Published online Nov 7, 2008. doi: 10.3748/wjg.14.6347
Revised: October 26, 2008
Accepted: November 2, 2008
Published online: November 7, 2008
AIM: To explore the mechanism for interactions of leptin with ghrelin and orexin in the arcuate nucleus (ARC) activating neuropeptide Y (NPY) neurons during physiological regulation of feeding.
METHODS: Single neurons from ARC of adult rats with matured feeding function were isolated. [Ca2+]i was measured to monitore their activities. The time course of leptin effects on ghrelin-induced versus orexin-induced [Ca2+]i increases in NPY neurons was studied.
RESULTS: Administration of ghrelin or orexin-A at 10-10 mol/L increased cytosolic Ca2+ concentration ([Ca2+]i) in NPY neurons isolated from the ARC of adult rats. Upon administration of leptin at 10-14-10-12 mol/L, ghrelin-induced [Ca2+]i increases were initially (< 10 min) inhibited but later restored, exhibiting a transient pattern of inhibition. In contrast, orexin-induced [Ca2+]i increases were inhibited by leptin in a long-lasting manner. Furthermore, a prior administration of leptin inhibited orexin action but not ghrelin action to increase [Ca2+]i.
CONCLUSION: Leptin counteracted ghrelin effects transiently and orexin effects long-lastingly in NPY neurons. The transient property with which leptin counteracts ghrelin action in NPY neurons may allow the fasting-associated increase in ghrelin levels to activate NPY neurons in the presence of physiological leptin and to stimulate feeding.
- Citation: Kohno D, Suyama S, Yada T. Leptin transiently antagonizes ghrelin and long-lastingly orexin in regulation of Ca2+ signaling in neuropeptide Y neurons of the arcuate nucleus. World J Gastroenterol 2008; 14(41): 6347-6354
- URL: https://www.wjgnet.com/1007-9327/full/v14/i41/6347.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6347
Food intake is controlled by the feeding regulatory centers, in which the arcuate nucleus (ARC) in the hypothalamus is considered the first order center that senses and integrates a variety of central and peripheral factors[1]. In the ARC, neuropeptide Y (NPY) neurons that coexpress agouti-related peptide (AgRP) are mandatory for feeding[2,3], while proopiomelanocortin (POMC) neurons are essential for satiety[1]. Orexin-A and -B (hypocretin-1 and -2) are orexigenic peptides[4] localized in neurons in the lateral hypothalamus (LH)[5], an area implicated in feeding behavior[6]. Fasting and lowering glucose concentrations increase prepro-orexin mRNA level[4] and cytosolic Ca2+ concentration ([Ca2+]i) in orexin neurons[7], suggesting a possible physiological role of orexins in feeding. Ghrelin is an orexigenic peptide released predominantly from the stomach[8-10] and also from the intestine and pancreas[11-13]. Ghrelin is the only one orexigenic peptide of the peripheral origin known. The plasma concentration of ghrelin increases before meals and rapidly declines upon food intake[14]. Orexin levels might also change during a day, since this peptide is implicated in regulation of sleep/wakefulness[15]. These rhythmic changes in ghrelin and orexin levels may alter their inputs on the feeding center and thereby regulate feeding. Both ghrelin[9,16-17] and orexin[18] stimulate food intake primarily by activating NPY neurons in the ARC.
Leptin, a powerful anorectic hormone produced and released from the adipocytes, is present constantly in the plasma at the nanomolar range[14] and is considered to enter the brain through the blood-brain barrier[19]. Therefore, leptin is likely to act continuously on the feeding center and thereby modulate the efficacy of orexigenic substances. The primary action of leptin in the feeding center is inhibition of NPY neurons as well as activation of POMC neurons in the ARC. The obesity syndrome in ob/ob mice resulting from lack of functional leptin is attenuated by the loss of neuropeptide Y[20]. Therefore, elucidation of the interaction of leptin with ghrelin and with orexin in the ARC NPY neurons may provide a clue to understand the neuronal mechanisms for physiological regulation of feeding. It has recently been shown that leptin counteracts ghrelin action to increase cytosolic free [Ca2+]i in NPY neurons in the ARC, and that the PI3-kinase (phosphatidylinositol 3-kinase)-PDE3 (phosphodiesterase 3) signaling plays a key role in the leptin action[17]. In the present study, we isolated single neurons from ARC of adult rats with matured feeding function and monitored their activities by measuring [Ca2+]i. We studied the time course of leptin effects on ghrelin-induced vs orexin-induced [Ca2+]i increases in NPY neurons.
