Published online Nov 28, 2008. doi: 10.3748/wjg.14.6817
Revised: November 4, 2008
Accepted: November 11, 2008
Published online: November 28, 2008
AIM: To investigate the most important aspects of hyperthermic intraperitoneal chemotherapy (HIPEC) that has been accepted as the standard treatment for pseudomyxoma peritonei (PMP), with special regard to morbidity, overall survival (OS) and disease free survival (DFS) over 10 years.
METHODS: Fifty-three patients affected by PMP underwent cytoreduction (CCR) and HIPEC with a “semi-closed” abdomen technique in our institution. The peritonectomy procedure and completeness of CCR were classified according to Sugarbaker criteria. Preoperative evaluation always included thoracic and abdominal CT scan to stage peritoneal disease and exclude distant metastases. Fifty-one patients in our series were treated with a protocol based on administration of cisplatinum 100 mg/m2 plus mitomycin C 16 mg/m2, at a temperature of 41.5°C for 60 min. Anastomoses were always performed at the end of HIPEC. The mean duration of surgery was 12 h including HIPEC. Continuous monitoring of hepatic and renal functions and hydroelectrolytic balance was performed in the postoperative period.
RESULTS: Twenty-four patients presented with postoperative complications: surgical morbidity was observed in 16 patients and 6 patients were re-operated. All complications were successfully treated and no postoperative deaths were observed. Risk factors for postoperative morbidity were considered to be gender, age, body surface, duration of surgery, Peritoneal Cancer Index (PCI) and tumor residual value (CC score). No statistically significant correlation was found during the multivariate analysis: only the CC score was statistically significant. The OS in our experience was 81.8%, with a DFS of 80% at 5 years and of 70% at 10 years.
CONCLUSION: In our experience, even if HIPEC combined with cytoreductive surgery involves a high risk of morbidity, postoperative complications can be resolved favorably in most cases with correct patient selection and adequate postoperative care, thus minimizing mortality. The association of CCR and HIPEC can be considered as the standard treatment for PMP. The OS and DFS results confirm the validity of this combined approach for the treatment of this rare neoplasm. The impact of preoperative chemotherapy on OS, in our opinion, is due to a major aggressiveness of tumors in treated patients.
- Citation: Cioppa T, Vaira M, Bing C, D’Amico S, Bruscino A, Simone MD. Cytoreduction and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis from pseudomyxoma peritonei. World J Gastroenterol 2008; 14(44): 6817-6823
- URL: https://www.wjgnet.com/1007-9327/full/v14/i44/6817.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6817
| Procedures | No. |
| Greater omentectomy | 48 |
| Left colectomy | 30 |
| Right colectomy | 34 |
| Total abdominal hysterectomy | 16 |
| Bilateral salpingo-oophorectomy | 20 |
| Splenectomy | 40 |
| Cholecystectomy | 34 |
| Small bowel partial resection | 27 |
| Total gastrectomy | 5 |
| Sub-total gastrectomy | 3 |
| Distal pancreatectomy | 6 |
| Rigth upper quadrant peritonectomy | 47 |
| Left upper quadrant peritonectomy | 38 |
| Pelvic peritonectomy | 43 |
| Variable | No. of cases | With complications | Without complications | P-value |
| Gender | NS | |||
| Male | 23 | 11 (46) | 12 (41) | |
| Female | 30 | 13 (54) | 17 (59) | |
| Age (yr) | 59 ± 10 | 56 ± 9 | NS | |
| Body mass index (Kg/m2) | 25.4 ± 5.3 | 26.8 ± 4.7 | NS | |
| Previous systemic chemotherapy | NS | |||
| Performed | 21 | 10 (42) | 11 (38) | |
| Not performed | 32 | 14 (58) | 18 (62) | |
| PCI | NS | |||
| > 16 | 36 | 18 (75) | 18 (62) | |
| < 16 | 17 | 6 (25) | 11 (38) | |
| Operative time (h) | 8.5 ± 3.0 | 7.1 ± 2.1 | NS | |
| Completeness of cancer resection | 0.017 | |||
| CCR-0 | 35 | 12 (50) | 23 (79) | |
| CCR-1 | 18 | 12 (50) | 6 (21) |
| Complication | No. of cases | Treatment (No. of cases) |
| Surgical | ||
| Wound infection | 4 | Drainage |
| Urinary tract perforation | 2 | Reoperation (1); Urinary stenting (1) |
| Intestinal fistula | 3 | Reoperation |
| Abdominal abscess | 2 | US-guided drainage (1); medical (1) |
| Prolonged ileous | 1 | Medical |
| Bleeding from gastric ulcer | 1 | Endoscopic haemostasis |
| Intraabdominal bleeding | 2 | Reoperation |
| abdominal wall dehiscence | 1 | Conservative |
| Medical | ||
| Grade ≥ 2 hematological toxicity | 3 | Medical |
| Acute renal failure | 1 | Medical |
| Arrhythmias | 1 | Medical |
| Cutaneous rush | 1 | Medical |
| Sepsis | 2 | Medical |
Peritoneal carcinomatosis (PC) is one of the most common routes of dissemination of abdominal neoplasms; it may be present at the time of diagnosis of a primary tumor, but more frequently it arises as tumor recurrence after surgical treatment[1].
