Published online Mar 7, 2008. doi: 10.3748/wjg.14.1378
Revised: October 30, 2007
Published online: March 7, 2008
AIM: To investigate the anti-neoplastic effect of MK615, an anti-neoplastic compound isolated from Japanese apricot, against human pancreatic cancer cells in vitro.
METHODS: Three human pancreatic cancer cell lines PANC-1, PK-1, and PK45H were cultured with MK615 at concentrations of 600, 300, 150, and 0 &mgr;g/mL. Growth inhibition was evaluated by cell proliferation assay, and killing activity was determined by lactate dehydrogenase (LDH) assay. Expression of Aurora A and B kinases was detected by real-time polymerase chain reaction (PCR) and Western blotting. Cell cycle stages were evaluated by flow cytometry.
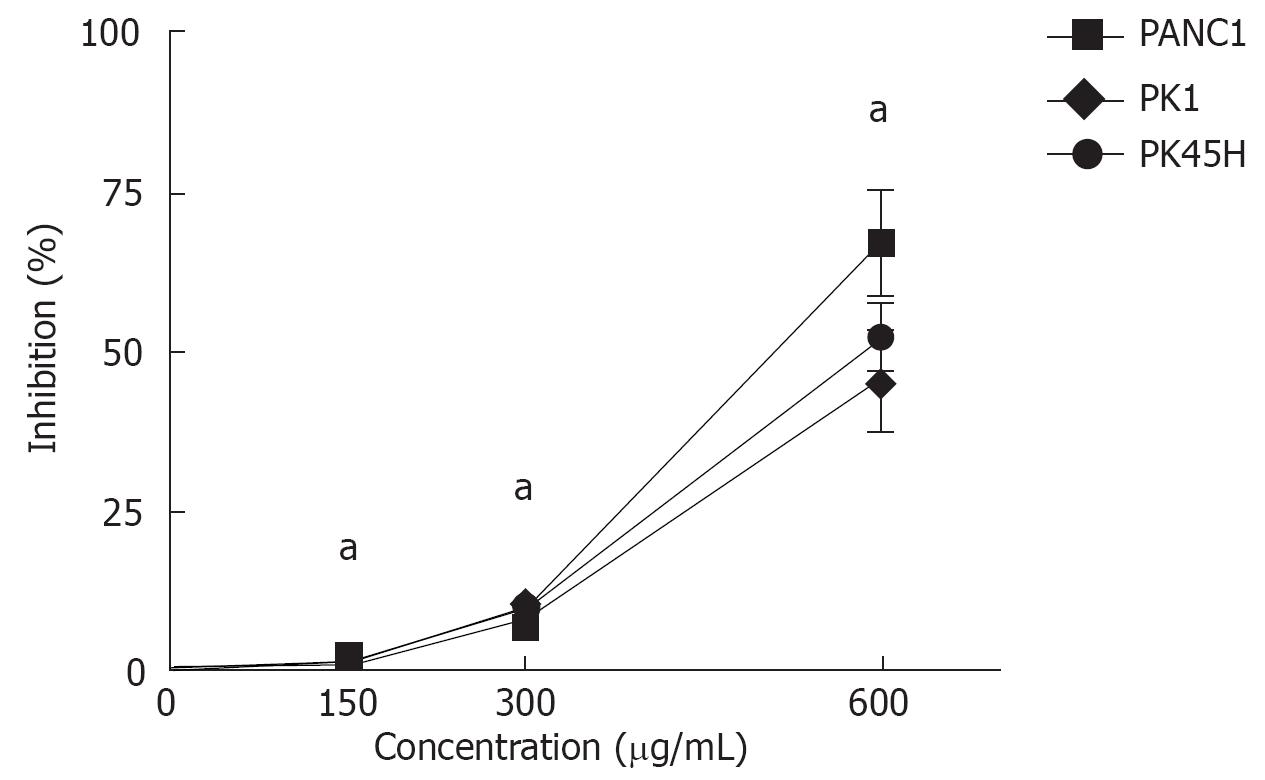
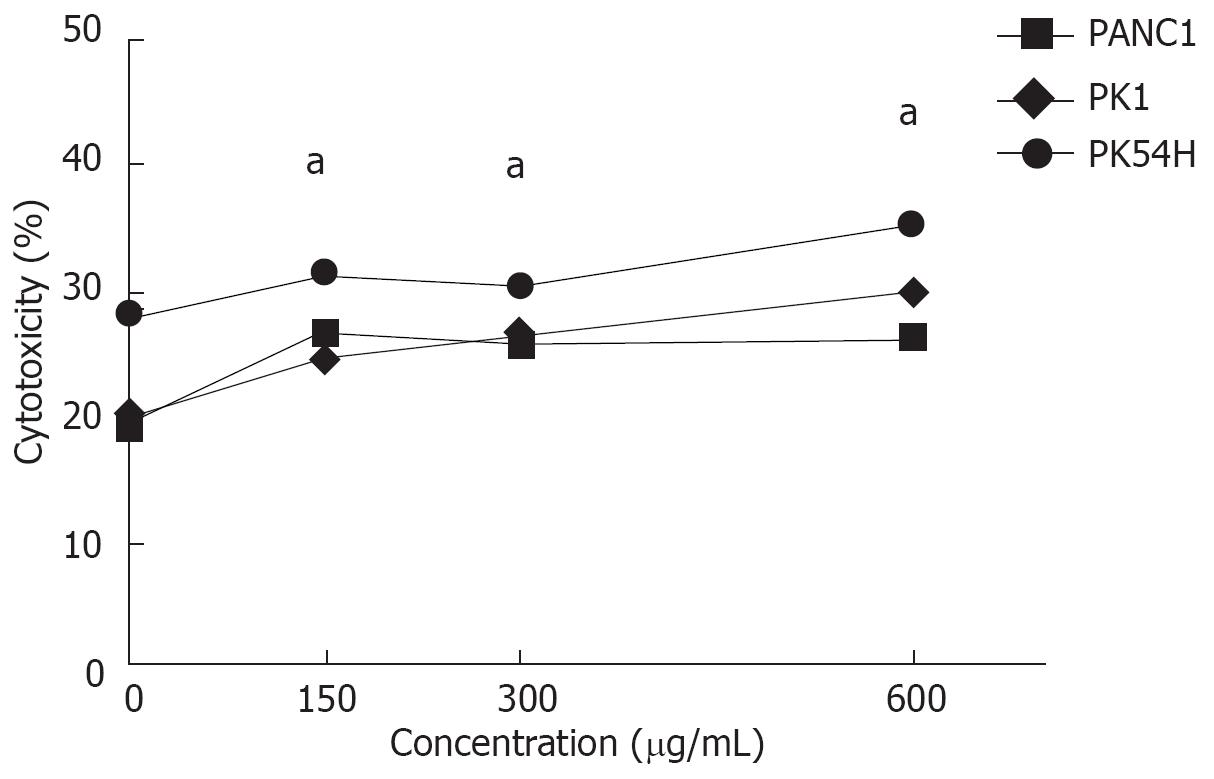
