Published online Feb 28, 2008. doi: 10.3748/wjg.14.1167
Revised: December 31, 2007
Published online: February 28, 2008
AIM: To develop a fusion vaccine of esophageal carcinoma cells and dendritic cells (DC) and observe its protective and therapeutic effect against esophageal carcinoma cell line 109 (EC109).
METHODS: The fusion vaccine was produced by fusing traditional polyethyleneglycol (PEG), inducing cytokine, sorting CD34+ magnetic microbead marker and magnetic cell system (MACS). The liver, spleen and lung were pathologically tested after injection of the fusion vaccine. To study the therapeutic and protective effect of the fusion vaccine against tumor EC109, mice were divided immune group and therapeutic group. The immune group was divided into P, E, D and ED subgroups, immunized by phosphate buffered solution (PBS), inactivated EC109, DC and the fusion vaccine respectively, and attacked by EC109 cells. The tumor size, weight, latent period and mouse survival period were recorded and statistically analyzed. The therapeutic group was divided into four subgroups: P, inactivated EC109, D and ED subgroups, which were attacked by EC109 and then treated with PBS, inactivated EC109, DC, and EC109-DC respectively. Pathology and flow cytometry were also used to study the therapeutic effect of the fusion vaccine against EC109 cells.
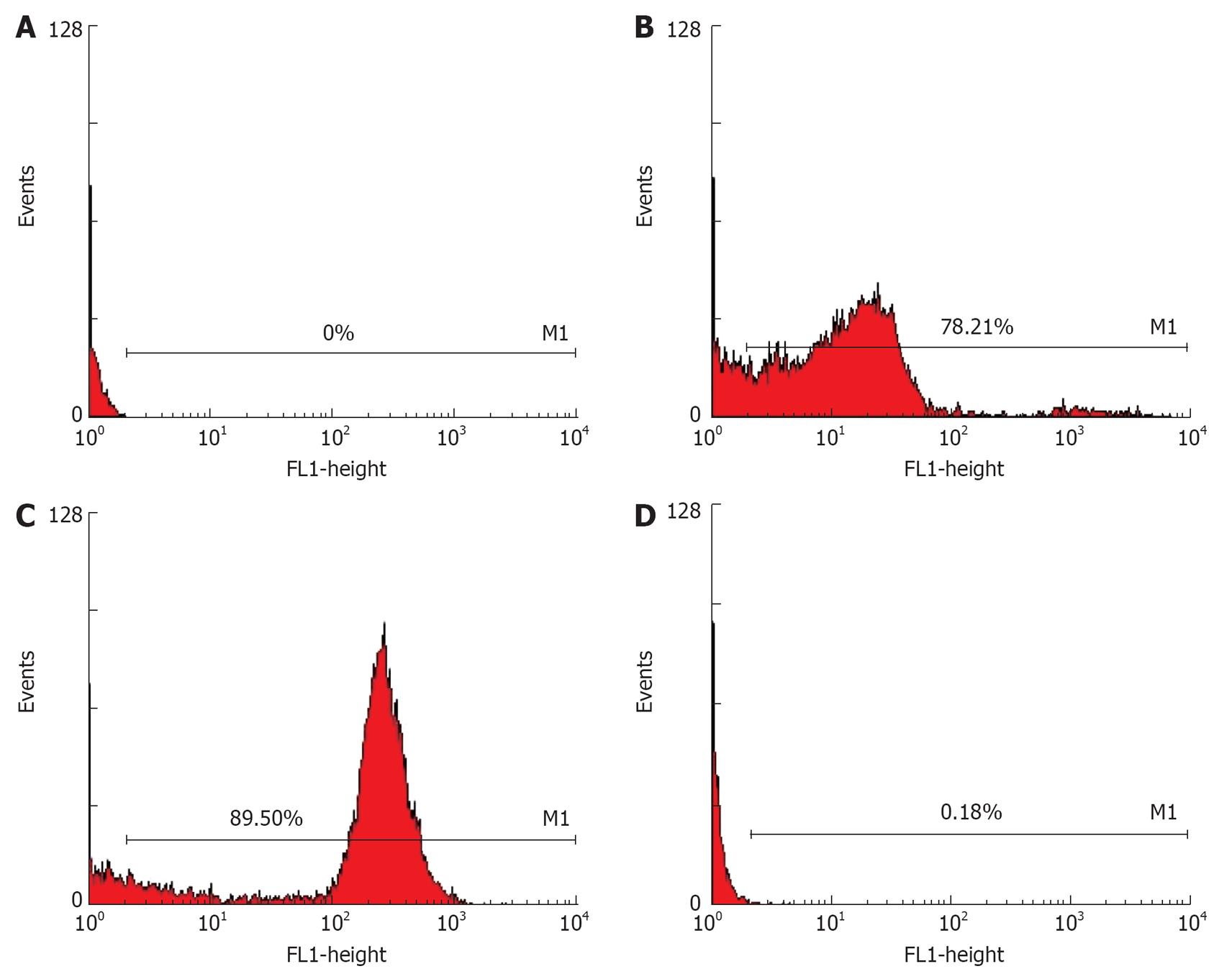
RESULTS: Flow cytometry showed that the expression of folate receptor (FR), EC109 (C), DCs (D) in human nasopharyngeal carcinoma cell line (HNE1) (B) was 78.21%, 89.50%, and 0.18%, respectively. The fusion cells (C) were highly expressed. No tumor was found in the spleen, lung and liver after injection of the fusion vaccine. Human IgG was tested in peripheral blood lymphocytes (PBL). In the immune group, the latent period was longer in EC109-DC subgroup than in other subgroups, while the tumor size and weight were also smaller than those in ED subgroup. In the therapeutic group, the tumor size and weight were smaller in ED subgroup than in P, inactivated EC109 and DC subgroups.
CONCLUSION: Fusion cells are highly expressed not only in FR but also in CD80. The fusion vaccine has a distinctive protective effect against tumor EC109 and can inhibit the growth of tumor in mice, and its immune protection against tumor attack is more significant.
-
Citation: Guo GH, Chen SZ, Yu J, Zhang J, Luo LL, Xie LH, Su ZJ, Dong HM, Xu H, Wu LB.