Adult male Sprague-Dawley (SD) rats were maintained on a 12-h light/dark cycle and given conventional food and water ad libitum. The ARC was isolated from the brain of 5-8-wk-old SD rats and single neurons were prepared according to the procedures reported previously[16,21] with slight modifications. Briefly, rats were anaesthetized with an intraperitoneal injection of carbamic acid ethyl ester (900 mg/kg) and decapitated, and their brains were removed. Brain slices containing the ARC were prepared and the whole ARC of the left and right sides was cut out. The dissected tissues were washed with 10 mmol/L HEPES-buffered Krebs-Ringer bicarbonate buffer (HKRB) containing 10 mmol/L glucose. Then they were incubated in HKRB supplemented with 20 U/mL papain (Sigma Chemical Co., St. Louis, MO), 0.015 mg/mL deoxyribonuclease, 0.75 mg/mL bovine serum albumin and 1mmol/L cysteine for 15 min at 36°C in a shaking water bath, followed by gentle mechanical trituration for 5-10 min. After trituration, the cell suspension was centrifuged at 100 ×g for 5 min. The pellet was resuspended in HKRB and distributed on coverslips. The cells were kept at 20°C in moisture-saturated dishes for up to 10 h. The animal protocols were approved by the Jichi Medical School Institute of Animal Care and Use Committee.
At 2 to 10 h after cell preparation, [Ca2+]i was measured by ratiometric fura-2 microfluorometry in combination with digital imaging as previously reported[16,21]. Briefly, following incubating with 2 μmol/L fura-2-AM for 30 min at room temperature, the cells were mounted in a chamber and superfused with HKRB at 1 mL/min at 34°C. Fluorescence images due to excitation at 340 nm and 380 nm were detected every 8.0 s with an intensified charge-coupled device (ICCD) camera, and the ratio image was produced by an Argus-50 system (Hamamatsu Photonics Co., Hamamatsu, Japan). Ratio values were converted to [Ca2+]i according to calibration curves. Data were taken from the cells identified as neurons by the procedures reported previously[16,21].
Neurochemical identification of the neurons that exhibited [Ca2+]i responses were performed according to the original method[22] with slight modification[16]. Briefly, the cells were fixed with 4% paraformaldehyde over night. They were blocked in 10% normal goat serum (NGS) and in 0.1 mol/L PBS for 1 h at room temperature. Primary antiserum against NPY (DiaSorin, Stillwater, MN) was diluted 1:1000 in PBS containing 1.5% NGS and was incubated 24 h at 4°C. The antiserum were then rinsed and incubated with biotinylated secondary antibody raised against rabbit IgG (Vector Laboratories Inc., Burlingame, CA; diluted at 1:400) for 1 h at room temperature. The secondary antibody was rinsed, and the sections were labeled with avidin-peroxidase complex (ABC kit, Vector) for 1 h and color-developed with 3,3'-diaminobenzidine (DAB). Control experiments were carried out by omitting the primary antiserum.
To correlate [Ca2+]i and immunocytochemical data, photographs of all the cells in the microscopic field subjected to [Ca2+]i measurements were taken at the end of [Ca2+]i imaging. Based on these photographs, the cells in which [Ca2+]i was recorded were correlated with their corresponding immunocytochemical results.
Ghrelin, orexin-A and leptin were administered to the superfusion solution. Amplitudes of [Ca2+]i increases in response to agents were calculated by subtracting pre-stimulatory basal [Ca2+]i levels from peak [Ca2+]i levels. When increases in [Ca2+]i took place within 5 min after addition of agents and their amplitudes were 150 nmol/L or larger, they were considered responses. Suppression by leptin was judged by the following criteria. In Figures 1 and 2, when the peak amplitude of ghrelin-induced [Ca2+]i increase was decreased to a level of 40% or smaller for at least 5 min and the recovery of [Ca2+]i increase was observed after washing out leptin, it was considered inhibition. In Figure 3, repetitive additions of ghrelin or orexin-A twice induced repeated [Ca2+]i increases, and the second challenge to ghrelin or orexin-A was performed in the presence of leptin. When the amplitude of the [Ca2+]i response to the second addition was less than 150 nmol/L, it was considered inhibition.
The measurements were carried out in HKRB solution composed of 129 mmol/L NaCl, 5.0 mmol/L NaHCO3, 4.7 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.8 mmol/L CaCl2, 1.2 mmol/L MgSO4, and 10 mmol/L N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) at pH 7.4. Fura 2-acetoxymethylester was obtained from Dojin Chemical (Kumamoto, Japan). Ghrelin and orexin-A were obtained from Peptide Institute, Inc. (Osaka, Japan), Leptin was from R&D Systems (Mineapolis, MN).