RESULTS: The growth inhibitory rates of MK615 at 150, 300, and 600 &mgr;g/mL were 2.3% ± 0.9%, 8.9% ± 3.2% and 67.1% ± 8.1% on PANC1 cells, 1.3% ± 0.3%, 8.7% ± 4.1% and 45.7 ± 7.6% on PK1 cells, and 1.2 ± 0.8%, 9.1% ± 2.1% and 52.1% ± 5.5% on PK45H cells, respectively (P <0.05). The percentage cytotoxicities of MK615 at 0, 150, 300, and 600 &mgr;g/mL were 19.6% ± 1.3%, 26.7% ± 1.8%, 25.5% ± 0.9% and 26.4% ± 0.9% in PANC1 cells, 19.7% ± 1.3%, 24.7% ± 0.8%, 25.9% ± 0.9% and 29.9% ± 1.1% in PK1 cells, and 28.0% ± 0.9%, 31.2% ± 0.9%, 30.4% ± 1.1% and 35.3 ± 1.0% in PK45H cells, respectively (P < 0.05). Real-time PCR and Western blotting showed that MK615 dually inhibited the expression of Aurora A and B kinases. Cell cycle analysis revealed that MK615 increased the population of cells in G2/M phase.
CONCLUSION: MK615 exerts an anti-neoplastic effect on human pancreatic cancer cells in vitro by dual inhibition of Aurora A and B kinases.
