Published online Feb 28, 2008. doi: 10.3748/wjg.14.1263
Revised: December 18, 2007
Published online: February 28, 2008
AIM: To investigate the antiviral effect of beta-L-enantiomer of 2’,3’-didehydro-2’,3’-dideoxyadenosine (β-L-D4A) on 2.2.15 cells transfected with the hepatitis B virus (HBV) genome.
METHODS: Lamivudine (3TC) as a positive control. Then, HBV DNA in treated 2.2.15 cells and the Hepatitis B surface antigen (HBsAg) in the culture supernatants were detected to determine the inhibitory effect of β-L-D4A. At the same time, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was used to detect the survival ratio of 2.2.15 cells.
RESULTS: β-L-D4A has a dose-dependent inhibitory effect on HBV DNA replication; this effect was apparent when the concentration was above 1 mol/L. When β-L-D4A was at the highest concentration, 100 mol/L, the HBsAg inhibition ratio was above 50%. The Therapeutic index (TI) of β-L-D4A was above 2.1.
CONCLUSION: β-L-D4A has a dose-dependent inhibitory effect on the replication of HBV DNA and the secretion of HBsAg at low toxicity.
- Citation: Gao LL, Wang XY, Lin JS, Zhang YH, Li Y. Efficacies of β-L-D4A against hepatitis B virus in 2.2.15 cells. World J Gastroenterol 2008; 14(8): 1263-1267
- URL: https://www.wjgnet.com/1007-9327/full/v14/i8/1263.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1263
Hepatitis B virus (HBV) is a partially double-stranded DNA virus, which is able to induce acute or chronic infection in humans[1]. Chronic HBV infection can last a lifetime, causing persistent hepatitis that frequently progresses to more-severe liver diseases[2], including cirrhosis and hepatocellular carcinoma[3–5]. Chronically infected patients also serve as sources of HBV transmission.
Traditionally approved therapies against HBV include treatment with alpha interferon and lamivudine (3TC). However, the efficacy of alpha interferon is limited by undesirable side effects, low sustained response rate and high cost[67]. While lamivudine (3TC) is well-tolerated and less costly, resistance emerges in approximately 20% of patients receiving monotherapy per year[8].
Adefovir-dipivoxil prodrug (ADV) and entecavir (ETV) are recently approved anti-HBV therapeutics. While these two agents produce multilog suppression of serum HBV DNA, they cause only modest rates of HBV surface antigen seroconversion and, thus, require long-term administration to control disease in most patients[9]. ADV retains clinical efficacy against lamivudine-resistant mutants[10], but selects the mutations rtN236T and rtA181V[11]. In addition, treatment with ADV is reported to be associated with dose-limiting nephrotoxicity[12]. Entecavir shows reduced susceptibility to lamivudine-resistant mutants in vitro and clinically, and an accelerated development of entecavir resistance mutations (rtI169T, rtM250V, rtS202I and rtT184S/G) in lamivudine-resistant patients[1213]. Therefore, more effective and safer therapies are needed for the treatment of chronic HBV infections.
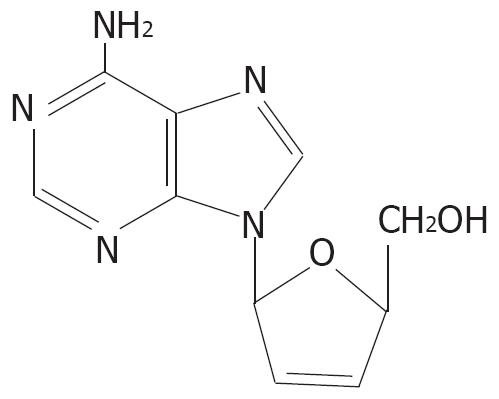
β-L-D4A is an L-enantiomer of the natural nucleoside deoxyadenosine. The structure of β-L-D4A is shown in Figure 1. In this report, we detected the efficacy of β-L-D4A against HBV replication in vitro.