In vivo anti-tumor effect of hybrid vaccine of dendritic cells and esophageal carcinoma cells on esophageal carcinoma cell line 109 in mice with severe combined immune deficiency. World J Gastroenterol 2008; 14(8): 1167-1174 - URL: https://www.wjgnet.com/1007-9327/full/v14/i8/1167.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1167
Dendritic cells (DCs) are the most potential and professional antigen presenting cells, which stimulate the native T cells and play an important role in the T lymphocyte-mediated immunoresponse[1]. It was reported that DCs activated by antigen display a good anti-tumor effect both in vitro and in vivo[2–4]. We have studied the biological characteristics and induced antitumor immunity of the cancer vaccine prepared by fusing esophageal carcinoma cell line with DCs derived from the cord blood in a previous study[5]. This study was to develop a fusion vaccine and observe its anti-oesophagus cancer effects on EC109 cells in mice with severe combined immune deficiency (SCID). It is expected that this paper may provide some worthy data for the prevention and treatment of oesophageal carcinoma.
EC109 cells were obtained from the Pathological Institute of Shantou University Medical College. Human nasopharyngeal carcinoma cell line (HNE1) was obtained from the Tumor Research Laboratory of Tumor Hospital of Shantou University Medical College.
CD34+ hematopoietic stem cells were isolated from the cord blood by Ficoll-Hypaque density-gradient centrifugation using the CD34+ progenitor cell isolation kit and the magnetic cell sorting system (MACS). The CD34+ cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum containing 300 ng/mL human granulocyte-macrophage colony-stimulating factor (rhGM-CSF), 150 &mgr;g/mL human tumor neucrosis factor α (rhTNF-α) and 200 ng/mL human stem cell factor (rhSCF) at 37°C, in an atmosphere containing 5% CO2 for two weeks and then a large number of DCs were harvested.
DCs were mixed with EC109 at a 5:1 ratio and incubated in serum free RPMI1640 containing 50% polyethylene glycol and the fusion cells were labeled with HLA-DR MicroBeads for 35 min at 6°C-12°C. The cells were centrifuged at 1000 r/min for 10 min. By using a MiniMACS separation column to enrich the magnetically positive cells, the enriched HLA-DR+ cells were washed, seeded in 12-well plates and cultured overnight. The adherent cells were regarded as fused cells EC109-DC.
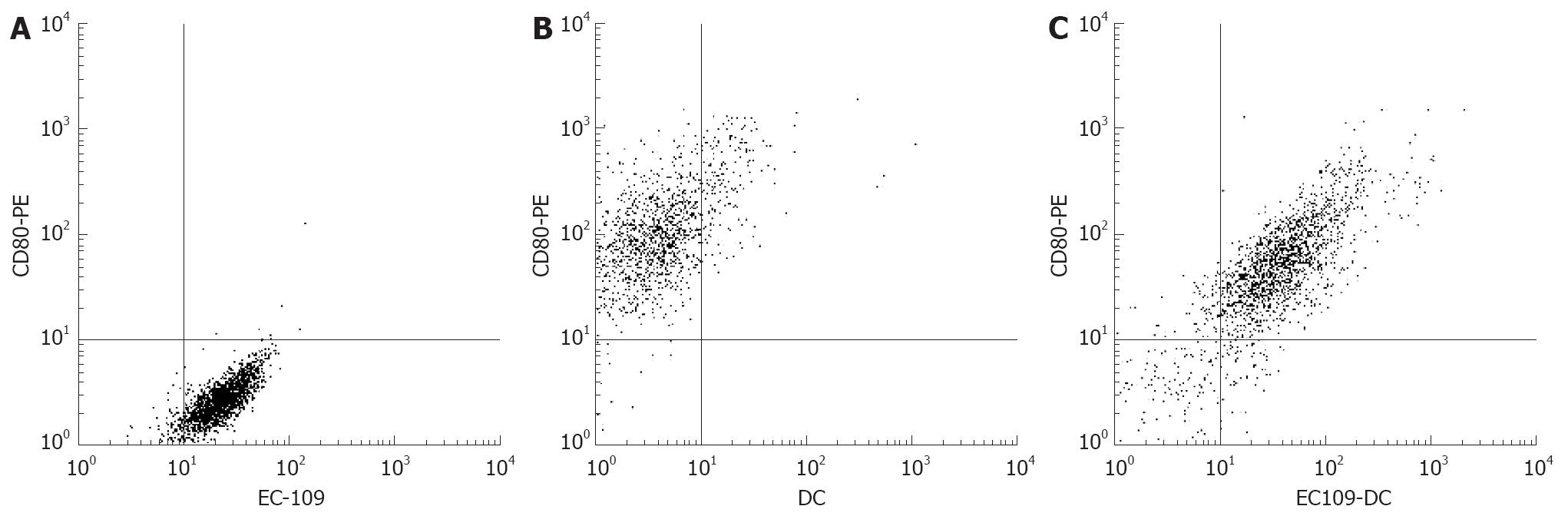
Peripheral blood lymphocytes (PBL) were isolated from the peripheral blood of normal donors by Ficoll-Hypaque density-gradient centrifugation. PBL, DC, EC109 and HNE1 were planted in RPMI 1640 medium supplemented with 10% fetal calf serum, washed with PBS and incubated with FA-FITC (which was obtained from Peixuan G, Purdue University, USA) for one hour. PBL were set up for negative cells and HNE1 for masculine cells as previously described[6]. EC109, DCs and EC109-DC were washed with PBS and incubated with FA-FITC and CD80-PE. Fluorescence intensity and positive cell percentage were analyzed by fluorescence-activated cell sorter analysis using FACScan (Beckman Coulter).
Preparation of SCID mice: SCID mice, 6-8 wk old, were purchased from the Animal Research Center of Zhongshan University. The animals were kept in micro-isolator cages in a separate barrier facility under a well-controlled pathogen-free environment with ambient temperature monitored and cycles of light and darkness regulated. The mice were fed with laboratory chow and water at the Tumor Research Laboratory of Tumor Hospital of Shantou University Medical College. The guidelines for animal care and use were approved by the experiment animal monitor of Guangdong Province.
Oncogenicity of fusion vaccine: Experiments were divided into setsIand II. There were 5 mice in setI. Live fusion vaccine (0.2 mL) was then injected into vena caudalis of the 5 mice. Twenty-five mice in set II were divided into 5 groups (A-E). Group A was injected intraperitoneally with 1 × 106 cells each of EC109-DC. The other 4 groups were injected with the same quantity of cells of EC109 + DC, EC109, DC and PBS. Whether tumor was induced in the mice of setIwas checked. The development time and size of the tumor, as well as the survival time of the mice were observed every day for 60 d. Organs, such as the spleen, liver and kidney were removed and fixed in neutral formalin, embedded in paraffin and stained with hematoxylin and eosin (HE) for routine light microscopic study. The mice in set II were killed on d 28 after inoculation. The removed tumors were weighed and fixed in neutral formalin, embedded in paraffin and stained with HE for routine light microscopic study.