- Citation: Okada T, Sawada T, Osawa T, Adachi M, Kubota K. MK615 inhibits pancreatic cancer cell growth by dual inhibition of Aurora A and B kinases. World J Gastroenterol 2008; 14(9): 1378-1382
- URL: https://www.wjgnet.com/1007-9327/full/v14/i9/1378.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1378
MK615, an extract from the Japanese apricot, Prunus mume Siebold et Zuccarini (ume in Japanese), contains several triterpenoids and has been shown to exert an anti-neoplastic effect against human cancers. Previous studies have revealed that MK615 has anti-neoplastic effects against gastric cancer[1], breast cancer[2], hepatocellular carcinoma[3], and colon cancer[4]. The mechanisms responsible for the anti-neoplastic effect of MK615 include induction of apoptosis[12] and autophagy[4], and suppression of Aurora A kinase[3] in cancer cells. However, the entire mechanisms of the anti-neoplastic effects of MK615 have not been elucidated.
In the present study, we cultured human pancreatic cancer cell lines in the absence and presence of MK615 for the first time, and found that MK615 had an anti-neoplastic effect that was exerted by dual inhibition of Aurora A and B kinases.
MK615 was provided by Japan Apricot Co., Ltd. (Gunma, Japan). MK615 is derived from Japanese apricot fruit[1].
Three human pancreatic cancer cell lines PANC-1 (PANC1), PK-1 (PK1) and PK-45H (PK45H) were obtained from the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University (Miyagi, Japan). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 100 mL/L heat-inactivated fetal calf serum (FCS) and incubated at 37°C in a humidified atmosphere consisting of 50 mL/L CO2 in air.
Cells were plated at 5 × 103/well in 96-well plates in DMEM containing 100 mL/L FCS and cultured with or without MK615 at concentrations of 600, 300, or 150 &mgr;g/mL. For the negative control wells, cells were cultured with 10 mL/L DMSO alone. After 48 h, 3-(4, 5-dimethylthiazol-2-yl)-5-(3carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTT; 5 g/L) was added to each well and the cells were incubated for 4 h. Then according to the manufacturer’s recommendation, 100 &mgr;L of solubilization solution/stop mix was added and the plates were left to stand for 60 min. The absorbances at 570 nm (A570) and 630 nm (A630) were then measured with an enzyme-linked immunoassay (ELISA) reader. The actual counts were calculated by subtracting A570 from A630. Each assay was performed in triplicate and the average absorbance was calculated. The growth inhibition rate was calculated using the ratio of absorbance at each drug concentration relative to the absorbance without drug.
Lactate dehydrogenase (LDH) assay was performed using the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI). Briefly, cells were plated at 5 × 103/well in 96-well plates in DMEM containing 100 mL/L FCS. Cells were cultured with or without MK615 at concentrations of 600, 300, or 150 &mgr;g/mL. For the negative control wells, cells were cultured with 10 mL/L DMSO alone. The medium was removed and 100 &mgr;L of CytoTox-ONETM Reagent was added. The cells were then incubated at 22°C for 10 min and 50 &mgr;L of stop solution was added. The plates were shaken for 10 s and the absorbances at 560 nm (A560) and 590 nm (A590) were measured with an ELISA reader. To obtain the maximum LDH release, 10 &mgr;L of lysis solution (10 ×) was added to the positive control wells 45 min prior to harvest. After 48 h, 50 &mgr;L of supernatant was transferred into a fresh 96-well plate and incubated with 50 &mgr;L of substrate mix for 30 min at room temperature. Fifty microliters of stop solution was added, and the absorbance at 490 nm (A490) was measured with an ELISA reader. Each assay was performed in triplicate and the average absorbance was calculated. The percentage cytotoxicity was calculated using the formula: Percentage cytotoxicity = 100 × (Experimental - Culture medium background)/(Maximum LDH release - Culture medium and lysis solution background).
The total RNA of each cell line was isolated using a Total RNA Isolation kit (MACHEREY-NAGEL, Düren, Germany). Reverse transcription reactions were performed using a Rever Tra Ace α-First Strand cDNA Synthesis kit (TOYOBO, Osaka, Japan). Briefly, 1 &mgr;g of total RNA, oligo dT-primer, and dNTPs were incubated at 65°C for 5 min, followed by addition of 10 &mgr;L of cDNA synthesis mixture and further incubation at 50°C for 50 min. The reaction was terminated by adding 1 &mgr;L of RNaseH and incubating the mixture at 37°C for 20 min.
