may be that fibroblasts obtained from palmar aponeurosis react more sensitively to TGF-β1 than CLPF.
Myofibroblast differentiation by TGF-β1 is dependent on cell adhesion and integrin signaling via FAK. TGF-β1 induces tyrosine phosphorylation of the autophosphorylation site Tyr-397 of FAK, an effect that is dependent on cell adhesion and is delayed relative to early Smad signaling. Pharmacologic inhibition of FAK or expression of kinase-deficient FAK, mutated by substituting Tyr-397 with Phe, inhibited TGF-β1-induced α-SMA production and stress fiber formation[34]. In the current study we found that long term incubation with TGF-β1 reduced FAK phosphorylation and this was associated with a decreased migratory potential. TGF-β1 untreated CLPF displayed a PDGF-dependent increase of FAK-phosphorylation that correlated with an enhanced migration.
FAK is a nonreceptor protein tyrosine kinase involved not only in adhesion but also in cell migration. FAK-deficient fibroblasts exhibit defects in cell migration and elevated numbers of cell-substratum contact sites[35]. The reduced migratory behavior of CD- and ulcerative colitis (UC)-CLPF is accompanied by a reduction of FAK and FAK phosphorylation[29].
TGF-β1 production is increased in myofibroblasts at sites of fibrosis in experimental enterocolitis and CD[9]. In CD, increased TGF-β1 expression is transmural whereas in UC, the increase is confined to the lamina propria and submucosa. The distribution of TGF-β1 coincides with the distribution of the inflammatory infiltrate as well as an increase in the collagen type III:I ratio in both CD and UC[36]. Additionally, TGF-β1, -β2, -β3 and their receptors are increased in fibrotic CD mucosal tissue samples[13]. Therefore, it may be assumed that a reduced migratory potential of inflammatory bowel disease (IBD)-CLPF[29] is a result of enhanced α-SMA production and enhanced formation of focal contacts induced by increased levels of TGF-β1 in the tissue. However, we could not find higher α-SMA levels in ex vivo cultures of CD- and UC-CLPF compared to cultures isolated from control mucosa (data not shown). Therefore, a differentiation of IBD-CLPF into myofibroblasts was not the reason for the reduced migratory potential of these cells.
Contrasting migration-modulating effects of TGF-Β are described in the literature. Ellis et al[7] report a diverse pattern of motogenic response to the three TGF-Β isoforms. Migration of subconfluent fibroblasts into 3D collagen gels was inhibited by all three TGF-Β-isoforms, whereas migration of confluent cells was unaffected by TGF-β1 and TGF-β2, but stimulated by TGF-β3[7]. Postlethwaite et al[8] reported that TGF-β1 is a potent chemoattractant in the Boyden chamber for human dermal fibroblasts. Inhibitory effects of TGF-β1 on fibroblast migration into 3D collagen gels are in marked contrast to reports indicating that TGF-β1 stimulates the migration of human skin fibroblasts in the modified Boyden chamber[6].
Previously we investigated the effect of TGF-β1 on CLPF migration in the modified Boyden chamber after a 6 h incubation period[28] and found a significant migration-inducing effect of TGF-β1. Here we report that long term incubation for 6 d with TGF-β1 induces a marked reduction of migration in subsequent migration assays. This discrepancy obviously was determined by the time TGF-β1 could act on the target cells.
Addition of 10 ng/mL and 50 ng/mL TGF-β1 resulted in a dose-dependent increase of FN, FN ED-A, and FN ED-B expression. After two days incubation, expression of FN and the splice forms significantly increased. Enhanced mRNA expression was also observed after 6 d incubation with TGF-β1 but was not as high as after 2 d. On the other hand, after six days higher protein levels of total FN and FN isoforms were observed in comparison to the unstimulated two day control. Therefore, the reduced migration of CLPF after six days incubation with TGF-β1 is accompanied by enhanced protein levels of FN and FN isoforms. This increased production of FN and FN isoforms might lead to enhanced cell adhesion. Other groups also report that fibroblast attachment is significantly increased after TGF-β1 treatment[36]. Nevertheless, cell adhesion was not addressed in our investigations.