2.2.15 cells and 3TC were kindly provided by Professor Yung-Chi Cheng (Yale University) and plasmid P3.6 was offered by Professor Wang Yuan (Shanghai Institute of Biochemistry, Chinese Academy of Sciences). β-L-D4A was synthesized by the Department of Pharmacology of Wuhan University. G418 and MTT were purchased from Sigma (USA) and DMEM was from Gibco (USA). Fetal bovine serum was purchased from Hyclone (USA), random primers were purchased from Takara (Japan) and DIG-labeled dUTP was purchased from Roche (Germany). An EIA Kit for the detection of HBsAg was purchased from Kehua (Shanghai). HBV DNA fluorescent quantified PCR (FQ-PCR) Kit was purchased from Biotronics (USA).
Cell culture: 2.2.15 cells were maintained in DMEM (supplemented with 10% fetal bovine serum, 200 g/mL G418, 2 mmol/L glutamine, 50 U of penicillin per milliliter and 50 &mgr;g of streptomycin per milliliter) at 37°C and 5% CO2. The medium was changed every three days.
Intracellular HBV DNA analysis: 2.2.15 cells were digested by parenzyme, seeded at a density of 1 × 105 cells per well on 24-well plates and maintained in 1.5 mL DMEM at 37°C and 5% CO2 for 48 h. Then, 2.2.15 cells were treated with freshly prepared medium containing β-L-D4A or 3TC. The following concentrations were used: 0.0001, 0.001, 0.01, 0.1, 1, 10, 100 mol/L. Negative control cells were treated with DMEM only. Media were changed every three days; on day 8, both the supernatants and cells were collected and stored at -20°C.
Then, the cells were lysed at room temperature with 1 mL of lysis buffer [50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 5 mmol/L MgCl2, 0.2% Nonidet P-40]. Removal of cellular debris was accomplished by a 5-min microcentrifuge spin at 16 000 r/min. Lysates were then incubated with 20% PEG 8000 and 1 mol/L NaCl at room temperature for 2 h, followed by a 1-min microcentrifuge spin at 16 000 r/min. The resulting pellets containing HBV core particles were resuspended in solution added with proteinase K [10 mmol/L Tris (pH 7.6), 10 mmol/L EDTA, 0.25% SDS, 1 mg/mL proteinase K] at 56°C for 2 h, followed by extraction with phenol-chloroform and ethanol precipitation. Nucleic acids were dissolved in ddH2O, electrophoresed through 1% agarose, transferred to a positively charged nylon membrane and hybridized to a probe, which was prepared from a full-length HBV DNA genome template excised from plasmid P3.6 and DIG-labeled using a random primer. Then, the membrane was incubated with anti-DIG and developed with NBP/ BCIP.
The concentration of extracted HBV DNA was also determined by real-time fluorescent quantitative Polymerase Chain Reaction(FQ-PCR). The inhibition ratio was calculated according to the following formula: inhibition ratio = (HBV DNA concentration of negative control - HBV DNA concentration of experimental group)/HBV DNA concentration of negative control × 100%. Then, the 50% inhibition concentration (IC50) was calculated according to the Reed-Muench method[14].
Detection of HBsAg: 2.2.15 cells were treated with antiviral compounds and lysed as described above. The collected supernatants were detected using an EIA Kit for the detection of HBsAg according to the manufacturer’s instructions, and A450 was observed. Inhibition ratio to HBsAg (%) = (A450 of negative control -A450 of experimental group) / (A450 of negative control -A450 of blank control) × 100%.
