Published online Nov 7, 2008. doi: 10.3748/wjg.14.6355
Revised: September 16, 2008
Accepted: September 23, 2008
Published online: November 7, 2008
AIM: To elucidate the metabolism and the effect of the cyclosporin A (CyA) as a representative immunosuppressive drug used in transplantation in a partially hepatectomized rat model.
METHODS: CyA was administered to rats that underwent a 70% hepatectomy. These rats were randomly assigned into three groups according to the dose of CyA administration as follows; (group 1) water, (group 2) 5 mg/kg CyA, (group 3) 10 mg/kg CyA. On postoperative days-1, 3, 7 and 14, the rats were killed to analyze the serum concentration of CyA, the liver regeneration ratio, biochemical or histological markers, and mRNA expression using reverse transcriptase-polymerase chain reaction method to determine albumin and cytochrome p450 expression.
RESULTS: The serum concentration of CyA in group 3 was significantly higher than group 2 during liver regeneration. CyA enhanced the liver regeneration in a dose dependent manner. The mRNA expression associated with CyA metabolism was significantly decreased on day 14, while preserving the albumin producing activity.
CONCLUSION: These data indicate that the p-450 activity required to metabolize the CyA may be reduced during regeneration of the remnant liver after a hepatectomy, which may, therefore, be linked to difficulty in controlling the optimal dose of CyA during early period of LDLT.
- Citation: Nagayoshi S, Kawashita Y, Eguchi S, Kamohara Y, Takatsuki M, Miyamoto S, Mochizuki S, Soyama A, Tokai H, Hidaka M, Tajima Y, Kanematsu T. Metabolism for cyclosporin A during liver regeneration after partial hepatectomy in rats. World J Gastroenterol 2008; 14(41): 6355-6359
- URL: https://www.wjgnet.com/1007-9327/full/v14/i41/6355.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6355
| Gene | Sequence (5’-3’ sense/antisense) | Reaction condition | Product size | Cycles | ||
| Denaturation | Annealing | Elongation | ||||
| GAPDH | TTCAACGGCACAGTCAAG | 95°C, 1 min | 60°C, 1 min | 72°C, 2 min | 240 bp | 26 |
| CACACCCATCACAAACAT | ||||||
| CYP3A2 | TACTACAAGGGCTTAGGGAG | 94°C, 1 min | 60°C, 1 min | 72°C, 2 min | 348 bp | 27 |
| CTTGCCTGTCTCCGCCTCTT | ||||||
| ALB | ATACACCCAGAAAGCACCTC | 94°C, 1 min | 60°C, 1 min | 72°C, 2 min | 305 bp | 27 |
| CAGAGTGGAAGGTGAAGGTC | ||||||
Orthotopic liver transplantation is an established treatment for patients with end-stage liver disease. However, donor organ shortages remain extremely problematic. To address this issue, living donor liver transplantation (LDLT) was developed[1]. During transplantation, the liver graft is subjected to a variety of potential hepatic injuries including ischemic injury associated with organ harvesting and the obligate storage before revascularization, reperfusion injury following revascularization, immunological attack caused by the immune system of the recipient, toxicity of certain drugs used during the post-transplant period, and certain infections[2,3]. After transplantation the liver graft goes into a regeneration process, which may be important for the overall success of the transplant procedures. Notably, the liver graft must be capable of normal growth, repair, and regeneration in the presence of immunosuppressive drugs such as calcineurin inhibitors. The aim of the present study was, therefore, to investigate the pharmacokinetics of cyclosporin A (CyA)[4] and its effect on liver regeneration and metabolic activity to elucidate the mechanism of metabolic activity and serum concentration of cyclosporin A as an example of calcineurin inhibitors administered during liver regeneration in a rat model.
Adult male Sprague Dawley rats, weighting 250-320 g (CRJ Charles River Japan, Kanagawa, Japan), were provided with water, and a standard laboratory diet ad libitum. All of the studies were performed according to the rules and regulations of the University of Nagasaki Research Animal Resources Guidelines.
