Published online Jan 7, 2009. doi: 10.3748/wjg.15.102
Revised: November 12, 2008
Accepted: November 19, 2008
Published online: January 7, 2009
AIM: To find the current seroepidemiology of hepatitis A virus (HAV) in Kuwait.
METHODS: A total of 2851 Kuwaitis applying for new jobs were screened.
RESULTS: HAV-positive cases were 28.8%; 59% were males and 41% were females. The highest prevalence was in the Ahmadi area. High prevalence was among the group of non-educated rather than educated parents. This is the first study in Kuwait demonstrating the shifting epidemiology of HAV.
CONCLUSION: This study reflects the need of the Kuwaiti population for an HAV vaccine.
- Citation: Alkhalidi J, Alenezi B, Al-mufti S, Hussain E, Askar H, Kemmer N, Neff GW. Seroepidemiology of hepatitis A virus in Kuwait. World J Gastroenterol 2009; 15(1): 102-105
- URL: https://www.wjgnet.com/1007-9327/full/v15/i1/102.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.102
Hepatitis A virus (HAV) infection is often a self-limiting disease that can be associated with fulminant hepatic failure (FHF). The mortality rate tends to increase with age, in particular, when greater than 40 years of age[1]. Various data from the world shows that with the improvement in hygiene and quality of life there is a shift in the epidemiology of HAV to older people, exposing them to the risk of serious HAV infection.
Hepatitis A is often considered a benign disease in our area. The idea is that almost 100% of the adult population has been infected in early childhood[2]. However, there are no available data about the current epidemiology of the disease in Kuwait. The aim of this study was to find out the current sero-prevalence of this disease and to decide the need of the Kuwaiti population for HAV vaccine.
The study population was healthy adults attending a medical checkup that was required before applying for a new job. The study was performed in two places in Kuwait; the first one was the General Medical Council, which accepts adults from both sexes and all nationalities applying for civilian jobs. Only Kuwaiti nationals were included in the study. The second place was the Armed Forces Hospital, which accepts Kuwaiti adult males recruited for military service. The study population belong, to the total six governorates of the country of Kuwait, which includes the Capital, Hawali, Farwania, Mubarak, Ahmadi and Jahra.
The study was approved by the Ministry of Health and the local ethical committee of the Kuwait Institution for Medical Specialization (KIMS). Informed consent was obtained from each case. Each individual completed a questionnaire. Thereafter, 5 mL of blood was obtained. The identities of the subjects were kept confidential by assigning a code number for the questionnaire and the blood samples. The study was conducted during May 2003 to May 2004.
Blood samples were collected from different centers and sent to the Virology Unit-Public Health Laboratories. An Axsym HAVAB 2.0 kit (Abbott Laboratories) was used for the detection of IgG anti-HAV from all samples. The procedure was followed as indicated by the manufacturer. In addition, the samples were tested for anti-hepatitis B surface antigen (HBsAg), anti-hepatitis C virus and anti-HIV.
Data are expressed using descriptive statistical methods, namely counts and percentages of screened subjects.
There were a total of 2851 Kuwaiti cases screened, 2216 were from the Medical Council and the remaining were from the Military Hospital.
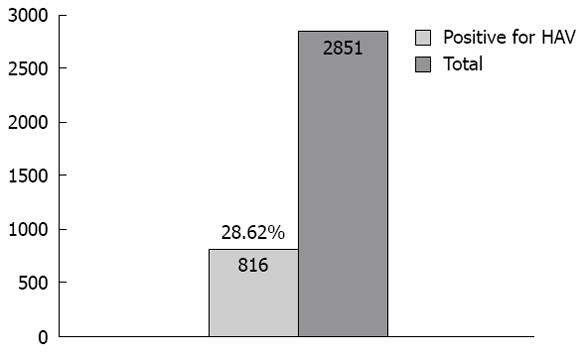
The screened cases were from the six governorates of Kuwait which included Farwania 528 cases (18.52%), Ahmadi 505 cases (17.71%), Hawali 467 (16.38%), Capital 433 (15.19%), Jahra 411 (14.42%), Mubarak 301 cases (10.56%) and 7.23% were from unidentified areas. Of 2851 cases screened, 816 (28.6%) cases were positive for HAV (Figure 1). The prevalence percentages of HAV in each governorate were higher in Ahmadi, 202 cases (24.5%); Farwania, 170 cases (20.6%); and Jahra, 164 cases (20%), than in others: Capital 89 cases (11%); Hawali, 84 cases (10%); Mubarak, 76 cases (9%); and 31 cases (3.8%) were unidentified. The 28% of cases which tested positive for HAV were in 94% of cases from the Medical Council and in 5% of cases from the Military Hospital.