PC is frequently associated with colorectal cancer, gastric cancer, ovarian carcinoma, and appendiceal cancer. Neoplasms with positive peritoneal cytology show high rates of peritoneal dissemination[2-6].
Pseudomyxoma peritonei (PMP) is a rare condition, with an incidence of 1/1 000 000 per year, characterized by copious mucus (so-called “jelly belly”) containing rare epithelial cells. According to Ronnet, PMP was histologically classified into disseminated peritoneal adenomucinosi (DPAM), peritoneal mucinous carcinomatosis (PMCA) and an intermediate or discordant feature group (ID)[7]. Recent studies show that most cases of PMP originate from ruptured appendiceal tumors with progressive dissemination in the peritoneal cavity of mucin-producing epithelial-cells[8,9]. Lymph-nodal or hematogenous metastases are rare in PMP and evidence suggests it has a poor prognosis.
PC from PMP is generally considered a lethal disease, with a limited response to conventional chemotherapeutic treatments[10]. While systemic chemotherapy has little impact on the treatment of peritoneal disease, some centers have reported encouraging results with intraperitoneal hyperthermic chemoperfusion (HIPEC)[11,12].
This technique is based on surgical cytoreduction (CCR) of the primitive cancer, peritonectomy and HIPEC. The principle of locoregional treatments is to obtain an elevated and persistent drug concentration for the tumor, with a limited systemic concentration. Many studies reported an impact on overall survival (OS) and disease-free survival (DFS) in patients affected by carcinomatosis of mucinous cancers such as PMP[13,14] and in recent trials this combined approach has been proposed as standard treatment for PMP.
In this study we report the results of a 10-year experience with this type of treatment in our institution, where 53 patients with PMP were treated with CCR and HIPEC, with special reference to follow-up and risk factors for postoperative complications.
For the present study, 53 patients (23 male and 30 female, mean age 58 years, range 32-72) with PMP who underwent surgical treatment and HIPEC between October 1998 and June 2008 at the Department of General Surgery and Surgical Oncology, San Giuseppe Hospital, were considered. Preoperative evaluation always included thoracic and abdominal CT scan to stage peritoneal disease and exclude distant metastases; upper digestive endoscopy and colonoscopy generally completed tumor staging. A careful preoperative evaluation of the patient’s general condition was always performed, and included complete blood tests, electrocardiogram, cardiac ultrasound, and spirometry. The presence of hepatic or extra-abdominal metastases, poor general condition or performance status > 2 according to the Eastern Cooperative Oncology Group (ECOG) and an age of > 72 years were generally considered contraindications to the treatment. Informed consent was obtained from all patients[15].
Just after laparotomy, a complete intraoperative staging of peritoneal disease was performed using the peritoneal cancer index (PCI)[16]; the mean PCI was 22.