A 70% hepatectomy was carried out according to the method described by Higgins and Anderson[5] under light ether anesthesia. Surgery was performed between 9:00 and 12:00 a.m. to avoid diurnal variation in the regenerative responses. The rats were randomly assigned to three groups, and treated daily by gavage beginning immediately after the hepatectomy. Group 1 animals were given water. Group 2 animals received 5 mg/kg CyA (Neoral®, Novartis Pharma, Basel) and group 3 animals received 5 or 10 mg/kg CyA. These CyA doses were selected based on the results reported by Morii et al[6].
In each group, five rats were killed before and at day 1, 3, 7 and 14 after the hepatectomy. Immediately before they were sacrifices, blood samples were obtained from the inferior vena cava. The remnant liver was removed to investigate hepatic restoration. The experimental protocol is demonstrated in Figure 1.
The serum concentration of CyA was measured in the whole blood by fluorescence polarization according to the manufacturer’s protocols (AxSYM® analyzer, Abbott, Tokyo)[7].
The liver regeneration ratio in each experiment was defined as the ratio of the remaining liver weight to the initial body weight.
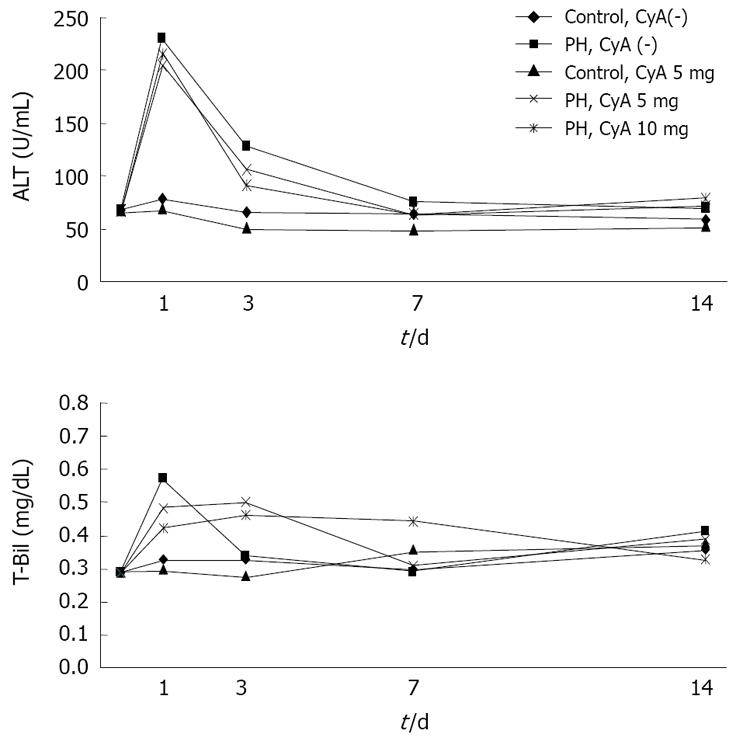
To evaluate liver toxicity of CyA administration, plasma concentrations of alanine aminotransferase (ALT) and total bilirubin (T-Bil) were examined using an automated analyzing system according to the manufacturer’s protocol.
Total hepatic RNA was prepared by the method as described previously[8] and used for the determination of the expression levels of albumin (ALB) and cytochrome-P 3A2 (CYP3A2). In addition, the level of gene expression of glyceral-dehyde-3-phosphate-dehydrogenase (GAPDH) was measured as an internal control. Complementary DNA (c-DNA) was prepared from total RNA by the method described previously[8]. The primers used in the present study are listed in Table 1.
The data are expressed as the mean ± SD (n = 5). Statistical analyses were performed by unpaired, two tailed Student’s t-test. A P value less than 0.05 was considered to be significant.