There were 481 (59%) males and 335 (41%) females. The prevalence of HAV cases in each age group was 24% (561) in the age group 18-27 years, 51% (213 cases) in the age group 28-40 years, and 56.5% (26 cases) in the age group 41-60 years (Table 1).
| Age category | Pts with HAV+ve |
| Less than 27 yr (2385 cases screened) | 577 (24) |
| 28 to 40 yr (420 cases screened) | 213 (51 ) |
| 41 to 60 yr (46 cases screened) | 26 (56.5 ) |
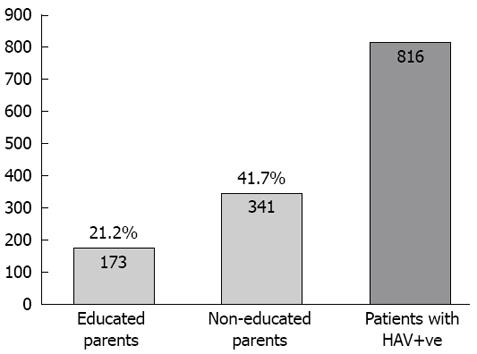
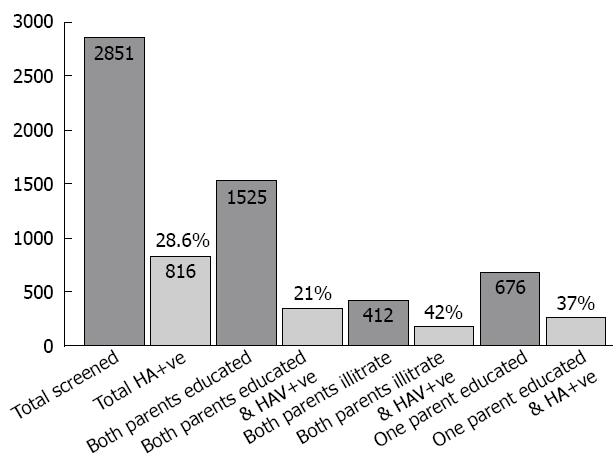
Of 2851 subjects screened, 1525 had both parents who were educated, while 412 had non-educated parents. The prevalence of HAV among the group with non-educated parents or a single educated parent was higher than the educated group (42%, 37% and 21%, respectively) (Figure 2). Of the 816 cases who had HAV, single parent education was determined as maternal education in 10%, while paternal education was 90%. Maternal education plays a greater role than paternal in the care of children and the family which is reflected in the social and health standards (Figure 3).
There was no risk factor in 2706 cases, household contacts with hepatitis in one case, surgery in two, blood transfusion in four, and unknown answer in 138 cases.
In this study, 2035 (71%) cases were not immune to HAV, 94% of them had no evidence of HBV or HCV infection. In 4.8% of cases, there was positive HBsAg serology, while 1.5% had positive anti-HCV results. Of 816 (28.6%) having immunity to HAV, 97.7% had no evidence of HBV, HCV or HIV; 1.7% had positive serology for HAV, HBV and HCV; 0.24% had positive serology for HBsAg, while 0.37% had positive serology for HCV. None of these cases were positive for HIV
| Anti HAV | HBsAg | Anti HCV | Count |
| Yes | Yes | Yes | 14 |
| Yes | Yes | No | 2 |
| Yes | No | Yes | 3 |
| Yes | No | No | 797 |
| No | Yes | No | 97 |
| No | No | Yes | 30 |
| No | No | No | 1908 |
| Anti HAV+ve | HBsAg+ve | Anti HCV+ve | Anti HIV+ve | Number of cases |
| Yes | Yes | Yes | No | 14 |
| Yes | Yes | No | No | 2 |
| Yes | No | Yes | No | 3 |
| Yes | No | No | No | 797 |
HAV is a non-enveloped, RNA-containing virus that belongs to the family Picornaviridae. It is a spherical 27-nm particle that was discovered by Feinstone in 1973[3]. The routes of infection are orofecal and percutaneous. The incubation period is about 28 d. The fecal shedding of virus is at a maximum during the late incubation period, just before or shortly after the onset of symptoms[4].
After oral inoculation of a chimpanzee with HAV, the viral antigen was detected first in the serum on day 14, in the tonsils on day 16 and in the liver on day 21. The viremia lasts for 2 wk[5]. In human studies, HAV RNA is detected for an average of 60 d after onset of clinical symptoms[6]. Risk factors that have been associated with reported HAV infection within the United States include sexual or household contact with another person with hepatitis (25%), contact with children attending a day-care center (15%), international travel (5%) and food or water-borne outbreak (5%). However, in 50% of cases, no risk factor can be identified[7].