The peritonectomy procedure was classified and performed according to Sugarbaker’s criteria: (1) Central peritonectomy consists of the removal of previous scars, greater omentectomy (performed by stripping the superficial peritoneal layer of the transverse mesocolon) and a close dissection to the greater curvature of the stomach. Sometimes splenectomy could be necessary en bloc with the greater omentum and the left diaphragmatic peritoneum; (2) Left upper quadrant peritonectomy consists of the stripping of the peritoneal tumor tissue from beneath the left hemidiaphragm, left adrenal gland, distal portion of the pancreas, and the cephalad half of Gerota’s fascia; (3) Right upper quadrant peritonectomy consists of right hemidiaphragmatic peritoneal stripping, removal of tumor from the right subhepatic space and from the surface of the liver by the stripping of the Glisson’s capsule. Peritonectomy is concluded with the removal of the peritoneum covering the right kidney and Morrison’s pouch; (4) Lesser omentum peritonectomy is performed after the cholecystectomy, and in this procedure the cancerous tissue which covers the common duct and hepatic artery is stripped from the base of gall bladder bed towards the duodenum. This phase is concluded by the stripping of omental bursa; (5) Pelvic peritonectomy with en bloc removal of pelvic peritoneum, sigmoid colon, rectum, uterus and salpingo-oophorectomy; (6) Peritonectomy of the lateral abdominal wall. Implants on the visceral serosa are removed by electrosurgical local dissection and the peritonectomies are variously combined with resections of viscera involved in tumor (total gastrectomy or total colectomy).
The completeness of CCR was also classified according to Sugarbaker’s criteria[17] as: CCR-0 (no residual tumor) in 35 cases, CCR-1 (no residual nodule greater than 2.5 mm in diameter) in 18 cases, CCR-2 (no residual nodules greater than 25 mm) in none of the cases and CCR-3 (residual nodules greater than 25 mm) in none of the cases.
HIPEC was performed according to the “semi-closed” abdomen technique[18]. Five drain tubes are placed in the abdominal cavity. There are 2 inflow tubes, and they have multiple holes. They present 2 diffusion lines for the homogeneous distribution of drugs into the abdominal cavity (1 in the sovramesocolic branch, 1 in the pelvis). Three outflow tubes are placed respectively in the pelvis and in the subdiaphragmatic spaces. Backhaus forceps are used to close the cranial and caudal portion of abdominal wound. The skin is then suspended by a self-retaining retractor, placed at more or less 15 cm from the abdomen, by plastic self-blocking strings. This kind of placement creates the virtual cavity needed to perform HIPEC. The central portion of the wound is suspended by the retractor too and covered with a laparoscopic device with sterile drapes on it, with a hole in the middle. The drain tubes are connected to a perfusion system formed by 2 pumps and a heat exchanger to heat the perfusion liquid. The inflow and outflow pumps are connected through a reservoir, so it is possible to achieve continuous circulation of the perfusate at the speed of more or less 1 L/min. The pumps are controlled by a computerized system that allows the checking of the flow rate and the temperature of the heat exchanger. Three intraperitoneal temperatures are checked by probes; the inflow temperature, outflow temperature, and the patient esophageal temperature. The amount of circulating perfusate required (solution for peritoneal dialysis) is calculated according to the patient’s body surface. During perfusion, the surgeon mixes the perfusate by hand through the hole in the sterile drapes. When the ideal intraperitoneal temperature is reached, the drugs are added to the circuit and HIPEC is performed for 60 min. Fifty-one patients in our series were treated with a protocol based on administration of cisplatinum 100 mg/m2 plus mitomycin C 16 mg/m2, at a temperature of 41.5°C. Two patients were treated with mitomycin C 35 mg/m2 for 60 min at a temperature of 40.5°C, according to the Netherland protocol, because of significant side effects from preoperative systemic chemotherapy with platinum. Anastomoses were always performed at the end of HIPEC. The mean duration of surgery was 12 h including HIPEC (range 8-16 h). At the end of the operation, the patient was admitted to the intensive care unit, and then returned to the surgical department when cardiovascular and pulmonary functions became stable. Continuous monitoring of hepatic and renal functions and hydroelectrolytic balance were performed afterwards. The primitive neoplasm was an appendicular adenocarcinoma in 37 patients (69.8%) and an appendicular adenoma in 16 patients (30.2%). Twenty-one patients (39.6%) with histological diagnosis of appendicular adenocarcinoma had been treated with systemic chemotherapy before our operation. Because of the massive involvement of viscera and peritoneum, in some selected patients we performed the treatment in steps. Three patients were treated in 2 steps, and 1 patient was treated in 3 steps. In these cases, we performed the upper abdominal CCR in the first step, then the patient was submitted to systemic chemotherapy for 2 or 3 mo. The second step consisted of lower abdominal CCR and peritoneal perfusion of the entire peritoneal cavity. The details of the CCR procedures are displayed in Table 1.