In CLPF treated for six days with TGF-β1, after wounding and PDGF-AB incubation increased protein levels of FN, FN ED-A, and FN ED-B were found when compared to untreated controls. In these cells FAK protein and FAK phosphorylation were reduced. This reduced FAK phosphorylation correlated with the observed reduced migratory potential after long term incubation with TGF-β1.
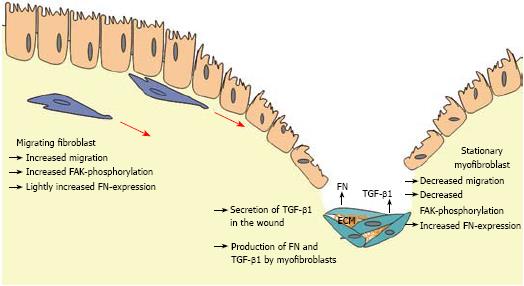
In conclusion, long term incubation of intestinal fibroblasts with TGF-β1 induced differentiation into myofibroblasts with an enhanced α-SMA production and a reduced migratory potential that was accompanied by decreased FAK production and phosphorylation. In contrast, short term contact of CLPF with TGF-β1 induced a dose-dependent increase of cell migration without induction of α-SMA. Therefore, the following scenario is conceivable: after wounding TGF-β1 is produced by different cell types and connective tissue cells, like CLPF, migrate towards the wound along the TGF-β1 gradient. Long-term contact with TGF-β1 in the wound allows the fibroblasts to differentiate into myofibroblasts. Myofibroblasts support wound healing via wound contraction and production of extracellular matrix deposition. In pathological situations the local increase in immigrated cell numbers with reduced dispersing potential and increased deposition of extracellular matrix may finally lead to tissue fibrosis. FN that is produced during the induction of migration and secreted at the site of injury may lead to an additional enhancement of the migratory gradient (Figure 9). The phosphorylation of FAK is reduced after longer contact with TGF due to the differentiation into ECM-producing myofibroblasts.
However, this model remains speculative, since the modulating influence of other factors on fibroblast migration and the complex interactions of fibroblasts with immune cells or epithelial cells certainly play an important role in wound healing, too.
The role of TGF-β and the mechanism of mucosal wound healing or intestinal tissue fibrosis require further investigation to develop therapies for the modulation of the mucosal healing response in IBD.
Migration of colonic lamina propria fibroblasts (CLPF) plays an important role during the progression of fibrosis and fistulae in Crohn’s disease. Transforming growth factor-β (TGF-β) is involved in the regulation of cell migration, cell differentiation, extracellular matrix deposition, and immune responses. In recent studies, the authors found that CLPF conditioned media induced migration of primary human CLPF and that fibronectin (FN) was mainly responsible for the autocrine induction of CLPF migration. Since the regulation of migration and differentiation of intestinal fibroblasts is an important mechanism during intestinal wound healing and fibrosis, the effect of TGF-β1 on these processes and on FN and FN isoform production was investigated in this study.
A high number of patients with Crohn’s disease have to undergo surgery because of fibrotic disease. The mechanism of stricture formation and the role of TGF-β in this are not well understood.
This study adds to the understanding of the role of TGF-β in intestinal wound healing and stricture formation. It shows that TGF-β can both be beneficial and detrimental depending on the duration of secretion.
Colon lamina propria fibroblasts (CLPF): CLPF are mesenchymal cells of connective tissue that are in an activated state and locally agile. After differentiation into myofibroblasts cells are in a less active state, concerned with maintenance. Transforming growth factor (TGF): TGF-β1 is a cytokine that on the one hand stimulates migration of fibroblasts and on the other hand potently stimulates the production of α-smooth muscle actin and stress fiber formation in fibroblasts and therefore their differentiation into myofibroblasts. Fibronectin (FN): FN is an extracellular matrix protein that is mainly responsible for the autocrine induction of CLPF migration and is essentially required for the induction of CLPF migration. Focal adhesion kinase (FAK): FAK is a nonreceptor protein tyrosine kinase involved not only in adhesion but also in cell migration. Enhanced migration of CLPF is associated with higher FAK phosphorylation and total FAK protein production. Myofibroblast differentiation by TGF-β1 is dependent on cell adhesion and integrin signaling via FAK.
