Published online Dec 7, 2008. doi: 10.3748/wjg.14.6981
Revised: July 27, 2008
Accepted: August 3, 2008
Published online: December 7, 2008
AIM: To investigate the impact of mucin production on prognosis in colorectal cancer, in terms of overall survival (OS) and time to disease progression (TTP) in patients with mucinous compared to those with non-mucinous colorectal cancer (NMCRC), matched for age, gender, and tumor stage.
METHODS: Thirty five patients with mucinous colorectal cancer (MCRC) were matched for age, gender, and tumor stage with 35 controls having NMCRC. OS and TTP were compared among 4 groups divided according to mucin content: group A (50%-75% mucin), group B (75%-100% mucin), group C or controls (< 50% mucin). Group D consisted of all patients with tumors having < 75% mucin (controls and groups A together).
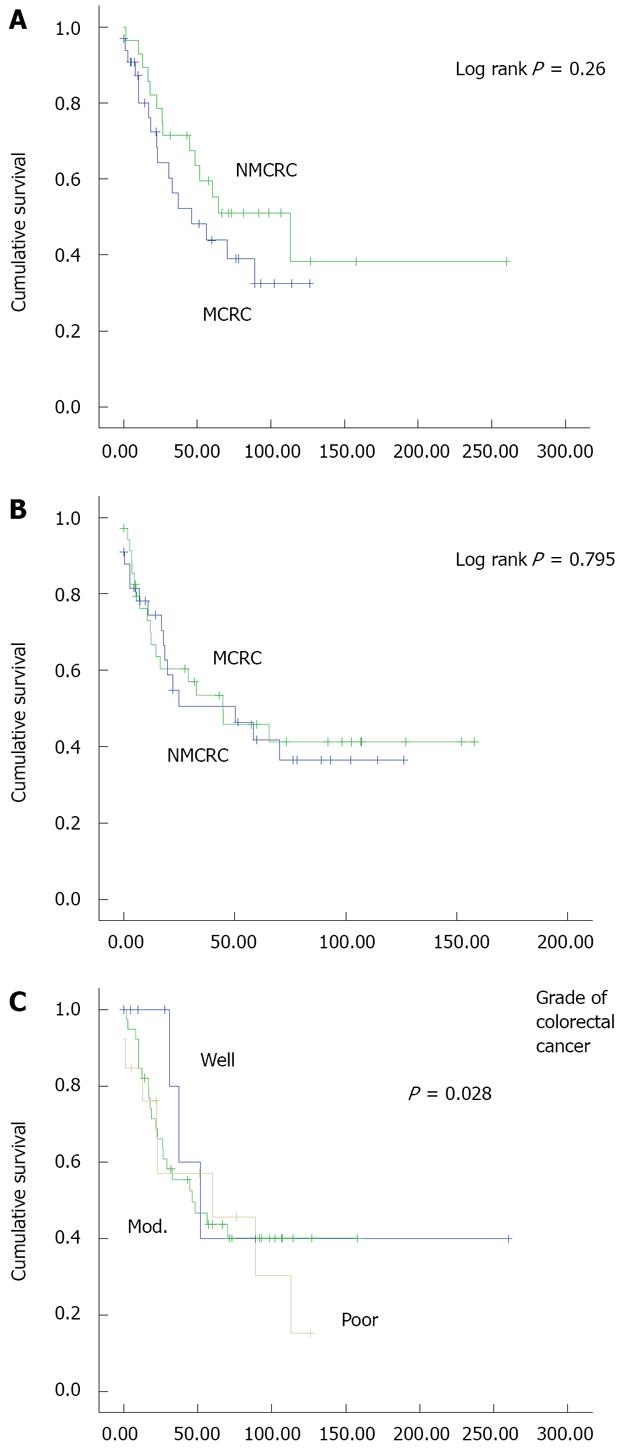
RESULTS: Median survival in MCRC and NMCRC groups was 46.2 and 112.9 mo, respectively (P = 0.26). OS in groups A and B was 70.1 and 32.8 mo (P = 0.46), and in groups B and D was 32.8 and 70.1 mo, respectively (P = 0.143). TTP in MCRC and NMCRC was 50.17 and 44.77 mo, respectively (P = 0.795). TTP in groups A, B, and D was 70.1, 24.8, and 65.5 mo, respectively. Twenty-eight percent of patients with MCRC had poorly differentiated adenocarcinoma versus 8.6% in NMCRC patients (P = 0.028).