In this study the statistical analyses focused on postoperative complications: the histopathological, clinical and follow-up data were stored in a database. The presence of postoperative complications was considered as the dependent variable whereas gender, age, body mass index, primary tumor, previous systemic chemotherapy, operative time, stage of PCI, and CCR were covariates.
Multivariate analysis of factors was performed by the Cox proportional hazard model. OS was dated from the day of surgery to the time of death due to any causes; progression-free survival (PFS) was dated from the day of the surgery to the time of postoperative disease progression. The survival curves for both OS and PFS were calculated according to the Kaplan-Meier method. The log-rank test was used to assess the significance of the comparison between survival curves.
The Statistical Package for the Social Sciences software (version 11.0) (SPSS, Chicago, IL, USA) was used for statistical analysis: P < 0.05 was considered significant.
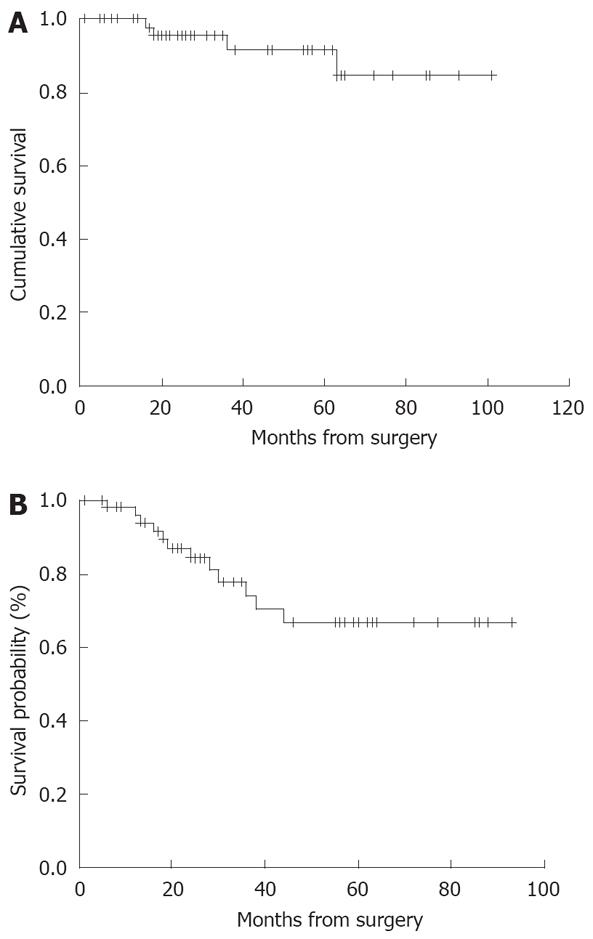
At the end of follow-up of the 53 patients, 5 and 10 year OS was 94% and 84.6%, respectively (Figure 1A). DFS was 80% and 70% at 5 and 10 years, respectively (Figure 1B).