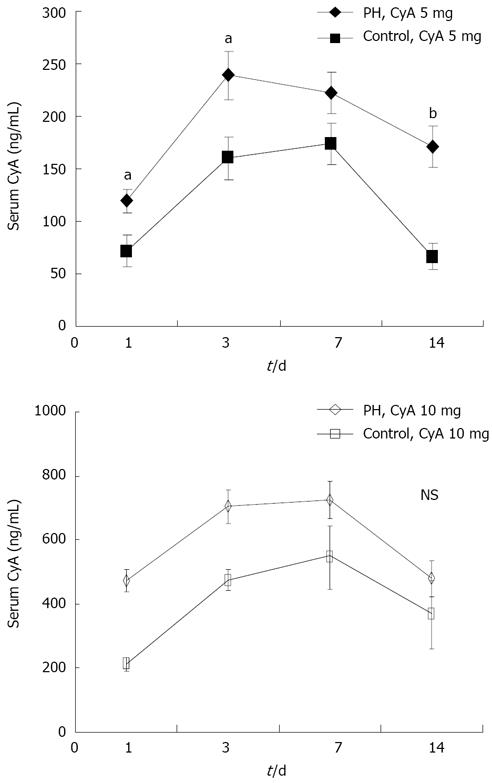
Figure 2 shows that the concentration of CyA reached a maximum during 3 to 7 d, and gradually declined thereafter. The levels of CyA in the PH group were significantly higher than that in control group.
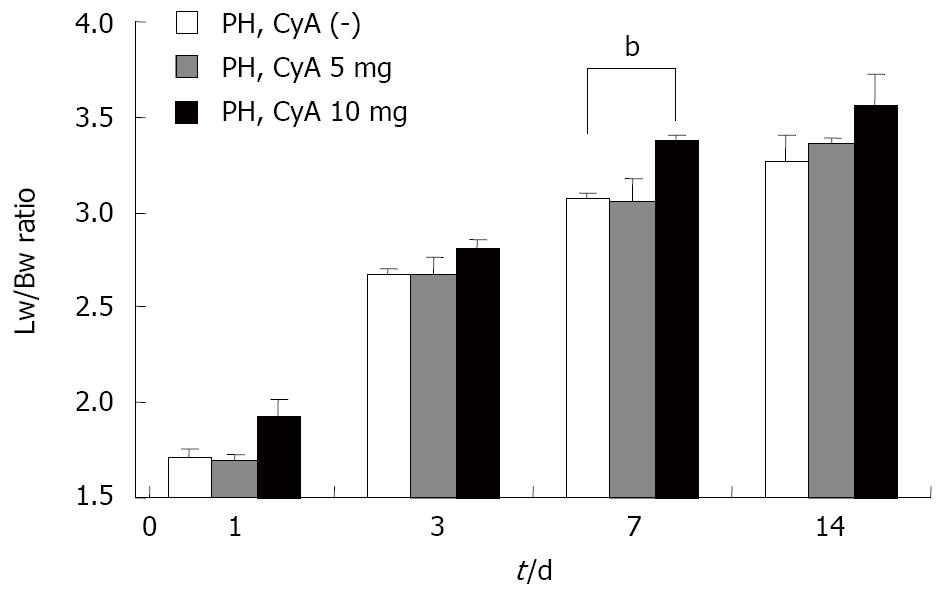
As shown in Figure 3, the lower concentration of CyA (5 mg) did not affect the liver regeneration potential during the observation period; however, the rate of liver regeneration was significantly higher than that in the low CyA group on postoperative day 7.
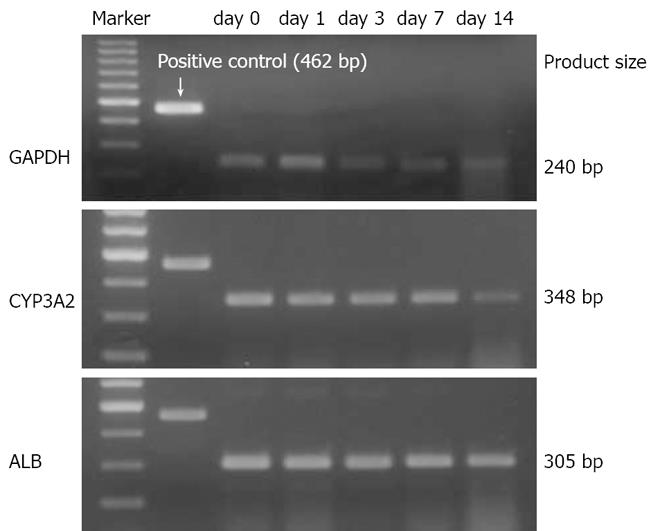
Alb mRNA expression remained constant during liver regeneration, while hepatocyte specific p450 activity-CYP3A2 was significantly reduced on postoperative day 14 (Figure 4).