Real-time PCR was performed with an ABI Prism 7700 Sequence Detector (Applied Biosystems, Warrington, UK). The PCR reaction was carried out in a final volume of 2 &mgr;L of cDNA, 12.5 &mgr;L of 2 × SYBR Green (Applied Biosystems), 0.5 &mgr;L of 25 nmol/L sense and antisense primers, and H2O up to 25 &mgr;L. The PCR conditions consisted of 40 amplification cycles at 95°C for 30 s and 60°C for 30 s. The sequences of the primers were as follows: GAPDH, 5’-CCACCCAGAAGACTGTGGAT-3’ (sense) and 5’-TTCAGCTCAGGGATGACCTT-3’ (anti-sense); Aurora A, 5’-TTGGAATATGCACCACTTGGA-3’ (sense) and 5’-ACTGACCACCCAAATCTGC-3’ (anti-sense); and Aurora B, 5’-GGGAGAGCTGAAGATTGCTG-3’ (sense) and 5’-GGCGATAGGTCTCGTTGTGT-3’ (anti-sense). The level of expression was calculated using the formula: Relative expression (t) = (Copy number of target molecule/Copy number of GAPDH) × 1000. Samples were assayed in triplicate. Means and standard deviations were calculated from the data obtained. The t value was calculated from the mean of three different assays.
Anti-Aurora A and Aurora B, nuclear factor-κB (NF-κB), and phosphorylated NFκB at serine residue 536 [pNF-κB (ser536)] antibodies were purchased from Cell Signaling Technology (Beverly, MA). Cells (5-10 × 106) were cultured with MK615 (300 &mgr;g/mL) for 48 h, washed twice with cold PBS, lysed with 200 &mgr;L of 5 g/L SDS, and centrifuged at 10 000 ×g. The supernatants were adjusted to contain equal amounts of protein by dilution, using a BCA Protein Assay kit (PIERCE, Rockford, IL). Samples (20 &mgr;g protein) were run on 125 g/L SDS-PAGE with 10% gel and electroblotted onto PVDF membranes. The blots were blocked for 1 h with 50 g/L nonfat milk powder and 1 mL/L Tween 20 in Tris-NaCl, followed by overnight incubation with primary antibody (dilution 1:1000) at 4°C. After extensive washing, the blots were incubated with the secondary horseradish-peroxidase-conjugated antibody (dilution 1:2000) for 2 h at room temperature. Immunoreactive bands were visualized by using an enhanced chemiluminescence detection system (Amersham Life Sciences, Arlington Heights, IL).
Cells were cultured with 300 &mgr;g/mL MK615 for 48 h, then collected by trypsinization. According to the manufacturer’s instructions, cell cycle analysis was performed with a Cycle TEST PLUS DNA Reagent kit (Becton-Dickinson, Mountain View, CA) on a FACScaliber (Becton-Dickinson). Results were analyzed with CellFIT software.
For the MTT assay, LDH assay, and real-time PCR, Student’s t test (two-sided) was used to compare the data obtained from MK615-treated and MK615-untreated groups. A P value less than 0.05 was considered statistically significant.
MK615 inhibited growth of the three pancreatic cancer cell lines (Figure 1). The growth inhibitory rates of MK615 at 150, 300 and 600 &mgr;g/mL were 2.3% ± 0.9%, 8.9% ± 3.2% and 67.1% ± 8.1% on PANC1 cells, 1.3% ± 0.3%, 8.7% ± 4.1% and 45.7% ± 7.6% on PK1 cells, and 1.2% ± 0.8%, 9.1% ± 2.1% and 52.1% ± 5.5% on PK45H cells, respectively. The growth inhibition induced by MK615 at all concentrations was significantly higher than that at 0 &mgr;g/mL (P < 0.05).
Cytotoxicity of MK615 against pancreatic cancer cell lines was evaluated by LDH assay (Figure 2). The percent specific lysis by MK615 at 0, 150, 300, and 600 &mgr;g/mL was 19.6% ± 1.3%, 26.7% ± 1.8%, 25.5% ± 0.9% and 26.4% ± 0.9% in PANC1 cells, 19.7% ± 1.3%, 24.7% ± 0.8%, 25.9% ± 0.9% and 29.9% ± 1.1% in PK1 cells, and 28.0% ± 0.9%, 31.2% ± 0.9%, 30.4% ± 1.1% and 35.3% ± 1.0% in PK45H cells, respectively. The percent specific lysis induced by MK615 at all concentrations was significantly higher than that at 0 &mgr;g/mL (P < 0.05).