CONCLUSION: MCRC is associated with a non-significant decrease in median survival and TTP, particularly when mucin content is > 75% of tumor volume. However, it tends to be more poorly differentiated. A larger study matching for stage and grade is needed.
- Citation: Farhat MH, Barada KA, Tawil AN, Itani DM, Hatoum HA, Shamseddine AI. Effect of mucin production on survival in colorectal cancer: A case-control study. World J Gastroenterol 2008; 14(45): 6981-6985
- URL: https://www.wjgnet.com/1007-9327/full/v14/i45/6981.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6981
| MCRC (%), n = 35 | NMCRC (%), n = 35 | P | |
| Mean age | 56.8 (22-83) | 56.7 (28-81) | |
| Gender (M:F) | 20:15 | 20:15 | |
| Comorbid conditions | |||
| Heart disease | 4 (11.4) | 2 (5.7) | 0.673 |
| Diabetes mellitus | 2 (5.7) | 2 (5.7) | 1.000 |
| Hypertension | 3 (8.6) | 9 (25.7) | 0.110 |
| Other cancer | 2 (5.7) | 1 (2.9 ) | |
| Family history | |||
| Colon cancer | 4 (11.4) | 2 (5.7) | 0.673 |
| Other cancer | 8 (22.9) | 11 (28.6) | 0.785 |
| MCRC (%), n = 35 | NMCRC (%), n = 35 | |
| Location | ||
| Right colon | 8 (22.8) | 7 (20) |
| Transverse colon | 2 (5.7) | 1 (2.9) |
| Left colon | 14 (40) | 16 (45.7) |
| Rectum/Rectosigmoid | 10 (28.6) | 10 (28.6) |
| Grade | ||
| Poor | 10 (28.6) | 3 (8.6) |
| Moderate | 16 (45.7) | 26 (74.3) |
| Well | 6 (17.1) | 3 (8.6) |
| Missing | 3 (8.6) | 3 (8.6) |
| Stage | ||
| I | 2 (5.7) | 2 (5.7) |
| II | 12 (34.3) | 12 (34.3) |
| III | 14 (40) | 13 (37.1) |
| IV | 7 (20) | 8 (22.8) |
| MCRC (%), n = 35 | NMCRC (%), n = 35 | |
| Colorectal recurrence | 6 (17) | 7 (20) |
| Metastasis on presentation | 5 (14.3) | 4 (11.4) |
| Metastasis on follow up | 7 (20) | 14 (40) |
| Sites involved by metastasis | ||
| Liver | 8 (22.8) | 12 (34.3) |
| Lung | 4 (11.4) | 4 (11.4) |
| Bone | 2 (5.7) | 1 (2.9) |
| Brain | 1 (2.9) | 2 (5.7) |
| Other | 7 (20) | 6 (17.1) |
| Lymph node involvement | 13 (37.1) | 13 (37.1) |
| MCRC (%), n = 35 | NMCRC (%), n = 35 | |
| Treatment | ||
| Surgery | 35 (100) | 35 (100) |
| Chemotherapy | 20 (57.1) | 19 (54.3) |
| Neoadjuvant | 5 (14.3) | 3 (8.6) |
| Adjuvant | 15 (42.9) | 16 (45.7) |
| Type | 5-Fu + leucovorin ± oxaliplatin | 5-Fu + leucovorin ± oxaliplatin |
| Radiotherapy | 11 (31.4) | 8 (22.9) |
| Case: control | Matched for | Survival in MCRC vs NMCRC | P | |
| Symonds et al[14] | 120/120 | Age, sex, stage | Decreased | Significant |
| Connelly et al[16] | 60/60 | Stage | Same | NS |
| Green et al[13] | 52/343 | Stage | Same | NS |
| Consorti et al[3] | 29/54 | Age, sex, location, stage | Decreased | NS |
| Kang et al[8] | 16 991/146 115 | Age, grade, stage, location | Same | NS |
| Farhat | 35/35 | Age, sex, stage | Decreased | NS |
Mucinous adenocarcinoma is a histological variant that accounts for 5% to 15% of cases of primary colorectal cancer[1]. It is defined as a tumor displaying extracellular mucin in more than 50% of the tumor volume. There is evidence that mucinous colorectal cancer (MCRC) may be a distinct biological and genetic entity compared with non-mucinous adenocarcinoma (NMCRC)[2,3]. Thus patients with MCRC may be younger[4,5], their tumors are more likely to be right-sided[6-8], and they present at a more advanced stage[3,4,6,8]. In addition, these tumors may have distinct mutations and cytogenetic abnormalities[2,9] and may be less likely to respond to chemotherapy[10-12]. There is, however, a lack of consensus in the published literature on whether the presence of mucin in colorectal tumors portends a poorer prognosis and worse survival or not.
