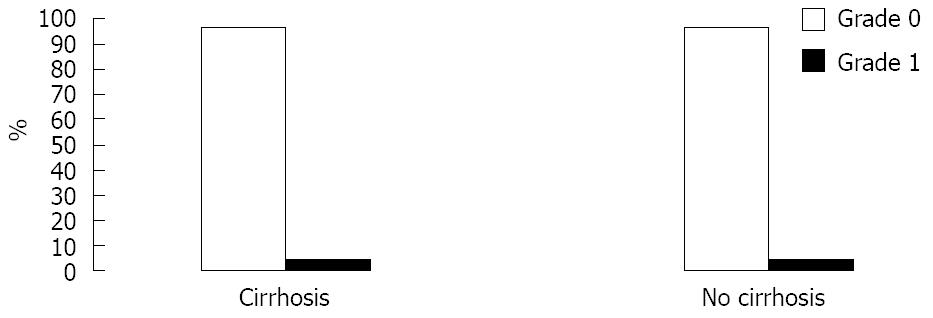Published online Nov 7, 2008. doi: 10.3748/wjg.14.6370
Revised: October 17, 2008
Accepted: October 24, 2008
Published online: November 7, 2008
AIM: To study the small bowel (SB) mucosa on biopsy in cirrhotic patients with portal hypertension and in non-cirrhotic controls and grade findings according to the Marsh criteria.
METHODS: We prospectively enrolled 51 consecutive patients undergoing an upper endoscopy for their routine medical care. Twenty five patients with cirrhosis and portal hypertension were compared to 26 controls. We obtained coeliac serology and multiple upper small bowel biopsies on all 51 patients. A GI pathologist interpreted biopsies and graded findings according to the Marsh criteria. We assessed equivalence in Marsh grade between cirrhotic and non-cirrhotic controls using the Mann-Whitney test for equivalence.
RESULTS: Gender, ethnicity and age were similar between both groups. Marsh grades were equivalent between the groups. Grade of 0 was present in 96% and grade of 1 was present in 4% of both groups and there was no villus atrophy or decrease in villus/crypt ratio in patients with portal hypertension.
CONCLUSION: This study provides evidence for the lack of villus atrophy in patients with cirrhosis and portal hypertension, and supports the continuous reliance on the Marsh criteria when the diagnosis of coeliac disease is to be made in the presence of cirrhosis.
- Citation: Wakim-Fleming J, Zein NN, Bennett A, Lopez R, Santisi J, Carey WD. Histological abnormalities of the small bowel mucosa in cirrhosis and portal hypertension. World J Gastroenterol 2008; 14(41): 6370-6375
- URL: https://www.wjgnet.com/1007-9327/full/v14/i41/6370.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6370
| Cirrhotic | Non-cirrhotic | P value | |
| n | 25 | 26 | - |
| Age | 57.5 ± 9.7 | 51.9 ± 15.3 | 0.12 |
| Gender | 0.49 | ||
| Male | 13 (52) | 11 (42.3) | |
| Female | 12 (48) | 15 (57.7) | |
| Ethnicity | 0.67 | ||
| Caucasian | 23 (92) | 22 (84.6) | |
| Other | 2 (8) | 4 (15.4) |
Studies of small bowel (SB) mucosa in cirrhosis and portal hypertension report a diverse spectrum of histological abnormalities[1-5]. These include increased capillary angiogenesis, mucosal edema, decreased villus to crypt ratio, villus atrophy and decreased total absorptive surface. Villus abnormalities resemble the abnormalities of coeliac disease (CD) and this may affect the interpretation of small bowel biopsy, and lead to confusion when CD is to be excluded in patients with cirrhosis and portal hypertension.
The diagnosis of CD is increasingly considered and work up recommended during the evaluation of abnormal liver enzymes[6-7]. This is in part due to the heightened awareness of the association of CD with a variety of liver disorders. Most commonly described associated liver disorders include autoimmune hepatitis, primary biliary cirrhosis, non-alcoholic fatty liver disease, unexplained abnormal liver tests and cirrhosis[8-15]. The mechanisms of this association are not clear and the prevalence of CD in patients with liver disease is variable depending on the associated liver disease. For example, while CD affects 1% of the general population, one study reports that about 4% of 185 cirrhotic patients who had undergone liver transplantation were found to have CD, and 3 of 4 patients presenting with severe liver disease were diagnosed with CD and were remitted as possible candidates for liver transplantation when placed on a gluten free diet[16]. Approximately 40% of patients with CD have abnormal liver enzymes, and these return to normal in 75% to 95% when a gluten free diet is instituted[16,17]. Based on these and other studies, it is recommended that clinicians should have a low threshold for testing for CD in patients with abnormal liver blood tests[6].