The authors investigated the effects of transforming growth factor-β (TGF-β) on the differentiation of colonic lamina propria fibroblasts (CLPF) into myofibroblasts in vitro. They found that long term incubation of CLPF with TGF-β1 induced differentiation into myofibroblasts with enhanced α-SMA, reduced migratory potential and FAK-phosphorylation, as well as increased FN production. In contrast, short term contact (6 h) of fibroblasts with TGF-β1 induced a dose-dependent increase of cell migration and FAK-phosphorylation without induction of α-SMA production. The study is well designed, the methods they use are sound and their results are reliable.
| 1. | Kay EP, Lee MS, Seong GJ, Lee YG. TGF-beta s stimulate cell proliferation via an autocrine production of FGF-2 in corneal stromal fibroblasts. Curr Eye Res. 1998;17:286-293. |
| 2. | Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V, van Kooten C, Müller G. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int. 2001;59:579-592. |
| 3. | Thannickal VJ, Aldweib KD, Rajan T, Fanburg BL. Upregulated expression of fibroblast growth factor (FGF) receptors by transforming growth factor-beta1 (TGF-beta1) mediates enhanced mitogenic responses to FGFs in cultured human lung fibroblasts. Biochem Biophys Res Commun. 1998;251:437-441. |
| 4. | Adelmann-Grill BC, Wach F, Cully Z, Hein R, Krieg T. Chemotactic migration of normal dermal fibroblasts towards epidermal growth factor and its modulation by platelet-derived growth factor and transforming growth factor-beta. Eur J Cell Biol. 1990;51:322-326. |
| 5. | Andresen JL, Ehlers N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res. 1998;17:79-87. |
| 6. | Ellis I, Grey AM, Schor AM, Schor SL. Antagonistic effects of TGF-beta 1 and MSF on fibroblast migration and hyaluronic acid synthesis. Possible implications for dermal wound healing. J Cell Sci. 1992;102:447-456. |
| 7. | Ellis IR, Schor SL. Differential motogenic and biosynthetic response of fetal and adult skin fibroblasts to TGF-beta isoforms. Cytokine. 1998;10:281-289. |
| 8. | Postlethwaite AE, Keski-Oja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987;165:251-256. |
| 9. | Simmons JG, Pucilowska JB, Keku TO, Lund PK. IGF-I and TGF-beta1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;283:G809-G818. |
| 10. | Shimao Y, Nabeshima K, Inoue T, Koono M. Role of fibroblasts in HGF/SF-induced cohort migration of human colorectal carcinoma cells: fibroblasts stimulate migration associated with increased fibronectin production via upregulated TGF-beta1. Int J Cancer. 1999;82:449-458. |
| 11. | Eickelberg O, Köhler E, Reichenberger F, Bertschin S, Woodtli T, Erne P, Perruchoud AP, Roth M. Extracellular matrix deposition by primary human lung fibroblasts in response to TGF-beta1 and TGF-beta3. Am J Physiol. 1999;276:L814-L824. |
| 12. | Wells RG. Fibrogenesis. V. TGF-beta signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2000;279:G845-G850. |
| 13. | McKaig BC, Hughes K, Tighe PJ, Mahida YR. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol. 2002;282:C172-C182. |
| 14. | Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP, Roberts AB. Functional characterization of transforming growth factor beta signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem. 2001;276:19945-19953. |
| 15. | Badid C, Mounier N, Costa AM, Desmoulière A. Role of myofibroblasts during normal tissue repair and excessive scarring: interest of their assessment in nephropathies. Histol Histopathol. 2000;15:269-280. |