Rats were anesthetized and blood samples were collected through the tail vein at the indicated time points. ALT and T-Bil levels were measured as indicators of liver function. On day 1, plasma ALT concentrations increased during the first 24 h after the hepatectomy and then decreased gradually returning to the preoperative values at 72 h. There was no significant difference between the groups (Figure 5).
As shown in Figure 5, the ALT level in control animals were slightly increased, and thereafter gradually reduced. There was no statistically significant difference in any of the groups.
The present study, investigated the pharmacokinetics of the CyA in a rat two thirds hepatectomy model, for the first time. The results yielded important information concerning the interrelationship between the CyA and regenerating liver. (1) The metabolism is retarded in a regenerating liver, which is actually seen in clinical partial liver transplantation. (2) CyA has possible hepatotrophic effect on the regenerating liver in a CyA-dose dependent manner. (3) The p450 activity of the regenerating liver was down-regulated after CyA administration.
As expected, the serum concentrations of CyA after a hepatectomy were significantly higher than that seen in the sham operated group as previously reported in clinical settings. There are several possible explanations for this, including increased absorption, decreased volume of distribution, or decreased clearance.
However, an increased absorption is not likely. The CyA used in this study was the microemulsified type, and the absorption is bile independent. Therefore, absorption of the CyA was table in both groups[9]. The volume of distribution should be smaller in the partially hepatectomized rats. In other words, a smaller volume of distribution could increase the relative blood level of an immunosuppressant for a given dose.
Another possibility for the higher levels of CyA after the partial hepatectomy is reduced hepatic immunosuppressive clearance, which may be explained by two possible mechanisms. One is simply because of the reduced hepatic mass available to metabolize the drugs. Another possibility is that immediately after hepatectomy, the hepatic mass is reduced because of the surgical excision of hepatic tissue. As a result, the ability to clear substances through the liver is reduced. For instance, the indocyanine green half-life is increased four-fold after a 60% hepatectomy and by 33% after a 40% hepatectomy[10]. In the rats after a two thirds hepatectomy, the whole-organ reduced form of- cytochrome c reductase and cytochrome p-450 activity are reduced by half. After a 90% hepatectomy, galactose clearance in rats was reduced by 90% within 24 h after surgery. The genetic data regarding the cytochrome p-450 gene suggested that the metabolic activity of specific enzymes responsible for drug metabolism is reduced in the remaining hepatic tissue. Marie et al reported that the cytochrome p-450 activity decreased soon after a two-thirds hepatectomy in rats, returning to 90% of the initial activity 2 wk postoperatively. In the present study, the expression analysis of cytochrome p450 activity was performed using RT-PCR method. The results showed the cytochrome p-450 activity remained at the initial stage of liver regeneration, finally declined in the late stages. Although, no other liver specific enzymes were examined, a previous study demonstrated that the levels of mRNA for enzymes responsible for gluconeogenesis and the acute phase proteins are increased up to four fold after a hepatectomy[11]. Therefore, there are adaptive changes in the hepatic tissue after a partial hepatectomy. Collectively, the activity of enzymes that support hepatic regeneration is increased, whereas the activity of the enzymes responsible for drug metabolism is reduced.
The present data also suggest that cyclosporine enhances the hepatic regenerative response without affecting the individual hepatocellular function. Among immunosuppressive drugs currently in clinical use, azathioprine and steroids have been reported to exert an antiproliferative action on the regenerating liver. Azathioprine inhibits the DNA or RNA synthesis of hepatocytes, acting as an antimetabolite, whereas the action of steroids is more complex. Several investigators have suggested a functional linkage between lymphoid tissues and hepatocytes. Craddock et al reported that a partial hepatectomy induced proliferation of hepatocytes as well as lymphoid tissues. Another study recently suggested a very close and positive interrelationship between hepatocyte replication and lymphocyte activities[12]. The new potent immunosuppressor, cyclosporin A has been extensively compared with azathioprine and steroids. It primarily inhibits T-lymphocyte responses, and has no functional effects on other hematopoietic cells or phagocytic cells[13-16]. The present study on hepatectomized rats confirmed the antimitotic action of these immunosuppressants on hepatocytes, although the degree of suppression was less than that seen in previous reports.