The diagnosis of CD is based on initial screening with coeliac serological tests (endomysial EMA and human tissue transglutaminase hTTG antibodies); but histological examination of SB biopsy is required for establishing a definite diagnosis[6,10]. However, in patients with cirrhosis, the diagnosis of coexisting CD is a challenge because of the reported similar changes on SB mucosa in both cirrhosis and CD. CD may, therefore, be under-diagnosed in cirrhosis. Studies of SB biopsy in cirrhosis[1-5], have thus shed doubt on the validity of biopsy in the diagnosis of CD in cirrhotic patients; but reported findings are poorly characterized, lack standardized grading, and of unclear significance. The current study was undertaken to determine if SB biopsies in cirrhosis show features that might mimic CD. Findings would determine if SB biopsy should be used in the diagnosis of CD in patients with cirrhosis and portal hypertension.
The aim of the study was to prospectively assess the histological abnormalities of the SB mucosa in patients with and without cirrhosis and portal hypertension by grading findings according to the grading system defined by Marsh[6].
This is a prospective case control study approved by the Institutional Board Review at the Cleveland Clinic. Eighty consecutive patients scheduled for an upper endoscopy EGD at the Cleveland Clinic between 9/1/2005 to 11/30/2005 were identified. Medical records were reviewed. Of 80 patients, 25 with cirrhosis and portal hypertension and 26 without cirrhosis, portal hypertension or liver disease fulfilled inclusion and exclusion criteria and were enrolled in the study.
Records reviewed included age, ethnicity, gender, indications for upper endoscopy EGD, imaging studies and laboratory tests. Laboratory tests obtained within the preceding 6 mo of the date of enrollment were reviewed for liver transaminases, alkaline phosphatase, protime/INR, celiac serology panel, complete blood count and differential, iron saturation and ferritin, and viral hepatitis panel.
Patients were included if they were older than 18 years old and able to give informed consent. Patients with cirrhosis and undergoing EGD were included regardless of the etiology of cirrhosis. Patients without cirrhosis and undergoing EGD for acid reflux, abdominal pain, dysphagia or vomiting were included.
Cirrhosis was defined histologically according to Batts and Ludwig staging system[18]. In patients without a liver biopsy, diagnosis of cirrhosis and portal hypertension was based on a combination of clinical data (jaundice, cutaneous spider angiomas, muscle wasting, ascites, and palmar erythema), biochemical data (decreased serum albumin and prolonged protime), imaging study (nodular surface on ultrasound or CT scan), and manifestations of portal hypertension (low platelets, splenomegaly, esophageal varices, hepatic encephalopathy or ascites) in the setting of chronic liver disease.
Patients were excluded if they were pregnant, on dialysis, had a bleeding disorder or were actively bleeding at the time of endoscopy, taking anticoagulants or had INR greater than > 1.5 or platelet count less than 30 × 103/mm3. Patients with a diagnosis of malabsorption, coeliac disease, patients taking corticosteroids or immunosuppressant drugs, patients with a history of Crohn’s disease, organ transplant, graft versus host disease, food allergies, iron deficiency anemia, osteoporosis, ataxia, and autoimmune disorders that could potentially be associated with CD such as thyroid disorders, dermatitis herpetiformis and type 1diabetes were excluded. In the control group, additional exclusions encompass individuals with a history of chronic liver disease or a history of abnormal liver tests.
Informed consent for SB biopsy and for blood draw for coeliac serology panel was obtained on all 51 patients. Severity of cirrhosis was assessed by calculating Child Turcotte Pugh (CTP) and Model for End-Stage Liver Disease (MELD) scores for all cirrhotic patients.
Coeliac serology panel included antibodies to human tissue transglutaminase (IgG and IgA for hTTG, QUANTA liteTM ELISA, Inova diagnostics, San Diego CA), endomysial antibodies (IgA EMA, Immunofluorescence Inova diagnostics San Diego CA), and total IgA levels by nephelometry (Beckman Coulter Immage/image 800 Immunochemistry system and Calibrator 1, Fullerton CA). Tests were consecutively analyzed in the immunology laboratory at the Cleveland Clinic. These tests are reported to be highly sensitive and specific in the diagnosis of CD in the general population with sensitivities and specificities above 85%, and they supplant the use of gliadin antibody testing as the preferred mean of serological detection[6]. Abnormal serology panel is any value for IgA EMA above 1:10 dilution, or any value for either IgG hTTG or IgA hTTG above 20 U.