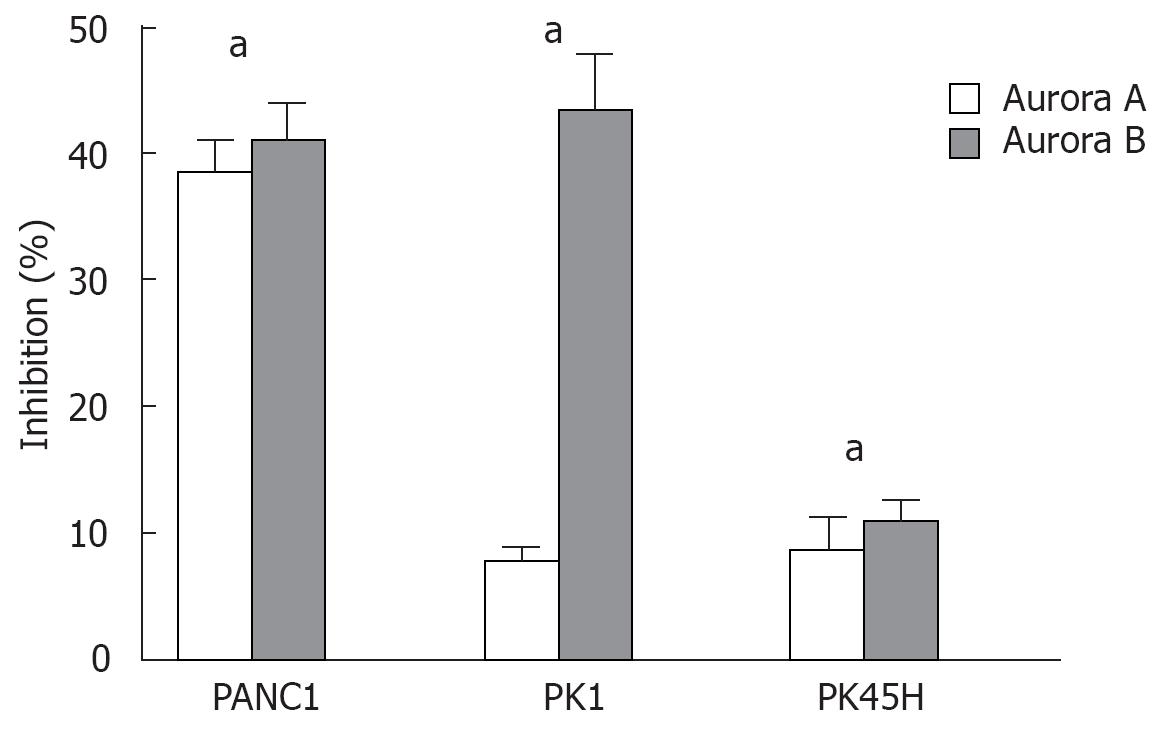
Moreover, we examined whether MK615 was able to inhibit Aurora kinases in the pancreatic cancer cell lines. Aurora kinases mRNA expression is shown in Figure 3. The inhibition rate of Aurora A and Aurora B mRNA was 38.4% ± 2.9% (P = 0.02) and 40.8% ± 3.2% (P = 0.03) in PANC1 cells, 7.6% ± 1.1% (P = 0.05) and 43.4% ± 4.5% (P = 0.03) in PK1 cells, and 8.7% ± 2.5% (P = 0.05) and 10.5% ± 2.1% (P = 0.05) in PK45H cells, respectively, indicating a significant inhibition of mRNA expression of both Aurora kinases by MK615.
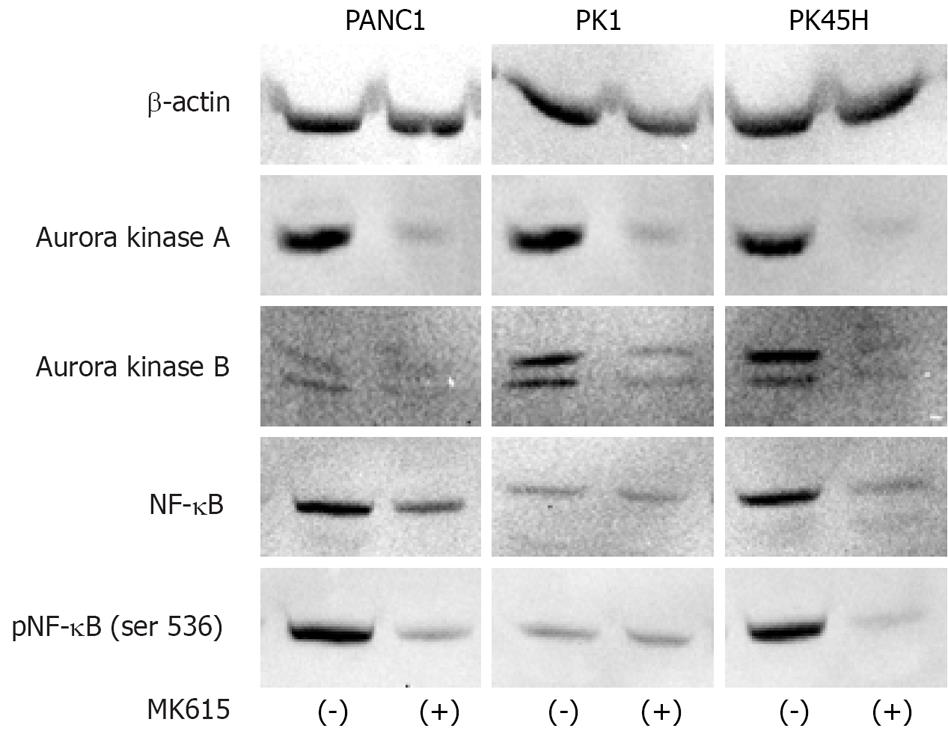
Figure 4 shows the protein expression of Aurora kinases. After 48 h of incubation with 300 &mgr;g/mL MK615, expressions of both Aurora A and B proteins were decreased in PANK1 and PK45H cells. Because endogenous Aurora A expression was low in PK1 cells, no significant decrease in its expression was observed. Consequently, MK615 decreased NF-κB expression in PANK1 and PK45H cells, but no evident decrease in NF-κB expression in PK1 cells was observed. The decrease in NF-κB expression was more evident with the use of anti-phosphorylated NF-κB antibody [pNF-κB (ser536)].
In addition, we analyzed the effect of MK615 on the cell cycle. MK615 increased the proportion of cells in G2-M phase (Table 1) from 7.8% to 13.0% in PANC1 cells, from 1.8% to 5.2% in PK1 cells, and from 22.9% to 29.3% in PK45H cells.
| MK615 | G0-G1 | S | G2-M | |
| PANC1 | (-) | 68.1 | 24.2 | 7.8 |
| (+) | 71.6 | 15.4 | 13.0 | |
| PK1 | (-) | 55.9 | 42.2 | 1.8 |
| (+) | 68.8 | 26.0 | 5.2 | |
| PK45H | (-) | 56.0 | 21.1 | 22.9 |
| (+) | 59.0 | 11.7 | 29.3 |