The data are presented as the mean ± SE (n: number of neurons). Each study was based on at least 7 neurons prepared from at least 3 rats. Student’s paired or unpaired t-test was used to evaluate differences and values of P < 0.05 were considered to be significant.
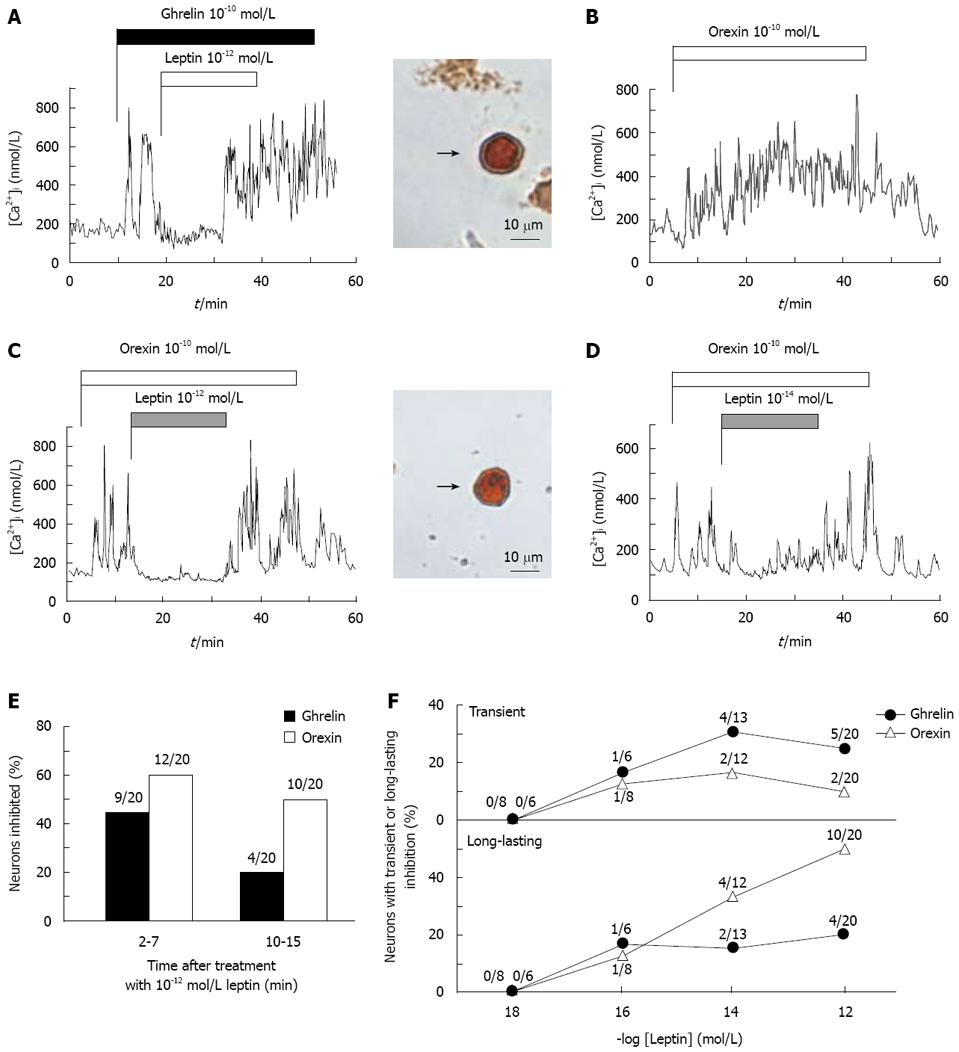
Single neurons isolated from the ARC were superfused with HKRB and subjected to measurements of [Ca2+]i with fura-2 fluorescence imaging. Administration of ghrelin or orexin-A at 10-10 mol/L for 40-50 min into superfusion solutions increased [Ca2+]i in a continuous manner (Figure 1A and B) as reported previously[16-18]. The [Ca2+]i increases in response to ghrelin and orexin took place in an oscillatory manner. The peaks of [Ca2+]i responses to ghrelin [486 ± 49 nmol/L (n = 33)] and orexin-A [433 ± 47 nmol/L (n = 27)] were significantly (P < 0.001) higher than the corresponding basal [Ca2+]i levels prior to administration of the peptides [107 ± 12 nmol/L (n = 33) for ghrelin; 110 ± 10 nmol/L (n = 27) for orexin].