Upper endoscopies were performed by gastroenterologists at the Cleveland Clinic. The gastroenterologists were not blinded to the study, and they were asked to obtain at least 3 biopsies from the second part of the duodenum or beyond on all subjects. Biopsy specimens were placed in vials containing 10% of buffered formalin solution for fixation. Paraffin sections were prepared and stained by hematoxylin and eosin (HE) stain. Pathology slides were interpreted by a Cleveland Clinic pathologist (A.B.) experienced with the spectrum of mucosal changes in CD. The pathologist was blinded to names and diagnosis. All slides were batched and read after samples from all 51 subjects collected and processed. The pathologist graded findings according to the Marsh grading system[6]. Marsh 0 is defined by normal mucosal and villus architecture, MarshIis defined by normal villus architecture, but increased numbers of intraepithelial lymphocytes, Marsh II shows increased intraepithelial lymphocytes, enlarged crypts and increased crypt cell division, Marsh III is defined by villus atrophy, shortened blunt villi and enlarged hyperplastic crypts. Marsh IV demonstrates hypoplastic mucosa.
The sample size estimation was based on the inference that the standard deviation of the Marsh grade would be equal to 1 for both groups. The study was designed to establish equivalence in small bowel mucosa (defined as a difference in mean Marsh grades no greater than1) between cirrhotics and non-cirrhotics with a significance level of 0.05 and a power of at least 90%. Therefore, it was estimated that a total of 25 subjects would be required in each group.
Descriptive statistics, such as frequencies for categorical factors and mean (SD) for continuous factors, were computed for all variables. A Student’s t-test was used to assess differences in age between cirrhotics with portal hypertension and non-cirrhotic controls. In addition, Pearson’s χ2 and Fisher’s exact test were used to compare gender and race between the groups.
In order to assess whether the SB mucosal architecture in cirrhotic patients with portal hypertension was indistinguishable from the mucosal architecture in non-cirrhotic controls, the Mann-Whitney test for equivalence was used[19]. It tests the null hypothesis (H0) of difference in SB mucosal architecture between groups versus the research hypothesis (Ha) of equivalent SB mucosal architecture between groups. A P-value < 0.05 was considered statistically significant. SAS version 9.1 software (SAS institute, Carey, NC) was used to carry out all analyses.
A total of 51 patients were enrolled. Twenty five had cirrhosis and portal hypertension and 26 controls had no evidence of liver disease or portal hypertension. Fifty patients had normal coeliac serology and one patient in each group had abnormal biopsy.
Baseline demographic characteristics of patients who fulfilled inclusion criteria are shown in Table 1. The mean age was 57 (± 10) years in the portal hypertension with cirrhosis group and 52 (± 15) years in the control group. Fifty two percent were males and 92% were Caucasians in the former group versus 42% and 84% respectively in the control group. There was no evidence to suggest statistically significant differences in the demographic characteristics between the groups (P > 0.05).
The most common indications for upper endoscopy in cirrhotic patients with portal hypertension were screening for or banding of esophageal varices (100%). In the control group, the most common indications were acid reflux (30.8%), dysphagia (23.1%), epigastric pain (23.1%), and nausea and vomiting in (15.4%).
All 25 patients with cirrhosis had evidence of portal hypertension, esophageal varices, thrombocytopenia and an imaging study showing cirrhotic liver. Fifteen patients had a liver biopsy. Twelve patients had CTP score A, 11 had CTP score B and 2 had CTP score C. The mean platelet count was 89.76 × 103/mm3, with a range between 45 and 148 × 103/mm3. The mean MELD score was 10 with a range between 5 and 17. The most common etiologies for cirrhosis were nonalcoholic steatohepatitis (24%), hepatitis C (24%) and cryptogenic cirrhosis (24%).