MK615 is isolated from Japanese apricot and contains many defined and undefined substances, including several triterpenoids[1], some of which exert an anti-neoplastic effect. In the present study, MK615 inhibited the proliferation of all three pancreatic cancer cell lines (Figure 1), and LDH assay showed that the anti-proliferative effect of MK615 on these cells was due not only to cell anti-proliferative effect, but also to direct cell killing effect (Figure 2).
In a previous study from our laboratory, MK615 was shown to be capable of inhibiting Aurora kinase A in human hepatocellular carcinoma[3]. This prompted us to investigate the effects of MK615 on Aurora B kinases. Aurora kinases are key mediators of cell division by controlling chromatid segregation[6–8]. There are three isoforms of Aurora kinases in mammals, Aurora A, Aurora B, and Aurora C. Structurally, all three share a similar domain organization, consisting of the N- and C-terminal domains, and a central region containing the catalytic domain, whose sequence is conserved among all three Aurora kinases[9]. Despite their sequence similarities, however, the functions and cellular localization of Aurora kinases differ. Aurora A is localized at the centrosome and forms the mitotic spindle apparatus that plays a crucial role in segregating chromosomes into daughter cells. Aurora B is localized in inner-centromeric chromatin during metaphase-anaphase transition, and relocalizes to microtubules in the spindle midzone during telophase, being required for accurate cytokinesis[1011]. Unlike Aurora A and Aurora B kinases, the function of Aurora C still remains unclear. Over-expression of Aurora A and Aurora B is frequently observed in various human cancers[12–15].