In Kuwait, the epidemiology of HAV in the 1980s was similar to developing countries with almost 100% of adults over the age of 20 years testing positive for anti-HAV. At that time, 90% of the screened cases with acute hepatitis A were below the age of 10 years and 70% below the age of 5 years[2]. Retrospective analysis of all charts of Kuwaiti patients presenting with acute HAV infectious between the ages of 0 and 15 years admitted to an infection disease hospital between 2000 and 2002 showed an incidence of 47 per 100 000. One third of these cases were from Jahra region, which is dominated by the Bedouin who live in large extended families. However, in 50% of these cases, there was no identified risk factor. Prolonged jaundice was found in 3% of cases and FHF in 0.4%[8].
There has been a dramatic drop in the incidence of HAV infection in children (47/100 000 vs 122/100 000) and a shift toward infection among older children (from 0-4 years to 7-12 years). Our study provides the most recent data about the prevalence of HAV in Kuwait over the last 20 years. The prevalence of HAV was 28% and one quarter of screened individuals below the age of 27 years had positive anti HAV. There were more cases in the areas dominated by Bedouin with extended families. Also, there were more cases among the group with uneducated parents, which reflects the relationship of the disease to low social background. These data show the shifting epidemiology of HAV in Kuwait toward intermediate to low endemicity, leaving 75% of the population below the age of 27 years non-immune, with a risk of exposure to HAV infection at a later age group, with increased morbidity. These changes have resulted from improvement in living standards and socio-economic progress. These data demonstrate the requirement of initiating an HAV vaccine program in Kuwait.
Areas in the Middle East like Qatar, United Arab Emirates and Saudi Arabia show a shifting pattern from high to intermediate endemicity for HAV. In Saudi Arabia, there are existing pockets of high HAV endemicity that may lead to outbreaks[9]. A study by Fathalla et al[10] showed that eastern Saudi Arabia still belongs to epidemiological pattern 1, which is characteristic of developing poor countries with low socioeconomic status and the country has a seroprevalence of 99%. The United Arab Emirates data showed that the seroprevalence of HAV was 60% and 90% for the ages of 16 and 40 years respectively which indicates a shift of HAV epidemic with infection towards an adult population[11]. In South-East Asia and China, there is shifting epidemiology of HAV from high to moderate or low endemicity. In China, this is associated with risk of outbreaks as a result of re-introduction of the virus from areas of high endemicity to low endemicity within a non-immune population[12] The incidence of HAV infection varies from high, moderate, low and very low endemicity areas. South-East Asia, India, Africa and Latin America were considered high endemic areas. The epidemiology of HAV is changing due to improvement in water supplies and sanitation conditions. Asian studies from Taiwan and India also show changing seroepidemiology of HAV infection and these countries are considering the use of HAV vaccine. The prevalence of HAV antibodies in Taiwan decreased in 1998 compared to 1992 reflecting the improvement of socioeconomic status and modernization of sanitation[1314]. Africa is still considered as an area with high endemicity for HAV[9]. In Latin America, the highest anti-HAV seroprevalence rates were found in Mexico and the Dominican Republic. In these countries over the last 15 years, there was a shift towards medium endemicity with the peak of infection occurring in later childhood and adolescence rather than in early childhood. Contaminated water and food supply were the strongest risk factors in Latin America[15].
In European industrialized countries like Italy, there is a markedly lower prevalence of HAV infection, especially among a younger age group, due to marked improvements in socioeconomic conditions and hygienic standards. In the same region, small outbreaks of HAV infection were associated with intravenous drug abusers travelling to endemic areas, shellfish consumption and with an increasing number of family members[16].
Patients with negative serology for HAV need HAV vaccine. Also, patients with chronic liver disease who are non-immune to HAV need HAV vaccine[17]. In our study, 2035 (71%) of cases were not immune to HAV, 4.8% of them had positive HBsAg serology, while 1.5% of them had positive anti-HCV serology.
HAV vaccines are highly purified and formalin-inactivated. The vaccine was shown to be safe and effective when tested by Werzberger et al[18] among seronegative children 2-16 years of age in Monroe in 1992. Inactivated HAV vaccine (VAQTA, Merck and Co Inc, West Point, PA, USA) is given in two doses (0 and 6-12 mo). The estimated protective efficacy of one or more doses of the vaccine is 98%. HAV vaccine provides long-term immunity lasting probably from 20 to 50 years[19]. The vaccine has been available in the USA since 1995, and is highly effective in preventing disease transmission in a community with recurrent epidemics. The adverse effects of the vaccine are mild and include fever, rash and injection site reaction. It proved safe with no adverse effects among 30 000 vaccine recipients[20]. The HAV vaccine VAQTA given in two doses to a group of infants at the age of 2 years and followed up for 9 years provided long-term protection. It was effective in preventing HAV epidemics in the community in spite of the exposure to sporadic cases in non-vaccinated individuals[21]. HAV vaccine is recommended for persons with increased risk of infection including international travellers, illegal drug users, persons with chronic liver disease, persons who have clotting factor disorders and homosexuals[17]. Vaccination against hepatitis is the most effective means of preventing sexual transmission of hepatitis A and B[22].