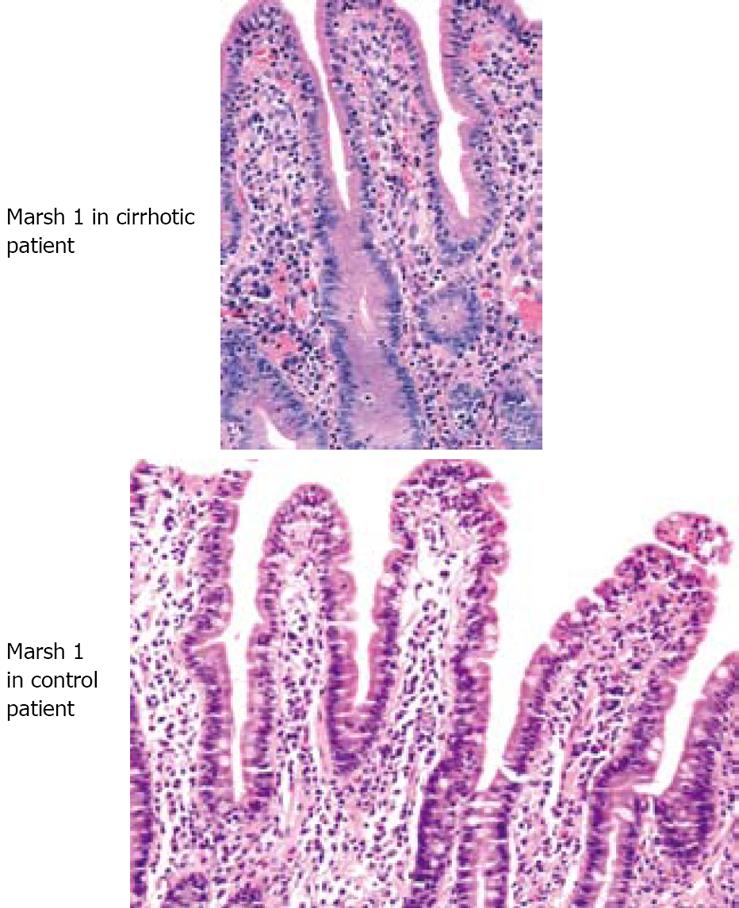
There was strong evidence to suggest that based on Marsh grade, cirrhotics and non-cirrhotics have indistinguishable SB mucosa (Mann-Whitney test of equivalence: P < 0.01). One patient in each group (4%) had an abnormal small bowel biopsy and 96% of patients from each group had a normal small bowel biopsy (Figures 1 and 2). Both patients with abnormal biopsy were females with normal coeliac serology. The two women had Marsh gradeIbased on 3 SB biopsies obtained for each. One of these women was 57 years old, and had a liver biopsy that was consistent with nonalcoholic steatohepatitis and cirrhosis. Her platelet level was 130 × 103/mm3, her MELD score was 10 and her CTP score was B. The other woman with Marsh gradeIon SB biopsy did not have cirrhosis. She was 51 years old and was scheduled for EGD for epigastric pain and bloating.
Portal hypertension and cirrhosis due to a variety of parenchymal liver diseases are associated with malnutrition[20,21]. The pathophysiologic mechanisms are not totally understood and several factors may be involved. Malabsorption has been implicated and this was presumed to be related to changes in the SB villi[2,4,5]. For example, Such et al[4] investigated 6 patients with cirrhosis using jejunal biopsies, and studied the mucosa under electron microscopy. The authors observed that “the microvilli were reduced in number and appeared shorter and thicker when compared to controls”, and their conclusion was that “the total absorptive surface may be reduced in cirrhotic patients”. Misra[2] found a significant decrease in villus/crypt ratio in cirrhotic patients when compared to healthy volunteers. On the other hand, Nagral[22] reports a significant number of patients with large vessels in duodenal mucosa of patients with portal hypertension in comparison with controls, but did not find a statistical difference in severity and type of infiltrate, edema of lamina propria or villus/crypt ratio between the groups. In the study of Barakat[5], abnormal villus changes were present in 11.4% of portal hypertensive patients. These were described as “shortened villi, decreased or even reversed villus to crypt ratio down to total villus atrophy”. The authors implied that these changes might have an effect on the intestinal absorptive functions, and in turn, might have a share in the pathogenesis of nutritional derangements in portal hypertensive patients.