Many studies suggest that MCRC is associated with poorer outcome and lower survival rates[10,13-15], but others deny this association[3,8,16,17]. The reasons for the conflicting reports are not clear. In many studies, there was no control for, age, sex, tumor stage or location[4,6,7,10]. In addition, the prognostic significance of having an extracellular mucinous component that is much larger than 50% is not known.
The aim of our study was to compare time to disease progression and overall survival (OS) of patients with mucinous to those with NMCRC after matching all patients for age, gender, and tumor stage. We also evaluated the effect of increasing mucin percentage in tumor body on survival.
A retrospective review of patients with colorectal cancer treated at the American University of Beirut-Medical Center between 1986 and 2003 identified 35 patients with MCRC and 750 patients with NMCRC.
For each patient with MCRC, a control with NMCRC was matched for age, gender and tumor stage, resulting in 70 cases and controls.
The patients’ gender, age, comorbid medical illnesses, tumor location, stage, grade, organ metastasis, surgical resection, chemotherapy regimen, radiological intervention, and survival data were obtained from hospital medical records and recorded for each patient.
Patients were classified as having MCRC if mucin constituted more than 50% of the tumor volume by histological examination.
An average of 4 slides per patient was reviewed to determine mucin content.
To determine the correlation between the percentage of mucin component and tumor stage and survival, histological slides from all patients (cases and controls) initially reported as mucinous tumors were reviewed by two pathologists from AUB-MC to determine the percentage of mucin in the studied sections.
To study the impact of increasing mucin content on survival, two cut-off points (50% and 75% mucin) were chosen. Accordingly, MCRC patients were further divided into 2 subgroups: group A (50%-75% mucin) and group B (75%-100% mucin). Furthermore, NMCRC patients (controls) were designated as group C (< 50% mucin). Moreover, all patients with less than 75% mucin (including groups A and C) were combined into a new group (group D).
Primary tumors were divided into four locations: right-sided (if arising in the cecum, ascending colon or hepatic flexure), transverse colon, left-sided (arising in the splenic flexure, descending colon, or sigmoid colon), and tumors arising in the rectum or rectosigmoid junction of the colon.
Patients with signet-ring colorectal cancer were excluded from the study.
The study design was a retrospective case-control study and the case-to-control ratio was 1:1. The Kaplan-Meier method was used to estimate survival. Univariate analysis was performed using the chi-squared testing in SPSS 14.0 software. P < 0.05 was considered statistically significant. Univariate analysis was performed to determine if increasing mucin production had an effect on survival that is independent of age, gender, and tumor stage. The median follow-up time was 42 ± 31 mo for non-mucinous (median: 27) and 38 ± 23 (median: 32) for mucinous tumors.
Patients with MCRC in our study accounted for 4.7% of all colorectal cancers. The study included 70 patients, 40 males and 30 females. The mean age at diagnosis for all patients was 56.8 (22-83) years, 56.8 (28-81) years for the MCRC group, and 56.7 (28-81) years for the NMCRC group. Males constituted 57% of the patients in both groups.
Patients in the two groups showed similar concomitant medical problems, mostly heart disease, diabetes mellitus type II, and hypertension (Table 1).
Both MCRC and NMCRC subgroups showed similar distributions of tumor location; the majority of tumors in both groups were located in the left colon and rectum (Table 2). The location of the tumor was not available in one patient.