Our results demonstrated that MK615 inhibited the growth of, and showed cytotoxicity against, all three pancreatic cancer cell lines. The former effect was dose-dependent, while the latter was not. Although the mechanism responsible for the anti-neoplastic effect of MK615 has not been fully elucidated, the results of real-time PCR (Figure 3) and Western blotting (Figure 4) indicated that MK615 inhibited the expression of Aurora A and B kinases. There are several inhibitors of Aurora kinases, including the anti-Aurora B inhibitor, hesperadin[16], anti-Aurora A and B, ZM447439[17], and anti-Aurora A, B, and C, VX-680[18]. As shown in the present study, MK615 dually inhibited both Aurora A and B kinases. Inhibition of Aurora kinases mRNA expression varied in three pancreatic cancer cell lines. In PANC1 and PK45H cells, Aurora A and Aurora B mRNA expression was inhibited to the same degree, whereas Aurora B mRNA was inhibited more efficiently by MK615 than Aurora A mRNA in PK1 cells. Consequently, MK615 efficiently inhibited Aurora A and Aurora B in PANC1 and PK45H cells at the protein level (Figure 4), whereas such inhibition was not evident in PK1 cells. Because the protein expression of Aurora A in PK1 cells was weak, the deficient inhibition of Aurora kinases may be due to the low expression of endogenous Aurora A in these cells. Although MK615 suppresses unknown molecules located upstream from Aurora A and B kinases, administration of MK615 at least down-regulates the expression of Aurora A and B kinases in a dual manner.
NF-κB plays a central role in cell proliferation[19–21] and is consistently activated in some human cancers[22–24]. Aurora A binds the E2 ubiquitin ligase UBE2N, and UBE2N phosphorylates IκBα. Activated IκB translocates NF-κB from the cytoplasm to the nucleus, resulting in activation of the NF-κB complex[25]. MK615 inhibits the expression of Aurora A, resulting in inhibition of NF-κB activation[26–28].
A previous study from our laboratory showed that MK615 induced cell-cycle arrest at the end of G2 phase in hepatocellular carcinoma[3]. In the present study, MK615 treatment increased the cell population in G2-M phase in all three pancreatic cancer cell lines. MK615 inhibits Aurora A and Aurora B kinases and may induce cancer cell death at the G2-arrest check point[2930].
In conclusion, MK615 exerts an anti-neoplastic effect on human pancreatic cancer cells in vitro via dual inhibition of Aurora A and B kinases. Although further investigation is needed, MK615 seems to have the property anti-Aurora kinase compound.
MK615 is an extract from the Japanese apricot. Previous studies have revealed that MK615 has anti-neoplastic effects against human cancers. The mechanisms of anti-neoplastic effect of MK615 include apoptosis, autophagy, and suppression of Aurora A kinase. However, the entire mechanisms of the anti-neoplastic effects of MK615 have not been elucidated.
Pancreatic cancer is one of the leading causes of death worldwide, and the 5-year survival rate is 2%-20%. There has been a need for chemotherapeutic drugs that are less toxic and can inhibit or suppress pancreatic cancer, and MK615 is a promising candidate.
There are not many inhibitors of Aurora kinases. MK615 is a natural compound which strongly inhibits Aurora A and B kinases.
MK615 shows an anti-neoplastic effect on human pancreatic cancer cells in vitro via dual inhibition of Aurora A and B kinases. MK615 may prove to be a therapeutic approach in pancreatic cancer therapy.
MK615 is an extract from the Japanese apricot, Prunus mume Siebold et Zuccarini (ume in Japanese). Aurora kinases are key mediators of cell division by controlling chromatid segregation. There are three isoforms of Aurora kinases in mammals: Aurora A, Aurora B, and Aurora C.
This is an in vitro study of an anti-neoplastic drug MK615 made from Japanese apricot. Its mechanism of action was studied and revealed to have inhibitory effects on Aurora A and B kinases. This is a good basic research on anti-neoplastic agent MK615 for pancreatic carcinoma.