Results of these studies implicate SB villus shortening and atrophy as underlying factors in the malabsorption and malnutrition observed in cirrhosis. Such conclusions need further validation and other diseases that may affect the SB mucosa should be considered in the differential diagnosis. Furthermore, histological abnormalities were reported descriptively and without systematic classification. Lack of a proper classification of abnormalities was once described by Marsh[23] as follows: “The system of qualitative terminology is not only inappropriate but also seems to have paralyzed any new intellectual activity that might elucidate afresh the immunopathogenic basis of mucosal response”. And in 1992, Marsh described the mucosal abnormalities of the SB by establishing a classification system that utilized inflammatory and atrophic grades. This grading system has since been adopted by clinicians and pathology researchers in the diagnosis and study of CD, and is the only available system to systematically grade mucosal abnormalities of the SB. In this unprecedented study, we prospectively analyzed and graded changes of SB mucosa on biopsy according to the unified and accepted grading system characterized by Marsh[6,10].
We found no difference in Marsh grade between the study groups. Our patients had either a grade of 0 or a grade ofI. One patient in each group had a Marsh grade ofI, but neither had abnormal coeliac serology. The presence of Marsh gradeIor intraepithelial lymphocytes is a non-specific finding especially in the absence of coexistent abnormal serology. We did not find any Marsh grades II, III or villus/crypt changes of CD among patients in either group. This provides evidence that histological abnormalities of the SB mucosa are equivalent between cirrhotic patients with portal hypertension and non-cirrhotic controls.
Since the etiology of cirrhosis has not been shown to influence the changes in mucosal architecture of the SB, we included all eligible cirrhotic patients regardless of the etiology of their cirrhosis. We excluded patients with clinical and laboratory evidence of malabsorption, patients with known SB mucosal disease and/or on therapy that included corticosteroids and immunosuppressants, and patients with autoimmune diseases that have the potential to be associated with CD or induce changes in the SB mucosa, because such cases may not necessarily yield the direct effects of cirrhosis on the SB mucosa.
We calculated the MELD and CTP scores on all cirrhotic patients. Although our study did not aim to examine whether the severity of liver disease would further exacerbate villus damage, we did not observe any correlation between Marsh grade and the degree of MELD and CTP scores. This has been reported previously[22].
An exact quantitative count for the intraepithelial lymphocytes IEL and immunohistochemical typing were not obtained because such stains are not utilized in routine clinical practice, and because one observer (pathologist A.B.) interpreted and analyzed all biopsy specimens.
Portal hypertensive enteropathy is receiving increased recognition in research studies of recent years. Studies have aimed at characterizing abnormalities on endoscopy, wireless capsule imaging, and SB biopsy. Small bowel biopsy findings describe villus changes and villus atrophy. Contrary to previously reported studies, our study did not show shortened villi, reversal of villus to crypt ratio, villus atrophy, crypt hyperplasia or changes suggestive of CD in patients with portal hypertension. This concurs with the study of Nagral[22].
Our study is unique and different from other studies of the effects of cirrhosis and portal hypertension on the SB mucosa because we used the validated Marsh grading system to grade for abnormalities and we excluded subjects with CD and other diseases that would potentially affect the SB mucosa such as Crohn’s and lymphoma etc, because we intended to study the sole effect of cirrhosis on the SB. SB villus changes and atrophy are characteristic, but are not specific for coeliac disease[24,25].
Coeliac serological tests have low sensitivities and specificities in chronic liver disease and cirrhosis[15,26-29] which emphasizes the importance of validating SB biopsy as the most important tool for the diagnosis of CD in this group of patients. Results of this study support the use of SB biopsy in patients with cirrhosis and portal hypertension when the diagnosis of CD is suspected. Furthermore, and since cirrhosis is not associated with significant inflammatory or atrophic changes of SB villi, we can extrapolate that there is a low probability that different results would be seen in the earlier stages of liver disease when cirrhosis has not yet developed.
Due to the estimated high prevalence of CD in patients with cirrhosis that is higher than in the general population[8,16,29,30], and the potential reversibility of liver dysfunction on a gluten free diet, we recommend prompt consideration and exclusion of SB mucosal diseases and CD (by using a combination of laboratory, clinical, pathologic and genetic examinations, and response to a gluten free diet), when shortened villi, reversal of villus to crypt ratio or villus atrophy are seen in patients with cirrhosis.
As to the mechanisms of malabsorption reported in patients with cirrhosis and portal hypertension, factors other than cirrhosis- related- villus changes should be considered. These may include bile salt deficiencies, motility disorders, protein losing enteropathy and other concomitant diseases of the SB. Further studies are needed.