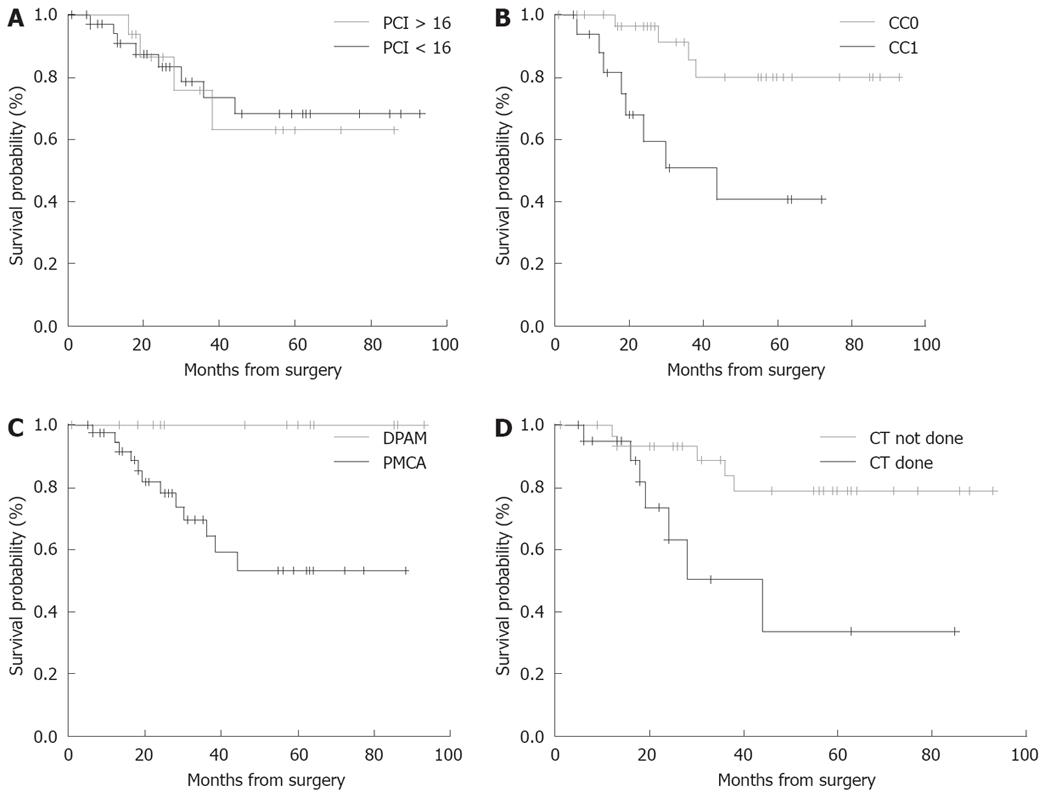
OS according to the PCI, completeness of CCR (CC-score), histological type, and pre-operative chemotherapy (done vs not done) are shown in Figure 2A-D. At the time of the present analysis 48 patients are alive without disease. Two patients died due to systemic disease progression at 16 and 63 mo, respectively, after the operation; 3 patients are alive with disease and intraperitoneal relapse but are not undergoing a further operation, with follow-up of 57, 28, 24, 19, 10 mo, respectively. Three patients had intraperitoneal relapse and were treated with tumor resection followed by HIPEC: 1 of those patients is alive without disease 17 mo after the second surgical procedure; the other 2 patients were treated only with CCR and they are alive without disease after 24 mo of follow-up.
For calculation of the morbidity rate, we considered postoperative complications occurring during the hospital stay or within 30 d of surgery. In 24 patients (45%) we observed postoperative complications: surgical morbidity was observed in 16 patients (3 intestinal fistulas, 2 urinary tract perforations, 2 abdominal abscesses, 4 wound infections, 1 prolonged ileus, 2 postoperative haemorrhages, 1 abdominal wall dehiscence, 1 bleeding from a gastric ulcer) and medical complications were observed in 8 cases (1 arrhythmia, 3 grade 2 hematological toxicities, 1 acute renal failure, 1 cutaneous rash, 2 cases of sepsis). Six patients were re-operated and 1 patient underwent ureteric stenting. One patient with abdominal abscess was submitted to ultrasound-guided drainage and 1 patient with bleeding from a gastric ulcer was treated by endoscopic haemostasis. All other complications were successfully treated by medical therapy (Table 2). No postoperative deaths were observed. An analysis of risk factors for postoperative morbidity rate was performed. Gender, age, body surface, duration of surgery, PCI and tumor residual value were considered to be risk factors. No statistically significant correlation between the analyzed variables and the incidence of postoperative complications was found except for CC-score (P < 0.017) (Table 3).
Final follow-up data from our experience indicated that survival probability may be good in patients with histological type appendicular adenoma who are optimally cytoreduced (CC-0). An interesting relief was related to whether preoperative chemotherapy was performed or not.
HIPEC associated with cytoreductive surgery is becoming a widely accepted procedure for the treatment of PMP.
Like reports in several studies, the results of our experience indicate that, even when combined with an aggressive surgical procedure, HIPEC is associated with an acceptable risk of postoperative complications and mortality[19-24]. The incidence of postoperative complications was similar to that of other reports[25-29] and major morbidity occurred in 45% of patients, which is also similar to other recent experiences[27,29,30]. All the complications were successfully treated with surgical or medical therapy. We believe that careful preoperative selection of patients, and adequate postoperative monitoring and care are crucial in order to minimize the incidence of postoperative complications in these patients.