| 1. | Vento S, Garofano T, Renzini C, Cainelli F, Casali F, Ghironzi G, Ferraro T, Concia E. Fulminant hepatitis associated with hepatitis A virus superinfection in patients with chronic hepatitis C. N Engl J Med. 1998;338:286-290. |
| 2. | Nordenfelt E, Atack W, Al-Kandani S, Al-Nakib W. Hepatitis A in Kuwait. J Kuwait Med Assoc. 1985;19:103-108. |
| 3. | Feinstone SM, Kapikian AZ, Purceli RH. Hepatitis A: detection by immune electron microscopy of a viruslike antigen associated with acute illness. Science. 1973;182:1026-1028. |
| 4. | Lemon SM. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985;313:1059-1067. |
| 5. | Cohen JI, Feinstone S, Purcell RH. Hepatitis A virus infection in a chimpanzee: duration of viremia and detection of virus in saliva and throat swabs. J Infect Dis. 1989;160:887-890. |
| 6. | Costa-Mattioli M, Monpoeho S, Nicand E, Aleman MH, Billaudel S, Ferre V. Quantification and duration of viraemia during hepatitis A infection as determined by real-time RT-PCR. J Viral Hepat. 2002;9:101-106. |
| 7. | Kemmer NM, Miskovsky EP. Hepatitis A. Infect Dis Clin North Am. 2000;14:605-615. |
| 8. | Husain E, Husain K. Hepatitis A injection in children in Kuwait. . |
| 9. | Tufenkeji H. Hepatitis A shifting epidemiology in the Middle East and Africa. Vaccine. 2000;18 Suppl 1:S65-S67. |
| 10. | Fathalla SE, Al-Jama AA, Al-Sheikh IH, Islam SI. Seroprevalence of hepatitis A virus markers in Eastern Saudi Arabia. Saudi Med J. 2000;21:945-949. |
| 11. | Dajani A, Boloushi S, Kashkosh A. HAV: The risk in UAE. . |
| 12. | Barzaga BN. Hepatitis A shifting epidemiology in South-East Asia and China. Vaccine. 2000;18 Suppl 1:S61-S64. |
| 13. | Wang SM, Liu CC, Huang YS, Yang YJ, Lei HY. Change in hepatitis A virus seroepidemiology in southern Taiwan: a large percentage of the population lack protective antibody. J Med Virol. 2001;64:104-108. |
| 14. | Das K, Jain A, Gupta S, Kapoor S, Gupta RK, Chakravorty A, Kar P. The changing epidemiological pattern of hepatitis A in an urban population of India: emergence of a trend similar to the European countries. Eur J Epidemiol. 2000;16:507-510. |
| 16. | Moschen ME, Floreani A, Zamparo E, Baldo V, Majori S, Gasparini V, Trivello R. Hepatitis A infection: a seroepidemiological study in young adults in North-East Italy. Eur J Epidemiol. 1997;13:875-878. |
| 17. | Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention. MMWR Recomm Rep. 1996;45:1-36. |
| 18. | Werzberger A, Mensch B, Kuter B, Brown L, Lewis J, Sitrin R, Miller W, Shouval D, Wiens B, Calandra G. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med. 1992;327:453-457. |
| 19. | Wiedermann G, Kundi M, Ambrosch F, Safary A, D'Hondt E, Delem A. Inactivated hepatitis A vaccine: long-term antibody persistence. Vaccine. 1997;15:612-615. |
| 20. | Averhoff F, Shapiro CN, Bell BP, Hyams I, Burd L, Deladisma A, Simard EP, Nalin D, Kuter B, Ward C. Control of hepatitis A through routine vaccination of children. JAMA. 2001;286:2968-2973. |
| 21. | Werzberger A, Mensch B, Nalin DR, Kuter BJ. Effectiveness of hepatitis A vaccine in a former frequently affected community: 9 years' followup after the Monroe field trial of VAQTA. Vaccine. 2002;20:1699-1701. |
| 22. | Sexually transmitted diseases treatment guidelines 2002. . Centers for disease control and prevention. MMWR Recomm Rep. 2002;51:1-78. |