In conclusion, our study provides evidence for the lack of villus atrophy in patients with cirrhosis and portal hypertension. Hence, small bowel biopsies can be interpreted reliably to exclude coeliac disease in these patients. Furthermore, findings of shortened villi and villus atrophy should trigger the exclusion of coeliac disease and other diseases of the small bowel in this group of patients.
Cirrhosis affects the small bowel mucosa in ways not totally elucidated. Villus shortening and atrophy are described. But these findings are reported descriptively and without proper classification, which may affect the interpretation of small bowel biopsy when the diagnosis of coeliac disease is to be excluded in patients with cirrhosis. We aimed to study the small bowel mucosa on biopsy in cirrhotic patients with portal hypertension and in non-cirrhotic controls and grade findings according to the standardized grading system described by Marsh.
Studies of recent years have shown that coeliac disease is increasingly recognized in association with chronic liver disease and cirrhosis, and coeliac disease is more common than previously thought but is under diagnosed. It is, therefore, recommended to exclude coeliac disease in patients who have cirrhosis and cryptogenic abnormality of liver tests, and the diagnostic tool of choice is a small bowel biopsy.
This study is different from other studies of the small bowel mucosa in cirrhosis and portal hypertension because we graded findings according to the standardized Marsh grading system and excluded subjects with diseases that could potentially affect the small bowel in order to assess the sole effect of portal hypertension on the small bowel mucosa. Additionally, we used the Mann-Whitney test of equivalence to support the equality of mucosal abnormalities in both study groups, and we estimated a sample size of 25 individuals in each group in order to confer a statistical power of at least 90%.
Today’s medical practice emphasizes evidence based medicine. Our study provides evidence for the lack of villus atrophy in cirrhosis and portal hypertension; hence SB biopsies can be interpreted reliably to exclude coeliac disease in these patients and in future studies of the mechanisms of liver disease in patients with coeliac disease. Additionally, small bowel mucosal diseases should be excluded when villus shortening and atrophy are seen in patients with cirrhosis and portal hypertension
This is a well designed study, and the data looks sound. Patients with liver disease of unknown etiology can have small bowel biopsies that reliably exclude coeliac disease as an association or cause of their hepatic disease.
Peer reviewer: Ned Snyder, MD, FACP, AGAF, Professor of Medicine, Chief of Clinical Gastroenterology and Hepatology, Department of Internal Medicine, The University of Texas Medical Branch, 301 University Blvd., Galveston, Texas 77555-0764, United States
S- Editor Tian L E- Editor Ma WH
| 1. | Baraona E, Orrego H, Fernandez O, Amenabar E, Maldonado E, Tag F, Salinas A. Absorptive function of the small intestine in liver cirrhosis. Am J Dig Dis. 1962;7:318-330. |
| 2. | Misra V, Misra SP, Dwivedi M, Gupta SC. Histomorpho-metric study of portal hypertensive enteropathy. Am J Clin Pathol. 1997;108:652-657. |
| 3. | Astaldi G, Strosselli E. Peroral biopsy of the intestinal mucosa in hepatic cirrhosis. Am J Dig Dis. 1960;5:603-612. |
| 4. | Such J, Guardiola JV, de Juan J, Casellas JA, Pascual S, Aparicio JR, Sola-Vera J, Perez-Mateo M. Ultrastructural characteristics of distal duodenum mucosa in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2002;14:371-376. |
| 5. | Barakat M, Mostafa M, Mahran Z, Soliman AG. Portal hypertensive duodenopathy: clinical, endoscopic, and histopathologic profiles. Am J Gastroenterol. 2007;102:2793-2802. |
| 6. | AGA Institute Medical Position Statement on the Diagnosis and Management of Celiac Disease. Gastroenterology. 2006;131:1977-1980. |