Reconstitution of SCID mice: Human lymphocytes to be transferred in SCID mice were isolated from the whole blood obtained from healthy adult volunteers as previously described[7–11]. Human peripheral blood lymphocytes (PBL) were isolated by Ficoll-Paque gradient centrifugation under sterile conditions. PBL were planted in RPMI 1640 medium supplemented with 10% fetal calf serum. The culture flasks were incubated in a humidified atmosphere containing 5% CO2 at 37°C. Thirty SCID mice were divided into two groups. Twenty-five mice in PBL group were injected intraperitoneally with 5 × 107 PBL. The other 5 mice in PBS group were injected with the same volume of PBS as a control group. Blood was obtained from the vena caudalis of mice on d 0, 7, 14, 21 and 28, and then centrifuged. The sera were collected for detecting the level of human IgG by avidin-biotin (ABG) ELISA. The successful marking of reconstitution is that the human lymphocytes or human IgG could be found in the peripheral blood of SCID mice.
Induction of anti-tumor immunoprotective effect: Twenty SCID mice with successful immune system reconstruction were divided into P, E, D and ED subgroups. PBS (0.2 mL) was injected into the vena caudalis of mice. EC109, DC and the fusion vaccine were inactivated with 1 × 106 cells each time, twice a day for 3 d. Five days later, all the mice were injected into the right armpit with 5 × 106 of live EC109. The tumor size, weight, latent period and survival period of mice were recorded at the same time point every day for 50 d. Two mice in each group were sacrificed 28 d after tumor attack. Tissues of the spleen, liver and kidney, were fixed in neutral formalin, embedded in paraffin and stained with HE for routine light microscopic study.