Most tumors in both the MCRC and NMCRC groups were moderately or poorly differentiated (Table 2). Interestingly, 28.6% of tumors in the MCRC group were poorly differentiated versus 8.6% of those in the NMCRC group. (P = 0.0028). Moreover, 40% of group B tumors were of poor grade as compared to 11% of group D tumors (P = 0.001). Lymph nodes were involved in 37% of patients in both subgroups (Table 3).
The liver was the most common organ affected by distant metastasis (22% vs 34%) followed by the lungs (11%) in cases and controls, respectively.
The two groups were similar in terms of local disease recurrence: 17% of MCRC patients versus 20% of NMCRC patients (P > 0.05).
Patients in both groups were treated similarly. All patients were treated with surgical resection. Twenty patients from the mucinous group (57%) and 19 patients (54%) from the control group received neoadjuvant or adjuvant chemotherapy. Chemotherapy regimens were all 5-FU based, as a monotherapy or in combination. Radiotherapy was mainly given to patients with rectal cancer (Table 4).
The exact mucin percentage was obtained on all 35 patients with MCRC.
Eleven patients had 50%-75% of tumor volume displaying mucin and were classified as group A. Mean age at presentation was 55 (36-73) years.
Twenty-Four patients had more than 75% of tumor volume displaying mucin and were classified as group B. Mean age at diagnosis was 59.5 (24-81) years.
Subgroup analysis shows that more than half of the patients in groups A, B, and controls (group C) presented at advanced disease stages (stages III and IV): 54.5%, 66.7%, and 60%, respectively.
Compared to patients with NMCRC, there was a non-significant decrease in survival in patients with MCRC. Median survival of patients in the MCRC subgroup was 46.2 mo compared to 112.9 mo in the NMCRC subgroup (P = 0.26) (Figure 1A). Mucinous subgroups A and B had median OS of 70.1 and 32.8 mo, respectively (P = 0.46). Median OS was 32.8 and 70.1 mo in groups B and D, respectively (P = 0.143). TTP in MCRC and NMCRC was 50.17 vs 44.77 mo, respectively (P = 0.795) (Figure 1B). TTP in groups A, B, and D was 70.1, 24.8, and 65.5 mo (P > 0.05), respectively.
When survival of all patients was analyzed in relation with tumor grade, no difference in survival was noted among the various degrees of differentiation (Figure 1C).
Our results show that MCRC is associated with a non-significant decrease in median OS and TTP, compared to NMCRC. Similar results were obtained when OS and TTP were compared in patients with more than 75% mucin to those with less than 75% mucin.
In our report, we matched patients for age, gender, and stage of disease at presentation. Moreover, we found that patients in the two subgroups were similar in terms of tumor location. Furthermore, there was no difference in both arms with respect to presence of comorbid illnesses, chemotherapy regimens received or radiotherapy administration. However, we noted that more MCRC patients presented with a higher tumor grade than NMCRC patients (P = 0.028).
The prognostic significance of mucin content in colorectal cancer remains a controversial issue. While some authors reported that patients with MCRC show no difference in survival outcome compared to patients with NMCRC[3,8,16-18], others found that MCRC is associated with worse survival[4-7,10,14].
The difference in survival was attributed to the advanced stage at which these tumors present[3,4,6,8,13], more invasion of adjacent viscera and more extensive lymph node involvement[7,15,16], increased incidence of distant metastasis[4,17], and decreased response to chemotherapy[10].
Most of the published series compared mucinous to nonmucinous subgroups of colorectal cancer without matching for age, stage, grade and location. Comparing non-matched groups may confound survival data since the difference in survival could be related to differences in tumor location, grade or stage, and not due to the presence of mucin in the tumor.
Five studies compared matched groups of patients[3,8,13,14,16] (Table 5). One study reported a statistically significant decrease in survival in MCRC[14] while the other four showed no difference in survival outcome[3,8,13,16].
The importance of matching for stage is reflected in the large population-based study done by Kang et al[8] where MCRC did show worse OS but similar stage-to-stage survival as the other non-mucinous subtypes of colorectal cancer. The difference in OS between MCRC and NMCRC was attributed to the difference in stage at presentation, with more mucinous tumors presenting at advanced disease stages. That study did not, however, examine the impact of high mucin content (> 75%) on survival, a factor that may be associated with an adverse prognosis.