| 1. | Adachi M, Suzuki Y, Mizuta T, Osawa T, Adachi T, Osaka K, Suzuki K, Shiojima K, Arai Y, Masuda K. The Japanese apricot Prunus mume Sieb. et Zucc (ume) is a rich natural source of novel anti-cancer substance. Int J Food Prop. 2007;10:375-384. |
| 2. | Nakagawa A, Sawada T, Okada T, Ohsawa T, Adachi M, Kubota K. New antineoplastic agent, MK615, from UME (a Variety of) Japanese apricot inhibits growth of breast cancer cells in vitro. Breast J. 2007;13:44-49. |
| 3. | Okada T, Sawada T, Osawa T, Adachi M, Kubota K. A novel anti-cancer substance, MK615, from ume, a variety of Japanese apricot, inhibits growth of hepatocellular carcinoma cells by suppressing Aurora A kinase activity. Hepatogastroenterology. 2007;54:1770-1774. |
| 4. | Mori S, Sawada T, Okada T, Ohsawa T, Adachi M, Keiichi K. New anti-proliferative agent, MK615, from Japanese apricot “Prunus mume” induces striking autophagy in colon cancer cells in vitro. World J Gastroenterol. 2007;13:6512-6517. |
| 5. | Agnese V, Bazan V, Fiorentino FP, Fanale D, Badalamenti G, Colucci G, Adamo V, Santini D, Russo A. The role of Aurora-A inhibitors in cancer therapy. Ann Oncol. 2007;18 Suppl6:vi47-vi52. |
| 6. | Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1-10. |
| 7. | Naruganahalli KS, Lakshmanan M, Dastidar SG, Ray A. Therapeutic potential of Aurora kinase inhibitors in cancer. Curr Opin Investig Drugs. 7(12):1044-1051. |
| 8. | Brittle AL, Ohkura H. Centrosome maturation: Aurora lights the way to the poles. Curr Biol. 2005;15:R880-R882. |
| 9. | Andrews PD. Aurora kinases: shining lights on the therapeutic horizon? Oncogene. 2005;24:5005-5015. |
| 10. | Vader G, Medema RH, Lens SM. The chromosomal passenger complex: guiding Aurora-B through mitosis. J Cell Biol. 2006;173:833-837. |
| 11. | Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842-854. |
| 12. | Adams RR, Carmena M, Earnshaw WC. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 2001;11:49-54. |
| 13. | Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189-193. |
| 14. | Sakakura C, Hagiwara A, Yasuoka R, Fujita Y, Nakanishi M, Masuda K, Shimomura K, Nakamura Y, Inazawa J, Abe T. Tumour-amplified kinase BTAK is amplified and overexpressed in gastric cancers with possible involvement in aneuploid formation. Br J Cancer. 2001;84:824-831. |
| 15. | Gritsko TM, Coppola D, Paciga JE, Yang L, Sun M, Shelley SA, Fiorica JV, Nicosia SV, Cheng JQ. Activation and overexpression of centrosome kinase BTAK/Aurora-A in human ovarian cancer. Clin Cancer Res. 2003;9:1420-1426. |
| 16. | Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281-294. |
| 17. | Ditchfield C, Johnson VL, Tighe A, Ellston R, Haworth C, Johnson T, Mortlock A, Keen N, Taylor SS. Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores. J Cell Biol. 2003;161:267-280. |
| 18. | Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A. VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med. 2004;10:262-267. |
| 19. | Kaltschmidt B, Kaltschmidt C, Hehner SP, Droge W, Schmitz ML. Repression of NF-kappaB impairs HeLa cell proliferation by functional interference with cell cycle checkpoint regulators. Oncogene. 1999;18:3213-3225. |
| 20. | Vermeulen K, Berneman ZN, Van Bockstaele DR. Cell cycle and apoptosis. Cell Prolif. 2003;36:165-175. |
| 21. | Courtois G, Gilmore TD. Mutations in the NF-kappaB signaling pathway: implications for human disease. Oncogene. 2006;25:6831-6843. |
| 22. | Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938-6947. |
| 23. | Sovak MA, Bellas RE, Kim DW, Zanieski GJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952-2960. |
| 24. | Greten FR, Karin M. The IKK/NF-kappaB activation pathway-a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193-199. |
| 25. | Briassouli P, Chan F, Savage K, Reis-Filho JS, Linardopoulos S. Aurora-A regulation of nuclear factor-kappaB signaling by phosphorylation of IkappaBalpha. Cancer Res. 2007;67:1689-1695. |
| 26. | Folmer F, Blasius R, Morceau F, Tabudravu J, Dicato M, Jaspars M, Diederich M. Inhibition of TNFalpha-induced activation of nuclear factor kappaB by kava (Piper methysticum) derivatives. Biochem Pharmacol. 2006;71:1206-1218. |
| 27. | Prajapati S, Tu Z, Yamamoto Y, Gaynor RB. IKKalpha regulates the mitotic phase of the cell cycle by modulating Aurora A phosphorylation. Cell Cycle. 2006;5:2371-2380. |
| 28. | Sun C, Chan F, Briassouli P, Linardopoulos S. Aurora kinase inhibition downregulates NF-kappaB and sensitises tumour cells to chemotherapeutic agents. Biochem Biophys Res Commun. 2007;352:220-225. |
| 29. | Marumoto T, Hirota T, Morisaki T, Kunitoku N, Zhang D, Ichikawa Y, Sasayama T, Kuninaka S, Mimori T, Tamaki N. Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cells. 2002;7:1173-1182. |
| 30. | Vogel C, Hager C, Bastians H. Mechanisms of mitotic cell death induced by chemotherapy-mediated G2 checkpoint abrogation. Cancer Res. 2007;67:339-345. |