Notably, there seems to be some discrepancy in these data showing that the statistical difference of the serum concentration of CyA between 5 mg treated and control animals was not observed in the groups treated with 10 mg of CyA treated as demonstrated in Figure 2. However, this is probably a reflection of-the fact that the liver regenerative effect of CyA at a higher dose may improve the impaired metabolic potential for CyA itself.
The limitation of this study is that this model potentially does not require immunosuppresion; therefore, further research will be needed to elucidate the underlining mechanism for these findings in a partial liver transplant model.
In conclusion, these results indicate that CyA levels in hepatectomized rats were significantly higher in control rats without a hepatectomy, probably because of the decreased volume of distribution, and/or decreased clearance by reduced metabolic activity. The possible hepatotrophic effect of CyA on the regenerating liver has also been confirmed.
Peer reviewer: Dr. Yogesh K Chawla, Professor, Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Tian L L- Editor Alpini GD E- Editor Ma WH
| 1. | Chen CL, Fan ST, Lee SG, Makuuchi M, Tanaka K. Living-donor liver transplantation: 12 years of experience in Asia. Transplantation. 2003;75:S6-S11. |
| 2. | Burton JR Jr, Rosen HR. Diagnosis and management of allograft failure. Clin Liver Dis. 2006;10:407-435, x. |
| 3. | Said A, Einstein M, Lucey MR. Liver transplantation: an update 2007. Curr Opin Gastroenterol. 2007;23:292-298. |
| 4. | Pichlmayr R, Neuhaus P, Ringe B, Wonigeit K, Burdelski M, Verner L, Lauchart W, Schmidt FW. Developments in liver transplantation. Jpn J Surg. 1985;15:409-419. |
| 5. | Higgins G, Anderson R. Experimental pathology of the liver. Arch Pathol. 1931;12:186-202. |
| 6. | Morii Y, Kawano K, Kim YI, Aramaki M, Yoshida T, Kitano S. Augmentative effect of cyclosporin A on rat liver regeneration: influence on hepatocyte growth factor and transforming growth factor-beta(1). Eur Surg Res. 1999;31:399-405. |
| 7. | Sabate I, Liron FJ, Gonzalez Alba JM, Ginard M, Virgili J, Baro S, Figueras J, Gonzalez Segura C, Jaurrieta E. Comparison of cyclosporine immunoassays (AxSYM and RIA) for assessing pharmacokinetic parameters in liver transplant patients. Transplant Proc. 1999;31:2421-2422. |
| 8. | Konno Y, Sekimoto M, Nemoto K, Degawa M. Sex difference in induction of hepatic CYP2B and CYP3A subfamily enzymes by nicardipine and nifedipine in rats. Toxicol Appl Pharmacol. 2004;196:20-28. |
| 9. | Tredger JM. Using cyclosporine Neoral immediately after liver transplantation. United Kingdom Neoral Pilot Study Group. Ther Drug Monit. 1995;17:638-641. |
| 10. | Prasse KW, Bjorling DE, Holmes RA, Cornelius LM. Indocyanine green clearance and ammonia tolerance in partially hepatectomized and hepatic devascularized, anesthetized dogs. Am J Vet Res. 1983;44:2320-2323. |
| 11. | Tygstrup N, Jensen SA, Krog B, Pietrangelo A, Shafritz DA. Expression of messenger RNA for liver functions following 70% and 90% hepatectomy. J Hepatol. 1996;25:72-78. |
| 12. | Sakai A, Pfeffermann R, Kountz SL. Liver regeneration and lymphocyte activation. Surg Gynecol Obstet. 1976;143:914-918. |
| 13. | White DJ, Calne RY, Plumb A. Mode of action of cyclosporin A: a new immunosuppressive agent. Transplant Proc. 1979;11:855-859. |
| 14. | Hellman A, Goldman JM. Effects of cyclosporin A on human granulopoiesis in vitro. Transplantation. 1980;30:386-387. |
| 15. | White DJ, Plumb AM, Pawelec G, Brons G. Cyclosporin A: an immunosuppressive agent preferentially active against proliferating T cells. Transplantation. 1979;27:55-58. |