Anti-tumor therapeutic effect: The EC109 cells at the logarithmic growth phase were collected and washed with PBS. The cell density was adjusted to 5 × 106/mL. Twenty SCID mice with successful immune system reconstruction were injected into the right armpit with 1 × 106 cells of live EC109. The model was successful when the tumor grew as big as a grain. The 20 mice in the model were divided into P, E, D and ED subgroups. PBS (0.2 mL) was injected into the vena caudalis. EC109, DC and the fusion vaccine were inactivated with 1 × 106 cells each time, twice a day for 3 d. Tumor size, weight, latent period and survival period of mice were recorded at the same time point every day. One mouse in each group was sacrificed 28 d after immunity acquirement. Others were observed for an additional 50 d. Organs, such as the spleen, liver and kidney, were fixed in neutral formalin, embedded in paraffin and stained with HE for routine light microscopic study.
SPSS10.0 was used for data variation analysis. P < 0.05 was considered statistically significant.
After fusion of EC109 and DCs, the resulting heterokaryons showed adherent growth and irregular shape. Flow cytometry displayed that the expression of FR, EC109 and DCs was 78.21%, 89.50% and 0.18%, respectively in HNE1. The fusion cells were highly expressed not only in FR but also in CD80 (Figures 1 and 2).
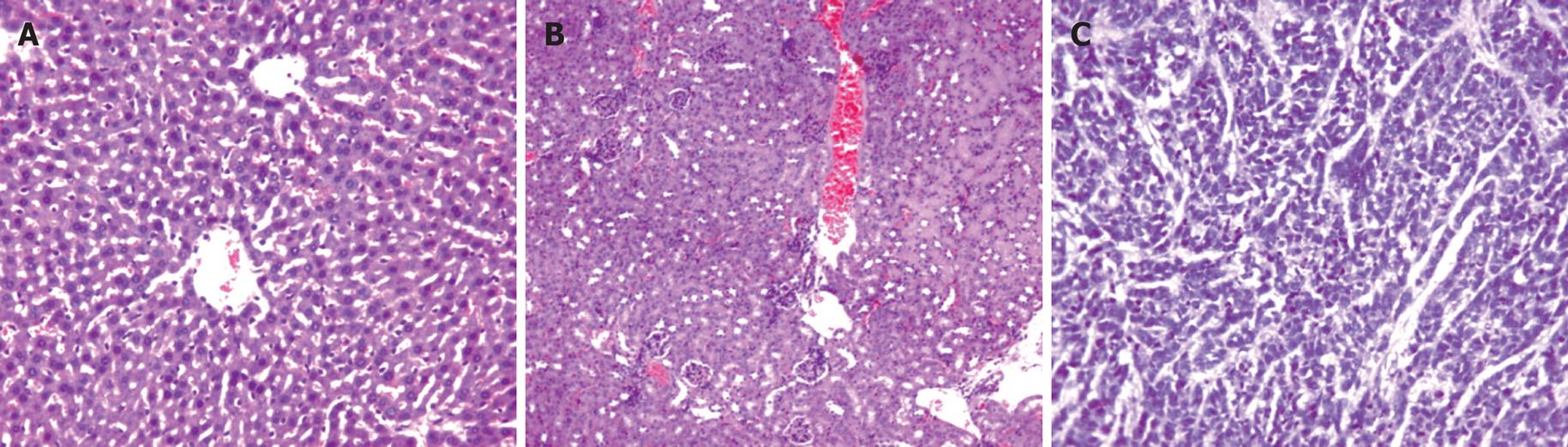
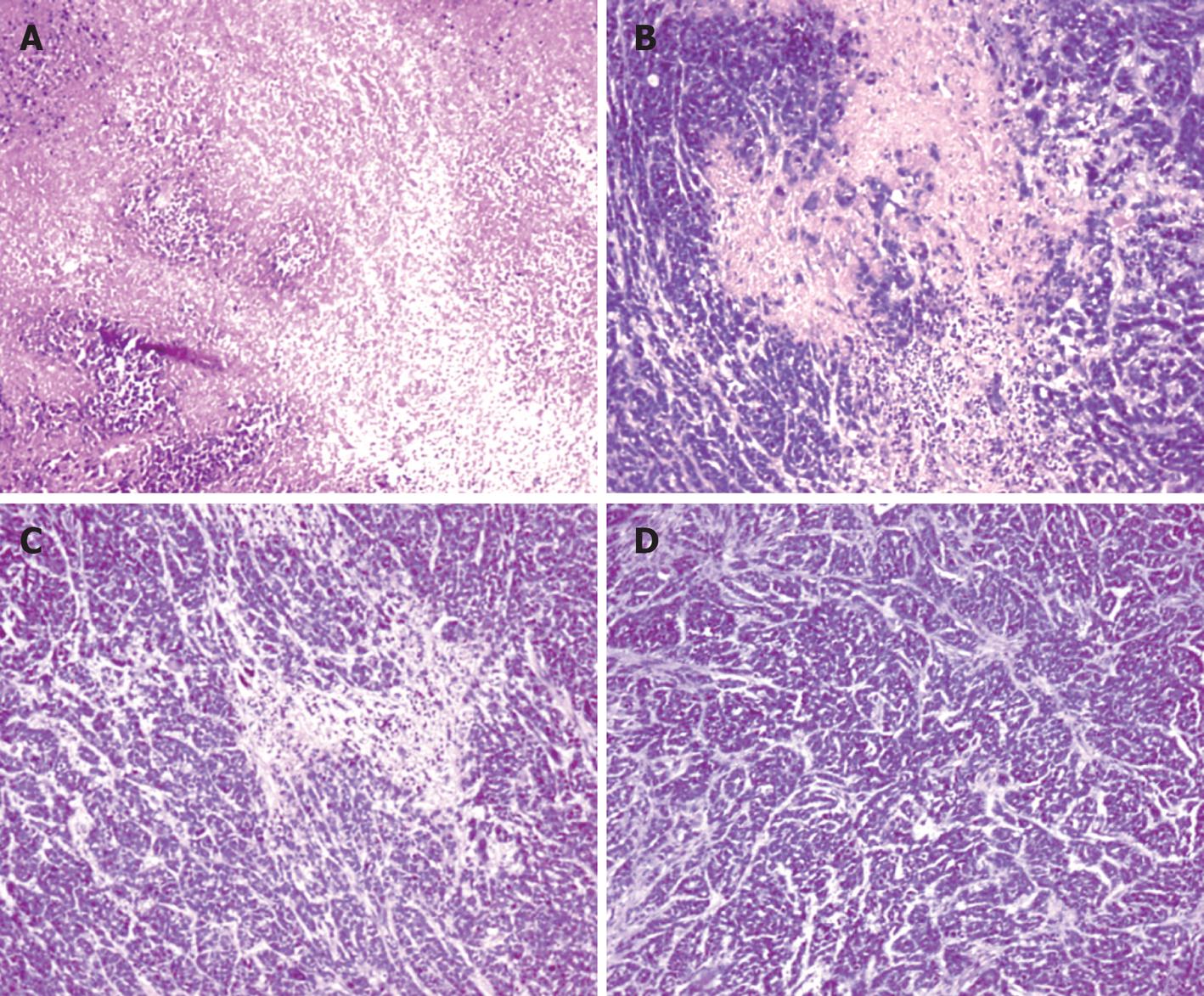
There was no formation of tumor 60 d after injection of EC109-DC in group 1. No formation of tumor tubercles was discovered in the heart, liver, lung, kidney and spleen (Figure 3).