Both ghrelin-induced (Figure 1A) and orexin-induced [Ca2+]i increases (Figure 1C) were inhibited by administration of 10-12 mol/L leptin in the ARC neurons, which were subsequently proven to be immunoreactive to NPY (Figure 1A and C, right panels). The results that leptin counteracts ghrelin and orexin actions on [Ca2+]i in the ARC NPY neurons confirm previous reports[16-18].
Typical results of the effects of leptin on [Ca2+]i responses to ghrelin and orexin-A are shown in Figure 1; leptin at 10-12 mol/L inhibited ghrelin-induced [Ca2+]i increases in a transient manner (Figure 1A) and orexin-induced [Ca2+]i increases in a longer-lasting manner (Figure 1C) during the 20 min period of leptin administration. Among 20 neurons that exhibited [Ca2+]i responses to ghrelin, administration of 10-12 mol/L leptin inhibited [Ca2+]i increases in 9 neurons (45%) during the earlier 2-7 min of leptin treatment, but only in 4 neurons (20%) later in the 10-15 min period of treatment (Figure 1E), showing attenuation of the counteracting effect of leptin for ghrelin in the later period (Figure 1F). In contrast, among 20 neurons that exhibited [Ca2+]i responses to orexin, administration of 10-12 mol/L leptin inhibited [Ca2+]i increases in 12 neurons (60%) during the 2-7 min of leptin treatment, and in 10 neurons (50%) in the 10-15 min period of treatment (Figure 1E), showing a long-lasting counteracting effect of leptin (Figure 1F). The long-lasting effect was also evoked by leptin at a lower concentration of 10-14 mol/L (Figure 1D). Leptin at both 10-14 mol/L and 10-12 mol/L counteracted ghrelin-induced [Ca2+]i increases predominantly in a transient manner and orexin-induced [Ca2+]i increases mainly in a long-lasting manner (Figure 1F).
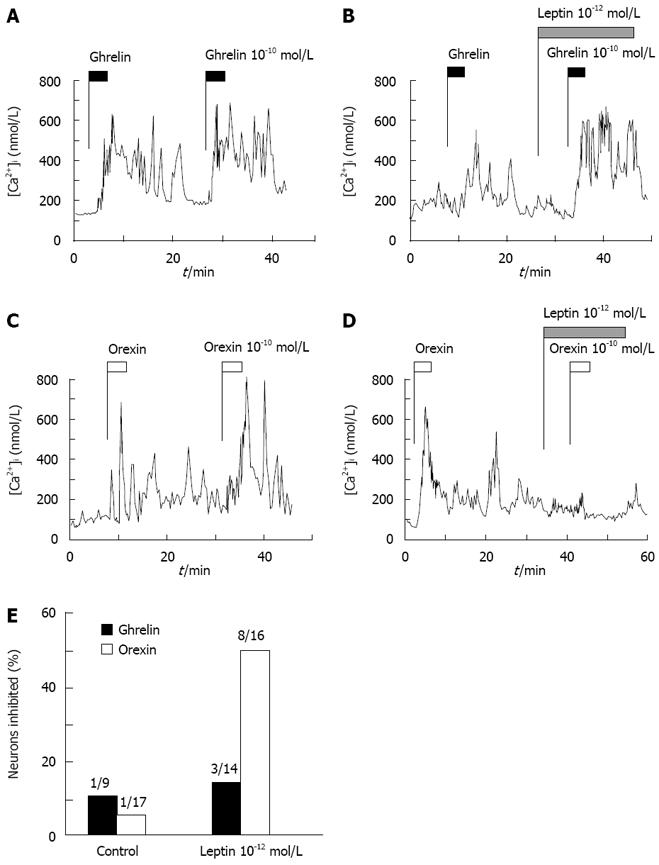
The data that leptin inhibited ghrelin-induced [Ca2+]i increases transiently prompted us to hypothesize that efficacy of leptin is attenuated by time. Therefore, we examined whether prior administration of leptin is less effective in counteracting ghrelin action. Repetitive additions of ghrelin or orexin-A twice induced repeated [Ca2+]i increases twice in a similar manner (Figure 3A and C). Following infusion of leptin that had started 8 min in advance, the addition of ghrelin induced [Ca2+]i increases with amplitudes comparable to those in the control without leptin (Figure 3B), and the similar result was observed in the majority of neurons (Figure 3E). This result indicates a marked attenuation of inhibitory ability of leptin by time. In contrast, infusion of leptin that started 8 min in advance inhibited [Ca2+]i responses to the addition of orexin-A in 8 of 16 orexin-responsive neurons (50%) (Figure 3D and E). This incidence of the inhibition by leptin administered in prior to orexin was comparable to the inhibition by leptin administered after orexin (12 of 20 neurons, 60%) (Figure 1E). These data indicate that the ability of leptin to counteract orexin action is well preserved without appreciable attenuation.