Analysis of cellular toxicity: MTT assays were used to detect the survival rates of 2.2.15 cells[15]. Briefly, 2.2.15 cells were seeded at a density of 1 × 105 cells per well on 24-well plates and treated with media containing β-L-D4A or 3TC of different concentrations. The following concentrations were used: 1000, 500, 250, 125, 62.5 and 31.25 mol/L. Three days later, the supernatants were discarded and 2 mL of MTT (1 mg/mL) was added per well. After incubation at 37°C and 5% CO2 for 4 h, the supernatants were discarded and 2 mL of acidified isopropanol (isopropanol:HCl = 500:1.68) was added per well. The survival ratio of 2.2.15 cells (%) = (A570 of experimental group/A570 of negative control) ×100%. Then, the 50% toxicity dose (TD50) was calculated according to the Reed-Muench method.
Evaluation of treatment: Therapeutic index (TI) = TD50/IC50. If the TI is above 2.1, the compound is high in efficacy and low in cellular toxicity. If TI is between 2 and 1, the compound is both considerably effective and toxic. If TI is below 1, the compound is low in efficacy, but high in cellular toxicity.
All statistics were analyzed using SPSS10.0, and differences were considered significant when the P value was < 0.05.
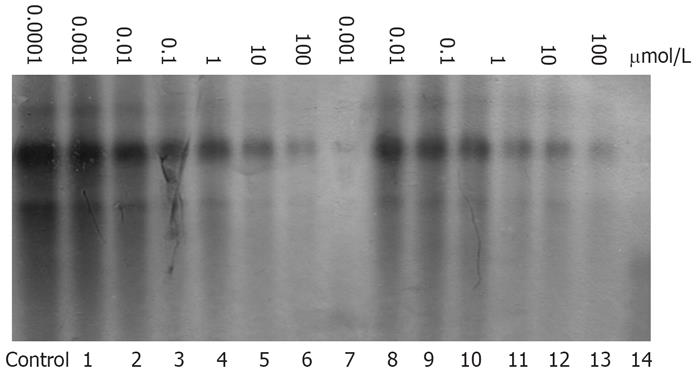
To investigate the potential of β-L-D4A to inhibit HBV viral replication, HBV DNA was isolated from treated 2.2.15 cells and detected by Southern hybridization. Figure 2 shows that the production of HBV DNA lessened following treatment of cells with β-L-D4A; this effect became more notable as the dose of β-L-D4A increased. A similar phenomenon was observed in cells treated with 3TC.
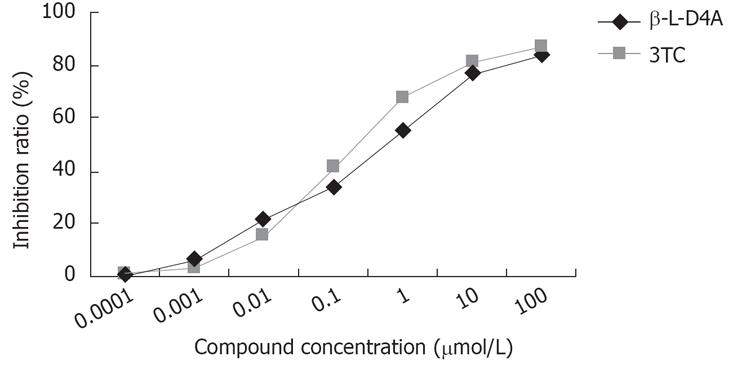
The concentration of HBV DNA in 2.2.15 cells was determined by real-time fluorescent quantitative PCR. As shown in Figure 3, β-L-D4A had an apparent inhibitory effect on the concentration of HBV DNA (P > 0.05 when compared to that of 3TC). Calculated according to the Reed-Muench method, the IC50 values of β-L-D4A and 3TC were 0.61 mol/L and 0.30 mol/L, respectively.