There were no ascites and lump organization 28 d after injection of EC109-DC in group 2. However, hemorrhagic ascites was discovered, grey tumor tissues were generated generally and widely adhered to the ambient organs in groups of EC109 + DC and EC109. The tumor tissues were mostly distributed on the abdominal wall, diaphragmatic muscle, liver and pelvic cavity with a diameter of 1-20 mm. Under light microscope, the size and shape of cancer cells were not extremely consistent, but polygonal and karyolobism lost the characteristics of epithelial cells in normal esophagus (Figure 3).
During the experiment, human IgG was tested in all the PBL groups and its highest level was 2580 &mgr;g/mL, compared with the PBS group (P < 0.05, Table 1).
| Class | n | D0 | D7 | D14 | D21 | D28 |
| PBS | 5 | 0.12 ± 0.03 | 0.05 ± 0.03 | 0.06 ± 0.01 | 0.09 ± 0.04 | 0.16 ± 0.12 |
| PBL | 25 | 0.21 ± 0.10 | 59.28 ± 12.61 | 260.82 ± 36.16 | 353.40 ± 69.23 | 482.7 ± 185.15 |
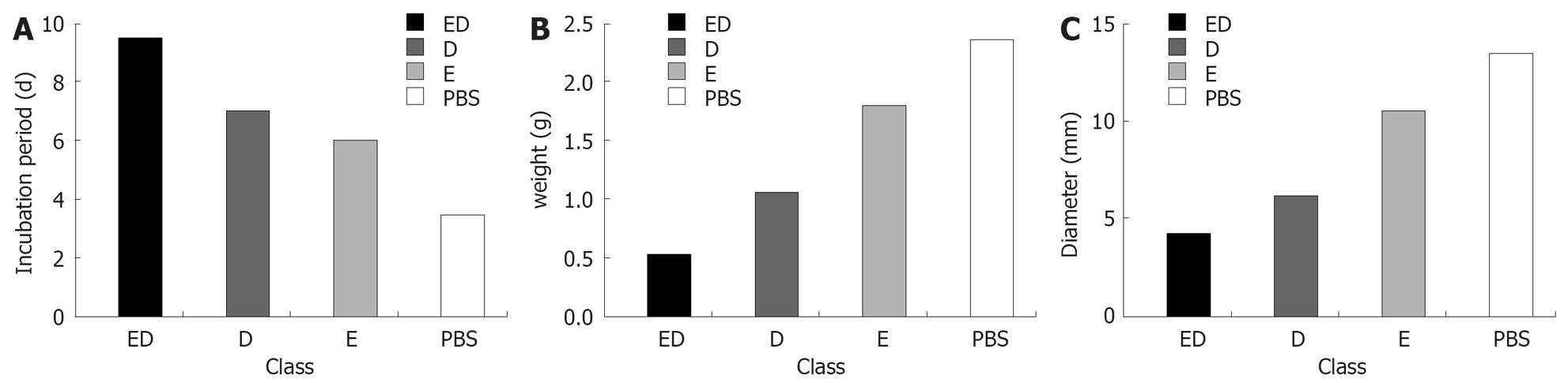
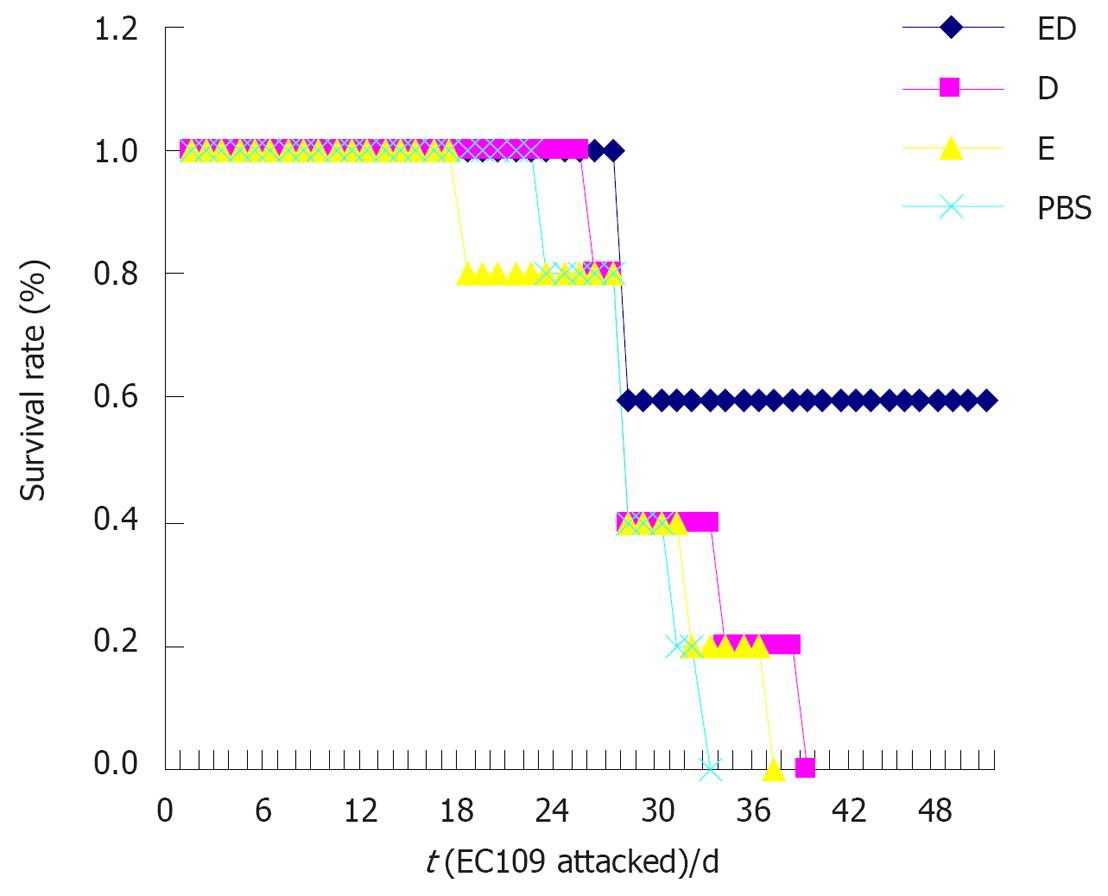
The incubation period of tumor cells after attacked by EC109 was P < E < D < ED (Figure 4A). The tumor weight and size were ED < D < E < P (Figure 4B and C). Except for 2 mice which were killed on d 28, the other mice in the ED group survived and their life span was obviously longer (P < 0.05). Compared with the PBS group, death occurred in the treatment group and the difference in the life span between the two groups was not significant (P > 0.05, Figure 5).
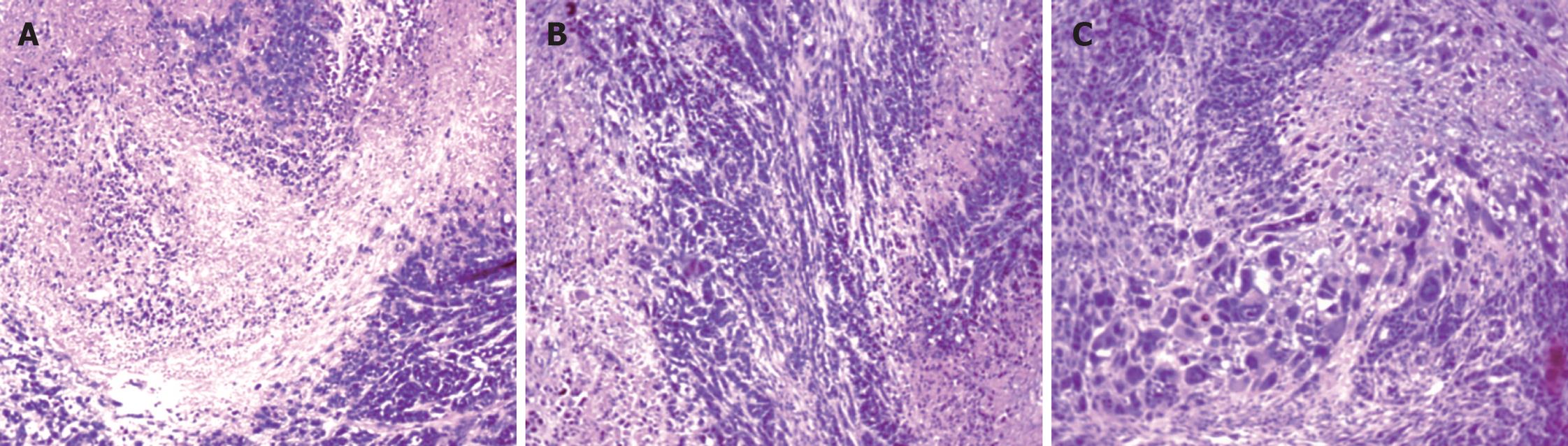
Pathology: Circlar, slippery and pellicular tumors in ED group were distended and movable with neither wide conglutination nor metastasis. Grey and hard tumors with an uneven surface were found in other groups. Some tumors were adhered to the ambient structure, even with distant metastasis. Tumor tissue sections were grey in color with diffuse punctiform necrosis. Under light microscope, tumor tissues from the ED, D and E groups attacked by EC109 for 28 d, except for P group, had different necrosis changes (Figure 6).