In patients with mucin producing colon cancer, few studies have examined the clinical impact of increasing mucin content in colorectal cancer on progression free and OS. Tumors with high mucin content (80%) were associated with worse clinicopathological behavior, more aggressive phenotype, and poorer prognosis[5,19,20]. By contrast, tumors with moderate mucin content (60%-80%) were found to be indistinguishable from non-mucinous tumors. However, the different subgroups were not matched for age, stage, grade, or location.
The prognostic value of tumor grade in MCRCs has not been adequately investigated in matched studies. In a non-matched study by Enríquez et al[21], MCRC showed higher tumor grade that did not affect survival, which is in agreement with our data. Moreover, Connelly et al[16] excluded the poorly differentiated histology from their MCRC study because tumors with high grade tend to exhibit a distinct biological behavior and a worse prognosis[22,23]. However, Kang et al[8] controlled for tumor grade and concluded that MCRC and NMCRC patients show similar risks of dying.
Although our patients with MCRC had more poorly differentiated tumors (P = 0.0028), there was no apparent significant effect of grade on prognosis. Due to the small number of patients in our study, matching for tumor grade was not possible.
There are several limitations in our study. First, this was a case-control study with a relatively small number of subjects. The inability to adequately perform more subgroup analyses or to control for additional confounders because of small sample size is to be noted.
Second, this is a retrospective assessment with all the attendant limitations of this approach including the missing data, which leads to fewer patients included in multivariable models, generally increasing the risk for both type one and type two errors. Third, the cases seen represent a single tertiary center experience which might reduce the prevalence rate of MCRC reported here as compared to the higher prevalence rates in some studies that used data from national registries. Fourth, some of the trends observed in the study might have reached statistical significance if the study sample had been larger. However, despite the limitations of our study, very few studies have examined the effect of increasing mucin content on survival in colorectal patients which adds more value to the results of our study and others’.
In conclusion, high mucin content in colorectal cancer is associated with a nonsignificant decrease in OS and TTP. However, the presence of mucin in more than 75% of tumor volume may indicate a more aggressive phenotype that presents with a higher tumor grade and a possible subsequent decrease in OS and TTP. Larger studies in patients with MCRC with matching for stage and grade are warranted to examine the impact of mucin content on survival, especially those with more than 75% mucin content.
Mucinous adenocarcinoma is a histological variant that accounts for 5% to 15% of cases of primary colorectal cancer. There is evidence that mucinous colorectal cancer (MCRC) may be a distinct biological and genetic entity compared with non-mucinous adenocarcinoma.
A retrospective review of patients with colorectal cancer treated at the American University of Beirut-Medical Center between 1986 and 2003 identified 35 patients with MCRC and 750 patients with NMCRC. To study the impact of increasing mucin content on survival, two cut-off points (50% and 75% mucin) were chosen. Univariate analysis was performed to determine if increasing mucin production had an effect on survival that is independent of age, gender, and tumor stage.
High mucin content in colorectal cancer is associated with a nonsignificant decrease in overall survival (OS) and time to disease progression (TTP). However, the presence of mucin in more than 75% of tumor volume may indicate a more aggressive phenotype that presents with a higher tumor grade and a possible subsequent decrease in OS and TTP.
MCRC is associated with a non-significant decrease in median survival and TTP, particularly when mucin content is > 75% of tumor volume. However, it tends to be more poorly differentiated. Larger studies in patients with MCRC with matching for stage and grade are warranted to examine the impact of mucin content on survival, especially those with more than 75% mucin content.
It is an interesting manuscript trying to answer the question if the mucin production is related with survival in colorectal cancer.