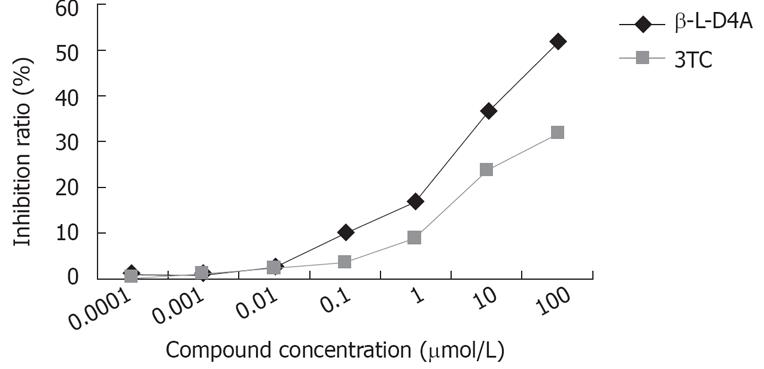
Following treatment of 2.2.15 cells with antiviral compounds for eight days, HBsAg was detected in the medium. As shown in Figure 4, when the concentration of β-L-D4A was 100 mol/L, the HBsAg inhibition ratio was above 50%. However, the HBsAg inhibition ratio with 3TC was less than 40% even at the highest concentration.
The cellular toxicity of both compounds was not evident at drug concentrations below 125 mol/L. The survival ratios were less than 50% when the drug concentrations were as high as 1000 mol/L, with both compounds. Calculated according to the Reed-Muench method, the TD50 values of β-L-D4A and 3TC were 417 mol/L and 243 mol/L, respectively.
HBV infection is one of the most prevalent viral diseases in the world and more than 350 million people are chronically infected[1617], with about 1 million deaths annually[18]. In order to screen anti-HBV compounds, researchers often purify HBV DNA polymerase. However, the purification procedure is very complicated and the quantity of polymerase is not satisfying[1920]. 2.2.15 cells can stably replicate HBV DNA and express HBsAg; thus, they can be used as an ideal cell model to screen the in vitro antiviral efficacy of medicines[2122].
In this study, we found that β-L-D4A has a dose-dependent inhibitory effect on HBV DNA replication that is apparent when the concentration is above 1 mol/L. However, the inhibition of the secretion of HBsAg was not as pronounced as that of HBV DNA replication. When β-L-D4A was at the highest concentration, 100 mol/L, the HBsAg inhibition ratio was slightly above 50%. The TI of β-L-D4A shows that it is high in efficacy and low in toxicity, which indicates that β-L-D4A is a promising anti-HBV compound.
β-L-D4A is a new type of L-nucleoside for which the natural counterpart is deoxyadenosine. Although there have been reports about L-nucleosides since 1960s, it was only after about 30 years that the merits of L-nucleoside became widely recognized[2324]. Previous studies revealed the bioactivities and securities of L-nucleoside analogues were superior to their counterpart enantiomers[25–27]. β-L 3/(2R, 5S)-1,3-oxathiolane-cytidine (lamivudine, 3TC), a typical L-nucleoside, was reported to be about 50 times more effective than its D-enantiomer[28–30].
The mechanism of action of β-L-D4A is speculated to be inhibition of HBV DNA polymerase and termination of DNA replication. In order to confirm this speculation, further study is necessary. It also remains to be seen whether β-L-D4A is effective against lamivudine-resistant HBV mutants and whether it will lead to HBV mutants resistant to β-L-D4A. Besides, much work remains to be done to gain a better understanding of the metabolism, toxicology and pharmacokinetic parameters of this compound. Our studies to date suggest β-L-D4A deserves further development as a potential treatment for chronic HBV infection.
Hepatitis B virus (HBV) is one of the causative agents of acute or chronic hepatitis in humans, and there are an estimated 350 million persistently HBV-infected carriers worldwide. Such prevalence of HBV demands the discovery of new effective therapeutics for the treatment of acute and chronic infections.
There have been articles studying the antiviral effects of Entecavir and Adefovir, which are analogs of deoxyguanosine and deoxyadenosine, respectively. While these two agents produce pronounced suppression of serum HBV DNA, they cause only modest rates of HBV surface antigen seroconversion and even induce resistant mutants. Thus, there is an urgent need to develop a new potent anti-HBV drug.