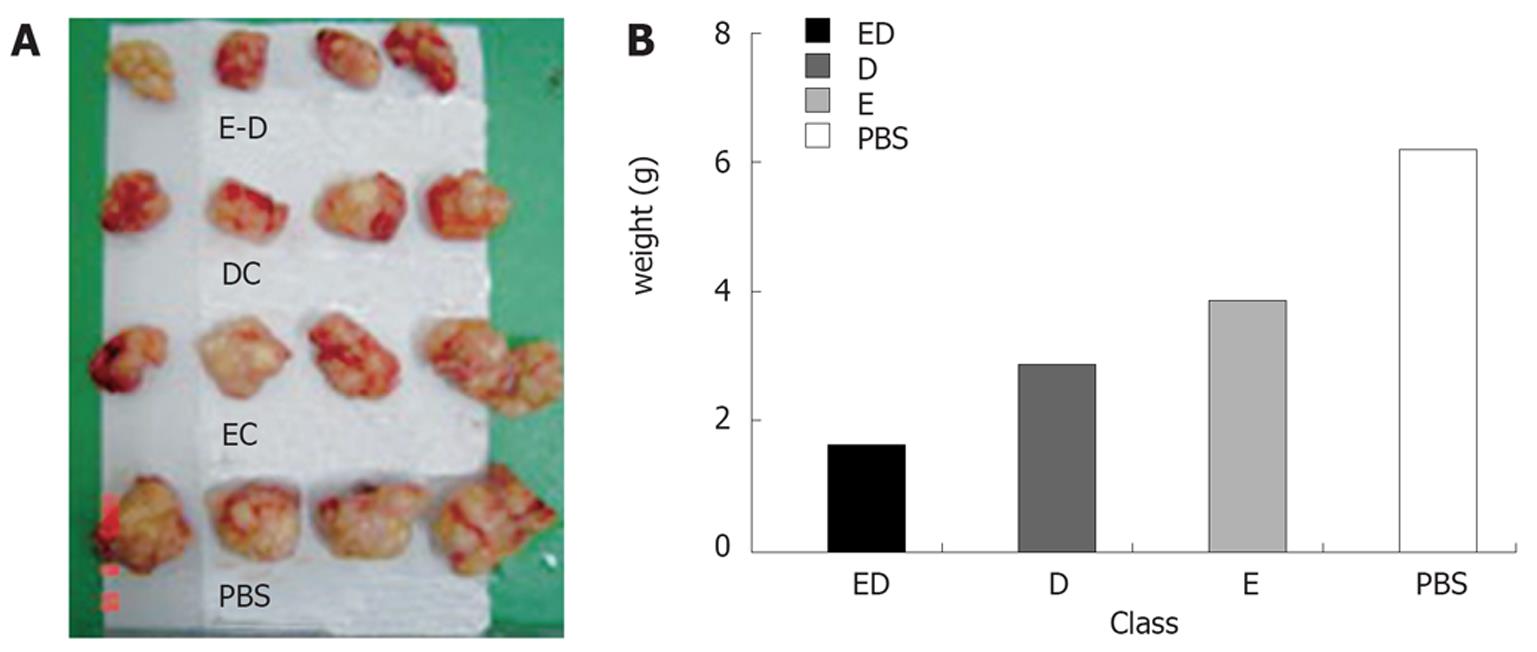
Immunotherapy: After treatment of EC109-DC, DC, inactivated EC109 and PBS, the tumor size was DE < D < E < P. On d 50, the growth time of tumors had no remarkable difference but the tumor weight did (P < 0.05, Figure 7).
The texture of tumors with a smooth surface was harder, partially adhered to the chest wall and the tumor tissue section was grey in color with diffuse punctiform necrosis. Under light microscope, the tumor tissue had different necrosis changes (Figure 8).
In this experiment, the fusion vaccine EC109-DC, was made from amalgamation of DC, which was induced by combining cytokine from CD34+ hematopoietic stem cells isolated from the cord blood, and the EC109 by traditional PEG fusing. The fusion vaccine, EC109-DC, integrated the protein, polypeptide and nucleic acid of EC109 and even the whole live cells with the antigen presenting DC, thus it can give immunoreaction to polyvalency tumor antigen, showing all possible antigens to EC109. With no specific antigen to the esophageal carcinoma cell line, it can effectively induce anti-tumor immune response. Consequently, it is more effective and practical to fuse vaccine with DC than a single antigen in theory[121314]. Some curative effects of fusion vaccine with DC have been seen in clinic experiments[15–25]. However, it cannot be brought into full use due to its lower fusion efficiency, shortage of effective methods of separation and purification as well as the difficulty in obtaining an enough-quantity and high-quality cancer vaccine by fusing cancer cell line with DC.
In this study, we developed the tumor fusion vaccine with HLA-DR mark and Mini MACS sorting method. The tumor fusion vaccine, EC109-DC, was double marked with FA-FITC and CD80-PE. The vaccine’s phenotype was accredited by flow cytometry amb-masc mark. After detection, the vaccine highly expressed FR and CD80 simultaneously. The principle of HLA-DR+ cells separated by Mini MACS separation unit is to abrupt the HLA-DR+ cells and the HLA-DR- cells which mark the magnetic by coupling the antibody to the cells, and HLA-DR monoclonal antibody to the HLA-DR+ cell membrane’s SA idio-discrimination. The results of our study have not only proved the successful fusion of DC with EC109, but also the separation effect of MACS. This may provide an effective way for the effective purification and separation of the vaccine, as well as for gaining an enough quantity and high-quality of hybrid DC.
Although the fusion vaccine was inoculated in the right abdominal cavity of SCID mice, we could not see hemorrhagic ascites and tumor tubercules in the abdominal cavity. There were hemorrhagic ascites and adhesive tumor organism in the control group. The fusion vaccine was injected into the SCID mice through vena caudalis, no tumor nodus was found in the liver, kidney and spleen of mice, indicating that the fusion vaccine has lost the oncogenicity of parent tumor cells, and is safe and reliable for its application in vivo.
In anti-tumor protective immunity in vivo experiment, tumor formulation was delayed in the ED and D groups compared with the immunoprotection groups. The time of tumor formulation in P group was the shortest. The tumor was the smallest and lightest in ED group, and the biggest and heaviest in D group, indicating that the fusion vaccine has the best immunity protective function. Our study used the cell-fusion technique to increase the number of cells fused with DC, which highly express MHC, co-stimulate molecules and adhesion molecules and also produce IL-12 and tumor cells which are short of information molecules, form the EC109-DC which change the bionomics of tumor cells. The fusion vaccine was re-infused in vivo in which DC carry all esophagus cancer cell antigen information. The fusion cells retain the MHC and co-stimulatory molecules in DC. The EC109-DC activate the CD4+ T and CD8+ T cells as MHC-Iand MHC-II, secrete homologous cytokines and activate the anti-tumor immune response, thus inducing special anti-tumor immunity reaction more effectively.