| 16. | Leeb SN, Vogl D, Grossmann J, Falk W, Schölmerich J, Rogler G, Gelbmann CM. Autocrine fibronectin-induced migration of human colonic fibroblasts. Am J Gastroenterol. 2004;99:335-340. |
| 17. | Brenmoehl J, Lang M, Hausmann M, Leeb SN, Falk W, Schölmerich J, Göke M, Rogler G. Evidence for a differential expression of fibronectin splice forms ED-A and ED-B in Crohn's disease (CD) mucosa. Int J Colorectal Dis. 2007;22:611-623. |
| 18. | Hynes R. Molecular biology of fibronectin. Annu Rev Cell Biol. 1985;1:67-90. |
| 19. | Owens RJ, Kornblihtt AR, Baralle FE. Fibronectin, the generation of multiple polypeptides from a single gene. Oxf Surv Eukaryot Genes. 1986;3:141-160. |
| 20. | Schwarzbauer JE. Fibronectin: from gene to protein. Curr Opin Cell Biol. 1991;3:786-791. |
| 21. | Vartio T, Laitinen L, Närvänen O, Cutolo M, Thornell LE, Zardi L, Virtanen I. Differential expression of the ED sequence-containing form of cellular fibronectin in embryonic and adult human tissues. J Cell Sci. 1987;88:419-430. |
| 22. | Zardi L, Carnemolla B, Siri A, Petersen TE, Paolella G, Sebastio G, Baralle FE. Transformed human cells produce a new fibronectin isoform by preferential alternative splicing of a previously unobserved exon. EMBO J. 1987;6:2337-2342. |
| 23. | Kornblihtt AR, Umezawa K, Vibe-Pedersen K, Baralle FE. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985;4:1755-1759. |
| 24. | MacLeod JN, Burton-Wurster N, Gu DN, Lust G. Fibronectin mRNA splice variant in articular cartilage lacks bases encoding the V, III-15, and I-10 protein segments. J Biol Chem. 1996;271:18954-18960. |
| 25. | Schwarzbauer JE, Tamkun JW, Lemischka IR, Hynes RO. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983;35:421-431. |
| 26. | Ffrench-Constant C, Van de Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989;109:903-914. |
| 27. | Nedelec B, Ghahary A, Scott PG, Tredget EE. Control of wound contraction. Basic and clinical features. Hand Clin. 2000;16:289-302. |
| 28. | Leeb SN, Vogl D, Falk W, Schölmerich J, Rogler G, Gelbmann CM. Regulation of migration of human colonic myofibroblasts. Growth Factors. 2002;20:81-91. |
| 29. | Leeb SN, Vogl D, Gunckel M, Kiessling S, Falk W, Göke M, Schölmerich J, Gelbmann CM, Rogler G. Reduced migration of fibroblasts in inflammatory bowel disease: role of inflammatory mediators and focal adhesion kinase. Gastroenterology. 2003;125:1341-1354. |
| 30. | Balza E, Borsi L, Allemanni G, Zardi L. Transforming growth factor beta regulates the levels of different fibronectin isoforms in normal human cultured fibroblasts. FEBS Lett. 1988;228:42-44. |
| 31. | Inoue T, Nabeshima K, Shimao Y, Koono M. Hepatocyte growth Factor/Scatter factor (HGF/SF) is a regulator of fibronectin splicing in MDCK cells: comparison between the effects of HGF/SF and TGF-beta1 on fibronectin splicing at the EDA region. Biochem Biophys Res Commun. 1999;260:225-231. |
| 32. | Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180-189. |
| 33. | Lijnen P, Petrov V, Rumilla K, Fagard R. Transforming growth factor-beta 1 promotes contraction of collagen gel by cardiac fibroblasts through their differentiation into myofibroblasts. Methods Find Exp Clin Pharmacol. 2003;25:79-86. |
| 34. | Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384-12389. |
| 35. | Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677-2691. |
| 36. | Lawrance IC, Maxwell L, Doe W. Inflammation location, but not type, determines the increase in TGF-beta1 and IGF-1 expression and collagen deposition in IBD intestine. Inflamm Bowel Dis. 2001;7:16-26. |