In this study, we find that β-L-D4A has a dose-dependent inhibitory effect on the replication of HBV DNA and the secretion of HBsAg at low toxicity.
The results of this study confirm the in vitro antiviral effect of β-L-D4A, which indicates it is a promising new drug against HBV infection.
β-L-D4A is an L-enantiomer of its natural nucleoside-deoxyadenosine.
This study aimed to investigate the antiviral effect of β-L-D4A on 2.2.15 cell line by comparing with 3TC. They found β-L-D4A has concentration-relative inhibitory effect to HBV DNA replication and the therapeutic index of β-L-D4A was above 2.1. It’s an interesting subject.
| 1. | Park SG, Jung G. Human hepatitis B virus polymerase interacts with the molecular chaperonin Hsp60. J Virol. 2001;75:6962-6968. |
| 2. | Delaney WE 4th, Yang H, Miller MD, Gibbs CS, Xiong S. Combinations of adefovir with nucleoside analogs produce additive antiviral effects against hepatitis B virus in vitro. Antimicrob Agents Chemother. 2004;48:3702-3710. |
| 3. | Jansen RW, Johnson LC, Averett DR. High-capacity in vitro assessment of anti-hepatitis B virus compound selectivity by a virion-specific polymerase chain reaction assay. Antimicrob Agents Chemother. 1993;37:441-447. |
| 4. | Delmas J, Schorr O, Jamard C, Gibbs C, Trepo C, Hantz O, Zoulim F. Inhibitory effect of adefovir on viral DNA synthesis and covalently closed circular DNA formation in duck hepatitis B virus-infected hepatocytes in vivo and in vitro. Antimicrob Agents Chemother. 2002;46:425-433. |
| 5. | Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733-1745. |
| 6. | Levine S, Hernandez D, Yamanaka G, Zhang S, Rose R, Weinheimer S, Colonno RJ. Efficacies of entecavir against lamivudine-resistant hepatitis B virus replication and recombinant polymerases in vitro. Antimicrob Agents Chemother. 2002;46:2525-2532. |
| 7. | Hoofnagle JH, di Bisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347-356. |
| 8. | Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, Brown N, Woessner M, Boehme R, Condreay L. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36:687-696. |
| 9. | Delaney WE 4th, Ray AS, Yang H, Qi X, Xiong S, Zhu Y, Miller MD. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006;50:2471-2477. |
| 10. | Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81-90. |
| 11. | Qi X, Xiong S, Yang H, Miller M, Delaney WE 4th. In vitro susceptibility of adefovir-associated hepatitis B virus polymerase mutations to other antiviral agents. Antivir Ther. 2007;12:355-362. |
| 12. | Tenney DJ, Levine SM, Rose RE, Walsh AW, Weinheimer SP, Discotto L, Plym M, Pokornowski K, Yu CF, Angus P. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to Lamivudine. Antimicrob Agents Chemother. 2004;48:3498-3507. |
| 13. | Yang H, Qi X, Sabogal A, Miller M, Xiong S, Delaney WE 4th. Cross-resistance testing of next-generation nucleoside and nucleotide analogues against lamivudine-resistant HBV. Antivir Ther. 2005;10:625-633. |
| 14. | Yang J, Yu CB, Xi HL, Xv XY. Effects of GL combined with Ara2AMP on HBsAg expression and survival rate of HepG2.2.15 cell lines. Guizhou Yiyao. 2006;30:483-485. |