Sixty days after treatment of tumor-bearing mice with the fusion vaccine, EC109, the size of tumor in ED group became obviously smaller than that in the control group. The tumor surface in ED group was smooth and divided by circum-tissues and had no conglutination. However, the tumor surface in control group was coarse and adhered to the chest wall and muscle tissues had no clear demarcation. Macro-lamellar necrosis was observed in ED group under light microscope, while punctiform necrosis was found in PBS group, indicating that the fusion vaccine has certain inhibitory effect on the tumor growth, which is in agreement with previous reports[2627]. At the same time, active immunotherapy for the tumor-bearing mice reduced the growth of the lump body, and the sphacelus became obvious in tumor in vivo, demonstrating that such immunity treatment has its certainly value and even cures the tumor-bearing mice if it is used in combination with operation or chemotherapy.
Tumors can escape the host immunity surveillance because tumor cells cannot express the information molecule required by the host immunity system. It was reported that induction of CD8+, CD4+ and B cells is also one of the most effective method to prevent and control chronic infections, diseases, tumors[28]. The immunoreactions in the host are to capture its antigen by APC. After processing, the antigen information is present in T and B lymphocytes, stimulating a series of specific cellular immunologic response and humoral immunoresponse. It is, therefore, directly related with the induction of immunological activation or immunological tolerance, and the key to the process is APC. DCs are a kind of the strongest APCs in which antigen processes and functions[2930], and can stimulate the native T cells. DCs are in a unique state of immune response.
This experiment adopted the technology of cell fusion to make DCs, which highly express the major histocompability complex (MHC), co-stimulate adhesion molecule, and produce IL-12, identify cells fused with tumor cells which are short of the information molecule. All these not only change the biological characteristics of tumor cells, but can effectively process presentation antigen and activate the anti-tumor immune response, and the host obtains the immunity protection strength by the resistance to parent tumor attack. During the procedure, it directly fuses the esophageal carcinoma cells (EC109) and DCs, passes the link of DCs insufficient to recognize EC109 which are low in immune function, causes the direct processing and presentation of tumor antigen in DCs.
At the same time, the fusion vaccine excretes corresponding cell factors, possibly combines the cell molecule with the specific acceptor on DCs surface, and increases the stimulation to DC, activates the low function of DCs, and also its function on antigen presentation. On the other hand, the conjugated tumor antigen is stably expressed on the cell surface, which also may be used as the extraneous antigen to the major histocompatibility complex (MHC-II), then activates the CD4+ Th, and induces the production of different antibodies. According to the difference in its secretion of Th1 and Th2 cell factors, the activated CD4+ Th can adjust the balance of immunity, enhance the intensity of cellular immunologic response and (or) humoral immunoresponse response. The tumor antigen to EC109 itself just lies in the DCs, which can be effectively captured by DCs, then presented to MHC-I, strengthens the reaction of CD8+ CTL, CD4+ Th cells, and the intensity of the cellular immunologic response and (or) humoral immunoresponse response. The fusion vaccine not only remarkably stimulates initial (naive) multiplication of T cells, but also confers upon the activated T cells for certain tumor memory, which may massively activate and expand under the situation of more contacting antigens[3132].
In addition, the fusion vaccine also participates in the nonspecific immunity. DCs which fuse tumor cells excrete cytokines, such as IL-1, IL-6, IL-8, IL-12, TNF-α, IFN-α and GM-CSF. Among them, IL-6 dissolves tumor cells by activating the complement mannose-binding lectin(MBL) pathway. The other cytokines such as IFN-α, can directly activate NK cells. After activation under the effect of chemotactic factors, the NK cells migrate to the tumor focus and directly kill and wound the responsive tumor cells, with no need to stimulate antigen and other factors. Inactivated macrophages have no lethal effect on tumor cells and can be activated by the stimulus of inflammation or other factors. LK secretes T cells and IFN. The activated macrophages as effector cells have no specificity to kill and inhibit tumors, but may have effect factors killing target cells, including hyperoxide, nitrogen monoxide, TNF and LYS, etc. Through the ADCC way, macrophages can also kill and wound target cells. In this experiment, the anti-tumor function of the fusion vaccine is due to the co-effect between specific immunity and non-specific immunity.
In conclusion, the fusion vaccine simultaneously expresses EC109 and DC specific antigen. Meanwhile, the fusion vaccine of DCs and EC109 has no in vivo oncogenicity, and has lost its parent characteristics of EC109 cells with malignant growth, becoming safe and reliable. Furthermore, the fusion vaccine has resistant functions against the attack of EC109 cells. Moreover, the fusion vaccine can restrain tumor growth in mice. The fusion vaccine may become a useful tool in preventing malignant tumors.
The present study demonstrated that immunization strategies for cancer vaccines based on mature dendritic cells (DCs) could present tumor peptide and induce specific anti-cancer immune response. No report is available on the fusion vaccine for human esophageal carcinoma cells fused with DCs derived from the cord blood.
We have studied the biological characteristics of cancer vaccine and induced its anti-tumor immunity.
The fusion vaccine of esophageal carcinoma cells and DCs was developed. Its protective and therapeutic effect on EC109 cells was studied. The EC109 highly expressed FR and DCs hardly express the FR.
To summarize the actual application values, the tumor vaccine could become a useful tool for preventing malignant tumors.