Peer reviewer: Damian Casadesus Rodriguez, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Li DL L- Editor Stewart GJ E- Editor Ma WH
| 1. | American Joint Committee on Cancer: Staging Manual, 6th ed. New York: Springer 2002. Available from: URL: http://www.cancerstaging.org/products/ajccproducts.html. |
| 2. | Zhang H, Evertsson S, Sun X. Clinicopathological and genetic characteristics of mucinous carcinomas in the colorectum. Int J Oncol. 1999;14:1057-1061. |
| 3. | Consorti F, Lorenzotti A, Midiri G, Di Paola M. Prognostic significance of mucinous carcinoma of colon and rectum: a prospective case-control study. J Surg Oncol. 2000;73:70-74. |
| 4. | Wu CS, Tung SY, Chen PC, Kuo YC. Clinicopathological study of colorectal mucinous carcinoma in Taiwan: a multivariate analysis. J Gastroenterol Hepatol. 1996;11:77-81. |
| 5. | Suma KS, Nirmala V. Mucinous component in colorectal carcinoma--prognostic significance: a study in a south Indian population. J Surg Oncol. 1992;51:60-64. |
| 6. | Papadopoulos VN, Michalopoulos A, Netta S, Basdanis G, Paramythiotis D, Zatagias A, Berovalis P, Harlaftis N. Prognostic significance of mucinous component in colorectal carcinoma. Tech Coloproctol. 2004;8 Suppl 1:s123-s125. |
| 7. | Nozoe T, Anai H, Nasu S, Sugimachi K. Clinicopathological characteristics of mucinous carcinoma of the colon and rectum. J Surg Oncol. 2000;75:103-107. |
| 8. | Kang H, O'Connell JB, Maggard MA, Sack J, Ko CY. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum. 2005;48:1161-1168. |
| 9. | Messerini L, Vitelli F, De Vitis LR, Mori S, Calzolari A, Palmirotta R, Calabro A, Papi L. Microsatellite instability in sporadic mucinous colorectal carcinomas: relationship to clinico-pathological variables. J Pathol. 1997;182:380-384. |
| 10. | Negri FV, Wotherspoon A, Cunningham D, Norman AR, Chong G, Ross PJ. Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol. 2005;16:1305-1310. |
| 11. | Glasgow SC, Yu J, Carvalho LP, Shannon WD, Fleshman JW, McLeod HL. Unfavourable expression of pharmacologic markers in mucinous colorectal cancer. Br J Cancer. 2005;92:259-264. |
| 12. | Takemura M, Osugi H, Lee S, Kaneko M, Tanaka Y, Fujiwara Y, Nishizawa S, Iwasaki H. [Choice of chemotherapeutic drugs for colorectal cancers by DPD and OPRT activities in cancer tissues]. Gan To Kagaku Ryoho. 2004;31:1053-1056. |
| 13. | Green JB, Timmcke AE, Mitchell WT, Hicks TC, Gathright JB Jr, Ray JE. Mucinous carcinoma--just another colon cancer? Dis Colon Rectum. 1993;36:49-54. |
| 15. | Yamamoto S, Mochizuki H, Hase K, Yamamoto T, Ohkusa Y, Yokoyama S, Ushitani Y, Tamakuma S. Assessment of clinicopathologic features of colorectal mucinous adenocarcinoma. Am J Surg. 1993;166:257-261. |
| 16. | Connelly JH, Robey-Cafferty SS, Cleary KR. Mucinous carcinomas of the colon and rectum. An analysis of 62 stage B and C lesions. Arch Pathol Lab Med. 1991;115:1022-1025. |
| 17. | Adell R, Marcote E, Segarra MA, Pellicer V, Gamon R, Bayon AM, Canales M, Torner A. [Is mucinous colorectal adenocarcinoma a distinct entity?]. Gastroenterol Hepatol. 2002;25:534-540. |
| 18. | Du W, Mah JT, Lee J, Sankila R, Sankaranarayanan R, Chia KS. Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum. 2004;47:78-85. |
| 19. | Sasaki O, Atkin WS, Jass JR. Mucinous carcinoma of the rectum. Histopathology. 1987;11:259-272. |
| 20. | Umpleby HC, Ranson DL, Williamson RC. Peculiarities of mucinous colorectal carcinoma. Br J Surg. 1985;72:715-718. |
| 21. | Enriquez JM, Diez M, Tobaruela E, Lozano O, Dominguez P, Gonzalez A, Muguerza JM, Ratia T. Clinical, histopatholo-gical, cytogenetic and prognostic differences between mucinous and nonmucinous colorectal adenocarcinomas. Rev Esp Enferm Dig. 1998;90:563-572. |
| 22. | Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979-994. |
| 23. | O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420-1425. |