| 7. | Morisco F, Pagliaro L, Caporaso N, Bianco E, Sagliocca L, Fargion S, Smedile A, Salvagnini M, Mele A. Consensus recommendations for managing asymptomatic persistent non-virus non-alcohol related elevation of aminotransferase levels: suggestions for diagnostic procedures and monitoring. Dig Liver Dis. 2008;40:585-598. |
| 8. | Bardella MT, Valenti L, Pagliari C, Peracchi M, Fare M, Fracanzani AL, Fargion S. Searching for coeliac disease in patients with non-alcoholic fatty liver disease. Dig Liver Dis. 2004;36:333-336. |
| 9. | Rubio-Tapia A, Murray JA. The liver in celiac disease. Hepatology. 2007;46:1650-1658. |
| 10. | National Institutes of Health Consensus Development Conference Statement on Celiac Disease, June 28-30, 2004. Gastroenterology. 2005;128:S1-S9. |
| 11. | Kingham JG, Parker DR. The association between primary biliary cirrhosis and coeliac disease: a study of relative prevalences. Gut. 1998;42:120-122. |
| 12. | Ludvigsson JF, Elfstrom P, Broome U, Ekbom A, Montgomery SM. Celiac disease and risk of liver disease: a general population-based study. Clin Gastroenterol Hepatol. 2007;5:63-69.e1. |
| 13. | Volta U, De Franceschi L, Lari F, Molinaro N, Zoli M, Bianchi FB. Coeliac disease hidden by cryptogenic hypertransaminasaemia. Lancet. 1998;352:26-29. |
| 14. | Hagander B, Berg NO, Brandt L, Norden A, Sjolund K, Stenstam M. Hepatic injury in adult coeliac disease. Lancet. 1977;2:270-272. |
| 15. | Germenis AE, Yiannaki EE, Zachou K, Roka V, Barbanis S, Liaskos C, Adam K, Kapsoritakis AN, Potamianos S, Dalekos GN. Prevalence and clinical significance of immunoglobulin A antibodies against tissue transglutaminase in patients with diverse chronic liver diseases. Clin Diagn Lab Immunol. 2005;12:941-948. |
| 16. | Kaukinen K, Halme L, Collin P, Farkkila M, Maki M, Vehmanen P, Partanen J, Hockerstedt K. Celiac disease in patients with severe liver disease: gluten-free diet may reverse hepatic failure. Gastroenterology. 2002;122:881-888. |
| 17. | Bardella MT, Fraquelli M, Quatrini M, Molteni N, Bianchi P, Conte D. Prevalence of hypertransaminasemia in adult celiac patients and effect of gluten-free diet. Hepatology. 1995;22:833-836. |
| 18. | Ludwig J, Batts KP, Moyer TP, Poterucha JJ. Advances in liver biopsy diagnosis. Mayo Clin Proc. 1994;69:677-678. |
| 19. | Wellek S. Testing Statistical Hypotheses of Equivalence. 1st ed. Boca Ration, London, New York, Washington, D.C. : Chapman & Hall/CRC Press 2003; 106-111. |
| 20. | Henkel AS, Buchman AL. Nutritional support in patients with chronic liver disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:202-209. |
| 21. | Hogenauer C, Hammer HF. Maldigestion and Malabsorption. 8th ed. Saunders: An Imprint of Elsevier 2006; 2200-2232. |
| 22. | Nagral AS, Joshi AS, Bhatia SJ, Abraham P, Mistry FP, Vora IM. Congestive jejunopathy in portal hypertension. Gut. 1993;34:694-697. |
| 23. | Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology. 1992;102:330-354. |
| 24. | Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731-1743. |
| 25. | Farrell RJ, Kelly CP. Celiac Sprue and Refractory Sprue. 8th ed. Saunders: An Imprint of Elsevier 2006; 2277-2307. |
| 26. | Bizzaro N, Tampoia M, Villalta D, Platzgummer S, Liguori M, Tozzoli R, Tonutti E. Low specificity of anti-tissue transglutaminase antibodies in patients with primary biliary cirrhosis. J Clin Lab Anal. 2006;20:184-189. |
| 27. | Vecchi M, Folli C, Donato MF, Formenti S, Arosio E, de Franchis R. High rate of positive anti-tissue transgluta-minase antibodies in chronic liver disease. Role of liver decompensation and of the antigen source. Scand J Gastroenterol. 2003;38:50-54. |
| 28. | Valera JM, Hurtado C, Poniachik J, Abumohor P, Brahm J. [Study of celiac disease in patients with non-alcoholic fatty liver and autoimmune hepatic diseases]. Gastroenterol Hepatol. 2008;31:8-11. |
| 29. | Lo Iacono O, Petta S, Venezia G, Di Marco V, Tarantino G, Barbaria F, Mineo C, De Lisi S, Almasio PL, Craxi A. Anti-tissue transglutaminase antibodies in patients with abnormal liver tests: is it always coeliac disease? Am J Gastroenterol. 2005;100:2472-2477. |
| 30. | Volta U. Pathogenesis and Clinical Significance of Liver Injury in Celiac Disease. Clin Rev Allergy Immunol. 2009;36:62-70. |