This manuscript is an original research article on the protective effect of the fusion vaccine. The findings are of great interest and the data are convincing.
| 1. | Trombetta ES, Ebersold M, Garrett W, Pypaert M, Mellman I. Activation of lysosomal function during dendritic cell maturation. Science. 2003;299:1400-1403. |
| 2. | Nestle FO, Banchereau J, Hart D. Dendritic cells: On the move from bench to bedside. Nat Med. 2001;7:761-765. |
| 3. | Kawada M, Ikeda H, Takahashi T, Ishizu A, Ishikura H, Katoh H, Yoshiki T. Vaccination of fusion cells of rat dendritic and carcinoma cells prevents tumor growth in vivo. Int J Cancer. 2003;105:520-526. |
| 4. | Gong J, Avigan D, Chen D, Wu Z, Koido S, Kashiwaba M, Kufe D. Activation of antitumor cytotoxic T lymphocytes by fusions of human dendritic cells and breast carcinoma cells. Proc Natl Acad Sci USA. 2000;97:2715-2778. |
| 5. | Guo G, Chen S, Zhang J, Luo L, Yu J, Dong H, Xu H, Su Z, Wu L. Antitumor activity of a fusion of esophageal carcinoma cells with dendritic cells derived from cord blood. Vaccine. 2005;23:5225-5230. |
| 6. | Wu M, Gunning W, Ratnam M. Expression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol Biomarkers Prev. 1999;8:775-782. |
| 7. | Walker W, Gallagher G. The development of a novel immunotherapy model of human ovarian cancer in human PBL-severe combined immunodeficient (SCID) mice. Clin Exp Immunol. 1995;101:494-501. |
| 8. | Schumacher U, Adam E, Horny HP, Dietl J. Transplantation of a human ovarian cystadenocarcinoma into severe combined immunodeficient (SCID) mice--formation of metastases without significant alteration of the tumour cell phenotype. Int J Exp Pathol. 1996;77:219-227. |
| 9. | Cui H, Li Y, Tong CR, Cheng YX, Feng J, Liu B, Ge H, Qian HN. The study of reconstitution of human ovarian carinoma severe combined immunodeficiency mice model. Beijing Yike Daxue Xuebao. 2000;32:488-491. |
| 10. | Zhu H, Ye DF, Chen HZ, Lv WG, Xie X. Development of intraperitoneally transplantated human ovarian carcinoma model with immune reconstruction in severe combined immunodeficient mice. Zhonghua Yixue Zazhi. 2002;82:630-633. |
| 11. | Li Y, Cui H, Ye X, Yao Y. The comparison of three kinds of enzymelinked immunosorbent assay on screening the “Leaky” phenotype SCID mice. Jiepou Xuebao. 2002;33:111-112. |
| 12. | Tary-Lehmann M, Saxon A, Lehmann PV. The human immune system in hu-PBL-SCID mice. Immunol Today. 1995;16:529-533. |
| 13. | Goddard RV, Prentice AG, Copplestone JA, Kaminski ER. In vitro dendritic cell-induced T cell responses to B cell chronic lymphocytic leukaemia enhanced by IL-15 and dendritic cell-B-CLL electrofusion hybrids. Clin Exp Immunol. 2003;131:82-89. |
| 14. | Jenne L, Schuler G, Steinkasserer A. Viral vectors for dendritic cell-based immunotherapy. Trends Immunol. 2001;22:102-107. |
| 15. | Gong J, Nikrui N, Chen D, Koido S, Wu Z, Tanaka Y, Cannistra S, Avigan D, Kufe D. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. J Immunol. 2000;165:1705-1711. |
| 16. | Parkhurst MR, DePan C, Riley JP, Rosenberg SA, Shu S. Hybrids of dendritic cells and tumor cells generated by electrofusion simultaneously present immunodominant epitopes from multiple human tumor-associated antigens in the context of MHC class I and class II molecules. J Immunol. 2003;170:5317-5325. |
| 17. | Chen D, Xia J, Tanaka Y, Chen H, Koido S, Wernet O, Mukherjee P, Gendler SJ, Kufe D, Gong J. Immunotherapy of spontaneous mammary carcinoma with fusions of dendritic cells and mucin 1-positive carcinoma cells. Immunology. 2003;109:300-307. |
| 18. | Soruri A, Fayyazi A, Neumann C, Schlott T, Jung T, Matthes C, Zwirner J, Riggert J, Peters JH. Ex vivo generation of human anti-melanoma autologous cytolytic T cells by dentritic cell/melanoma cell hybridomas. Cancer Immunol Immunother. 2001;50:307-314. |
| 19. | Suzuki T, Fukuhara T, Tanaka M, Nakamura A, Akiyama K, Sakakibara T, Koinuma D, Kikuchi T, Tazawa R, Maemondo M. Vaccination of dendritic cells loaded with interleukin-12-secreting cancer cells augments in vivo antitumor immunity: characteristics of syngeneic and allogeneic antigen-presenting cell cancer hybrid cells. Clin Cancer Res. 2005;11:58-66. |
| 20. | Krause SW, Neumann C, Soruri A, Mayer S, Peters JH, Andreesen R. The treatment of patients with disseminated malignant melanoma by vaccination with autologous cell hybrids of tumor cells and dendritic cells. J Immunother. 25(5):421-428. |
| 21. | Homma S, Matai K, Irie M, Ohno T, Kufe D, Toda G. Immunotherapy using fusions of autologous dendritic cells and tumor cells showed effective clinical response in a patient with advanced gastric carcinoma. J Gastroenterol. 2003;38:989-994. |
| 22. | Avigan D. Fusions of breast cancer and dendritic cells as a novel cancer vaccine. Clin Breast Cancer. 2003;3 Suppl 4:S158-S163. |
| 23. | Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337-344. |
| 24. | Koido S, Ohana M, Liu C, Nikrui N, Durfee J, Lerner A, Gong J. Dendritic cells fused with human cancer cells: morphology, antigen expression, and T cell stimulation. Clin Immunol. 2004;113:261-269. |
| 25. | Cerundolo V, Hermans IF, Salio M. Dendritic cells: a journey from laboratory to clinic. Nat Immunol. 2004;5:7-10. |
| 26. | Scanlan MJ, Jager D. Challenges to the development of antigen-specific breast cancer vaccines. Breast Cancer Res. 2001;3:95-98. |
| 27. | Miller G, Lahrs S, Shah AB, DeMatteo RP. Optimization of dendritic cell maturation and gene transfer by recombinant adenovirus. Cancer Immunol Immunother. 2003;52:347-358. |
| 28. | Zoller M. Immunotherapy of cancer for the elderly patient: does allogeneic bone marrow transplantation after nonmyeloablative conditioning provide a new option? Cancer Immunol Immunother. 2004;53:659-676. |
| 29. | Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245-3287. |
| 30. | Inaba K. Dendritic cells as antigen-presenting cells in vivo. Immnol Cell Biol. 1997;75:206-208. |
| 31. | Nouri-Shirazi M, Banchereau J, Fay J, Palucka K. Dendritic cell based tumor vaccines. Immunol Lett. 2000;74:5-10. |
| 32. | Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271-296. |