The advanced stage of neoplastic disease and immunodeficiency status of patients previously subjected to chemotherapy were important factors that probably contributed to the occurrence of septic complications after an extended surgical procedure. In our series 1 patient underwent ultrasound-guided drainage of an abdominal abscess and in the others septic complications were successfully treated with medical therapy.
Intestinal fistula has been reported to be an important cause of morbidity and mortality in patients submitted to HIPEC, with an incidence rate ranging from 6% to 27%[21-23,31-33]. Younan et al[32] reported that male gender, duration of surgery, and no previous systemic chemotherapy were independent predictors of bowel complications. The direct effect of HIPEC even in non-resective procedures can be associated with intestinal fistulas in the postoperative period[33,34]. We always performed anastomotic suture following HIPEC but in our series the 3 cases of intestinal fistula were due to intestinal perforation not involving anastomosis.
Recent studies have reported the duration and extent of surgery, visceral resections, PCI and incomplete CCR to be important risk factors for postoperative complications[21,26,31]. In our patients, we did not find this correlation, probably because of the limited number of cases. PCI > 16 was an independent predictor of postoperative morbidity only at univariate analysis and seemed to have an impact on the complication rate, but not on OS.
The largest series of PMP undergoing combined treatment was reported by Sugarbaker[35]: in this series completeness of CCR and Ronnet’s criteria were the most important factors correlated with survival and morbidity[36].
Complete CCR was obtained in most of our patients. In our series, we didn’t have patients with an elevated CC score (CC-2 or CC-3): as a consequence, even though the morbidity rate was high we did not find a correlation between cytoreductive status and complication rate. However, the CC-score in our experience was strictly correlated to DFS with an evident result between CC-0 and CC-1 patients (P < 0.003).
The limited number of patients in our series did not allow further stratifications and for these reasons, the potential impact of other factors on morbidity cannot be excluded.
Survival data indicate that high long-term survival could be achieved in patients with histological type DPAM vs PMCA (P < 0.014). Furthermore in the present series the adverse prognostic value of preoperative systemic chemotherapy was a unexpected finding that may be not easy to explain: the patient that received chemotherapy had a poor prognosis compared to those that did not undergo chemotherapy (P < 0.034). The same evidence was observed in the series of Baratti et al[37].
The hypothesis was that after chemotherapy, mucinous appendiceal tumors change to a more invasive process: it is quite possible that differences in chemotherapy penetration of mucinous and solid tumours may result in persistence and progression of the more solid components of a non uniform tumor. Appendiceal tumors are described as having large areas of adenomucinosis with small, even minute, areas of more aggressive tissue: the penetration of chemotherapy drugs into the mucin that contains adenomatous epithelial cells may eradicate these cells but the small foci of solid tumor may not be completely penetrated by chemotherapy. It is possible that this process selected resistant and more aggressive tumor cell clones but the explanation for the poor results of the treatment in these patients requires further investigation.
In conclusion the present study confirms that an aggressive approach can improve survival in selected patients with PMP. Although HIPEC combined with CCR has a high risk of morbidity, postoperative complications could be resolved favorably in most cases with correct patient selection and adequate postoperative care, thus minimizing mortality. Residual tumor (CC), preoperative chemotherapy and histological type PCMA significantly influence the prognosis of these patients[20,37].
To improve this encouraging survival outcome, it is very important to unify the surgical experience of expertise centers and adequate patient selection. Our results suggest also the need of an integrated approach to this rare neoplasm to identify the biological aspect of PMP that influences the prognosis and the evolution of the disease.
Peritoneal carcinomatosis is generally considered a lethal disease, with a mean survival time of 6 mo after conventional chemotherapeutic treatments. Systemic chemotherapy has little impact on treatment of peritoneal disease, but some centres have reported encouraging results with intraperitoneal hyperthermic chemoperfusion (HIPEC). Locoregional treatments are considered a new frontier in the management of this condition: it is possible to achieve an elevated and persistent drug concentration in the tumor, with limited systemic effects. Many studies reported an impact on overall survival and disease-free interval in patients affected by carcinomatosis, of mucinous cancers such as pseudomyxoma peritonei (PMP) and in recent trials this combined approach has been proposed as standard treatment for PMP.