| 15. | Situ ZQ, Wu JZ. Cell culture. Beijing: World Books Press 2001; 18. |
| 17. | Langley DR, Walsh AW, Baldick CJ, Eggers BJ, Rose RE, Levine SM, Kapur AJ, Colonno RJ, Tenney DJ. Inhibition of hepatitis B virus polymerase by entecavir. J Virol. 2007;81:3992-4001. |
| 18. | Qadri I, Siddiqui A. Expression of hepatitis B virus polymerase in Ty1-his3AI retroelement of Saccharomyces cerevisiae. J Biol Chem. 1999;274:31359-31365. |
| 19. | Lanford RE, Notvall L, Beames B. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J Virol. 1995;69:4431-4439. |
| 20. | Seifer M, Hamatake R, Bifano M, Standring DN. Generation of replication-competent hepatitis B virus nucleocapsids in insect cells. J Virol. 1998;72:2765-2776. |
| 21. | Deng JG, Zheng ZW, Wang Q, Yang K, Qin HZ, Li XJ, Wang CL. Effects of Eight Different Compound Chinese Medical Prescriptions on HBsAg and HBeAg Excreted by 2215 Cell. Guangxi Zhongyiyao. 2004;27:42-47. |
| 22. | Huang ZM, Yang XB, Cao WB, Chen HY, Zhang JZ, Teng L, Chen HS, CaiGM , LiuDP . Inhibition of Qinling Chongji on HBsAg and HBeAg secretion in cultured cell line 2215. Shijie Huaren Xiaohua Zazhi. 2000;8:184-186. |
| 23. | Cui L, Schinazi RF, Gosselin G, Imbach JL, Chu CK, Rando RF, Revankar GR, Sommadossi JP. Effect of beta-enantiomeric and racemic nucleoside analogues on mitochondrial functions in HepG2 cells. Implications for predicting drug hepatotoxicity. Biochem Pharmacol. 1996;52:1577-1584. |
| 24. | Zhu Y, Yamamoto T, Cullen J, Saputelli J, Aldrich CE, Miller DS, Litwin S, Furman PA, Jilbert AR, Mason WS. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001;75:311-322. |
| 25. | Jeong LS, Schinazi RF, Beach JW, Kim HO, Nampalli S, Shanmuganathan K, Alves AJ, McMillan A, Chu CK, Mathis R. Asymmetric synthesis and biological evaluation of beta-L-(2R,5S)- and alpha-L-(2R,5R)-1,3-oxathiolane-pyrimidine and -purine nucleosides as potential anti-HIV agents. J Med Chem. 1993;36:181-195. |
| 26. | Lin TS, Luo MZ, Liu MC, Zhu YL, Gullen E, Dutschman GE, Cheng YC. Design and synthesis of 2’,3’-dideoxy-2’,3’-didehydro-beta-L-cytidine (beta-L-d4C) and 2’,3’-dideoxy 2’,3’-didehydro-beta-L-5-fluorocytidine (beta-L-Fd4C), two exceptionally potent inhibitors of human hepatitis B virus (HBV) and potent inhibitors of human immunodeficiency virus (HIV) in vitro. J Med Chem. 1996;39:1757-1759. |
| 27. | Gumina G, Song GY, Chu CK. L-Nucleosides as chemotherapeutic agents. FEMS Microbiol Lett. 2001;202:9-15. |
| 28. | Schinazi RF, Chu CK, Peck A, McMillan A, Mathis R, Cannon D, Jeong LS, Beach JW, Choi WB, Yeola S. Activities of the four optical isomers of 2’,3’-dideoxy-3’-thiacytidine (BCH-189) against human immunodeficiency virus type 1 in human lymphocytes. Antimicrob Agents Chemother. 1992;36:672-676. |
| 29. | Chang CN, Skalski V, Zhou JH, Cheng YC. Biochemical pharmacology of (+)- and (-)-2',3'-dideoxy-3'-thiacytidine as anti-hepatitis B virus agents. J Biol Chem. 1992;267:22414-22420. |
| 30. | Schinazi RF, McMillan A, Cannon D, Mathis R, Lloyd RM, Peck A, Sommadossi JP, St Clair M, Wilson J, Furman PA. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2423-2431. |