The present study confirms that an aggressive approach can improve survival in selected patients with PMP. Although HIPEC combined with cytoreductive surgery involves a high risk of morbidity, postoperative complications can be resolved favorably in most cases with correct patient selection and adequate postoperative care, thus minimizing mortality. Residual tumor (CC), preoperative chemotherapy and histological type of PMP can be considered as independent variables able to significantly influence the prognosis of these patients. To improve this encouraging survival outcome, it is very important to unify the surgical experience of expertise centres. Our results suggest also the need for an integrated approach to this rare neoplasm to identify the biological aspects of PMP that influence the prognosis and the evolution of the disease.
In this paper we report a very important proof on the integrated approach to PMP. This lethal disease can be treated with good results: in fact 5 and 10 year overall survival was, respectively, 94% and 84.6% in our experience and disease free survival was 80% and 70% at 5 and 10 years, respectively.
On future application, the end point of this approach would be to improve a standard treatment for this particular disease to reduce the surgical risk of major complications. Correct patient selection and adequate postoperative care may minimize the considerable complication rate that is very high (45%).
This is a very interesting study on pseudomyxoma peritonei and its treatment with intraperitoneal hyperthermic chemoperfusion.
Peer reviewer: Gerardo Rosati, MD, “S. Carlo” Hospital, Via Potito Petrone, 1, Potenza 85100, Italy
S- Editor Tian L L- Editor O’Neill M E- Editor Zheng XM
| 1. | Sugarbaker PH. Peritoneal carcinomatosis: natural history and rational therapeutic interventions using intraperitoneal chemotherapy. Cancer Treat Res. 1996;81:149-168. |
| 2. | Marrelli D, Roviello F, de Manzoni G, Morgagni P, Di Leo A, Saragoni L, De Stefano A, Folli S, Cordiano C, Pinto E. Different patterns of recurrence in gastric cancer depending on Lauren's histological type: longitudinal study. World J Surg. 2002;26:1160-1165. |
| 3. | Roviello F, Marrelli D, de Manzoni G, Morgagni P, Di Leo A, Saragoni L, De Stefano A. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90:1113-1119. |
| 4. | Bando E, Yonemura Y, Takeshita Y, Taniguchi K, Yasui T, Yoshimitsu Y, Fushida S, Fujimura T, Nishimura G, Miwa K. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256-262. |
| 5. | Pestieau SR, Sugarbaker PH. Treatment of primary colon cancer with peritoneal carcinomatosis: comparison of concomitant vs. delayed management. Dis Colon Rectum. 2000;43:1341-1346; discussion 1347-1348. |
| 6. | Sugarbaker TA, Chang D, Koslowe P, Sugarbaker PH. Pathobiology of peritoneal carcinomatosis from ovarian malignancy. Cancer Treat Res. 1996;81:63-74. |
| 7. | Ronnett BM, Zahn CM, Kurman RJ, Kass ME, Sugarbaker PH, Shmookler BM. Disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. A clinicopathologic analysis of 109 cases with emphasis on distinguishing pathologic features, site of origin, prognosis, and relationship to "pseudomyxoma peritonei". Am J Surg Pathol. 1995;19:1390-1408. |
| 8. | Szych C, Staebler A, Connolly DC, Wu R, Cho KR, Ronnett BM. Molecular genetic evidence supporting the clonality and appendiceal origin of Pseudomyxoma peritonei in women. Am J Pathol. 1999;154:1849-1855. |
| 9. | Carr NJ, Emory TS, Sobin LH. Epithelial neoplasms of the appendix and colorectum: an analysis of cell proliferation, apoptosis, and expression of p53, CD44, bcl-2. Arch Pathol Lab Med. 2002;126:837-841. |
| 10. | Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358-363. |
| 11. | Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T, Isawa E, Sumida M, Ohkubo H. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer. 1997;79:884-891. |
| 12. | Witkamp AJ, de Bree E, Kaag MM, Boot H, Beijnen JH, van Slooten GW, van Coevorden F, Zoetmulder FA. Extensive cytoreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer. 2001;37:979-984. |
| 13. | Sugarbaker PH, Ronnett BM, Archer A, Averbach AM, Bland R, Chang D, Dalton RR, Ettinghausen SE, Jacquet P, Jelinek J. Pseudomyxoma peritonei syndrome. Adv Surg. 1996;30:233-280. |
| 14. | Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284-3292. |
| 15. | Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649-655. |
| 16. | Sugarbaker PH, Alderman R, Edwards G, Marquardt CE, Gushchin V, Esquivel J, Chang D. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol. 2006;13:635-644. |
| 17. | Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359-374. |
| 18. | De Simone M, Barone R, Vaira M, Aghemo B, Mioli P, Franco C, Scuderi S, Costamagna D, Dei Poli M. Semi-closed hyperthermic-antiblastic peritoneal perfusion (HAPP) in the treatment of peritoneal carcinosis. J Surg Oncol. 2003;82:138-140. |
| 19. | Glehen O, Mohamed F, Gilly FN. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 2004;5:219-228. |
| 20. | Roviello F, Marrelli D, Neri A, Cerretani D, de Manzoni G, Pedrazzani C, Cioppa T, Nastri G, Giorgi G, Pinto E. Treatment of peritoneal carcinomatosis by cytoreductive surgery and intraperitoneal hyperthermic chemoperfusion (IHCP): postoperative outcome and risk factors for morbidity. World J Surg. 2006;30:2033-2040; discussion 2041-2042. |
| 21. | Stephens AD, Alderman R, Chang D, Edwards GD, Esquivel J, Sebbag G, Steves MA, Sugarbaker PH. Morbidity and mortality analysis of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol. 1999;6:790-796. |
| 22. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. |
| 23. | Glehen O, Mithieux F, Osinsky D, Beaujard AC, Freyer G, Guertsch P, Francois Y, Peyrat P, Panteix G, Vignal J. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21:799-806. |
| 24. | Ahmad SA, Kim J, Sussman JJ, Soldano DA, Pennington LJ, James LE, Lowy AM. Reduced morbidity following cytoreductivPilati P, Mocellin S, Rossi CR, Foletto M, Campana L, Nitti D, Lise M. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol 2003; 10: 508-513e surgery and intraperitoneal hyperthermic chemoperfusion. Ann Surg Oncol. 2004;11:387-392. |
| 25. | Pilati P, Mocellin S, Rossi CR, Foletto M, Campana L, Nitti D, Lise M. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10:508-513. |
| 26. | Verwaal VJ, van Tinteren H, Ruth SV, Zoetmulder FA. Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol. 2004;85:61-67. |
| 27. | Cavaliere F, Perri P, Di Filippo F, Giannarelli D, Botti C, Cosimelli M, Tedesco M, Principi F, Laurenzi L, Cavaliere R. Treatment of peritoneal carcinomatosis with intent to cure. J Surg Oncol. 2000;74:41-44. |
| 28. | Schmidt U, Dahlke MH, Klempnauer J, Schlitt HJ, Piso P. Perioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31:53-58. |
| 29. | Elias D, Blot F, El Otmany A, Antoun S, Lasser P, Boige V, Rougier P, Ducreux M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71-76. |
| 30. | Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA, Levine EA. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11:178-186. |
| 31. | Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, Trillet-Lenoir V, Sayag-Beaujard AC, François Y, Vignal J. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863-869. |
| 32. | Younan R, Kusamura S, Baratti D, Oliva GD, Costanzo P, Favaro M, Gavazzi C, Deraco M. Bowel complications in 203 cases of peritoneal surface malignancies treated with peritonectomy and closed-technique intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2005;12:910-918. |
| 33. | Ryu KS, Kim JH, Ko HS, Kim JW, Ahn WS, Park YG, Kim SJ, Lee JM. Effects of intraperitoneal hyperthermic chemotherapy in ovarian cancer. Gynecol Oncol. 2004;94:325-332. |
| 34. | Makrin V, Lev-Chelouche D, Even Sapir E, Paran H, Rabau M, Gutman M. Intraperitoneal heated chemotherapy affects healing of experimental colonic anastomosis: an animal study. J Surg Oncol. 2005;89:18-22. |
| 35. | Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727-731. |
| 36. | González-Moreno S, Sugarbaker PH. Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg. 2004;91:304-311. |
| 37. | Baratti D, Kusamura S, Nonaka D, Langer M, Andreola S, Favaro M, Gavazzi C, Laterza B, Deraco M. Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol. 2008;15:526-534. |