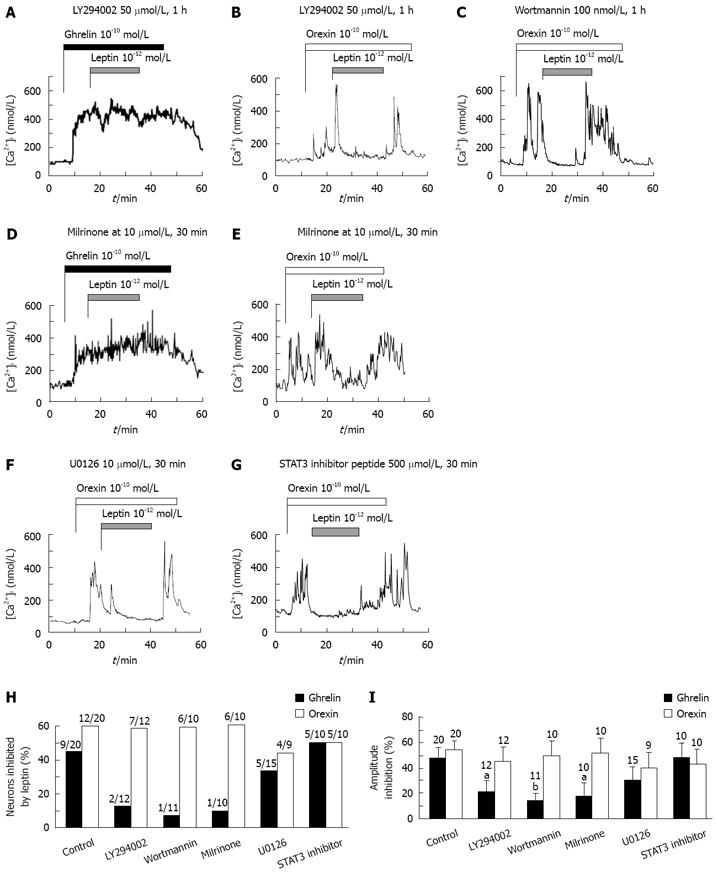
We next examined whether the difference in the time dependence of leptin action on ghrelin-vs orexin-induced [Ca2+]i increases could involve different leptin signaling mechanisms in NPY neurons. Leptin is linked to several signaling pathways, which include phosphatidylinositol 3 (PI3)-kinase and, its downstream effecter phosphodiesterase 3 (PDE3)[23], signal transducer and activator of transcription 3 (STAT3)[24], and mitogen-activated protein (MAP)-kinase[25]. We have previously shown that leptin suppresses ghrelin-induced [Ca2+]i increases via PI3-kinase- and PDE3-, but not MAP-kinase- and STAT3-, mediated pathway[17]. Therefore, whether these signaling mechanisms could be involved in the leptin action to counteract orexin-induced [Ca2+]i increases was examined. Pretreatment with inhibitors for PI3-kinase, LY294002 (Figure 2A) or wortmannin (data not shown), blocked the leptin action to suppress [Ca2+]i responses to ghrelin in both the response incidence (Figure 2H) and response amplitude (Figure 2I). Likewise, pretreatment with an inhibitor for PDE3, milrinone, blocked the leptin action against ghrelin (Figure 2D, H and I). These results confirm previous report[17]. In contrast, LY294002 (Figure 2B), wortmannin (Figure 2C) and milrinone (Figure 2E) failed to significantly affect the leptin suppression of orexin-induced [Ca2+]i increases in both the response incidence and amplitude (Figure 2H and I). Furthermore, pretreatment with a MAP kinase inhibitor U0126 (Figure 2F) or a STAT3 inhibitor peptide (Figure 2G) little altered the leptin ability to inhibit [Ca2+]i responses to orexin in both the response incidence and amplitude (Figure 2H and I), the results similar to those reported for ghrelin-induced [Ca2+]i increases[17].
The present data indicate that leptin inhibits ghrelin-induced [Ca2+]i increases in a transient manner and orexin-induced [Ca2+]i increases in a long-lasting manner. The transient action of leptin to inhibit ghrelin-induced [Ca2+]i increases is not due to insufficient concentration of leptin, since leptin at a lower concentration of 10-14 mol/L is already maximal in counteracting the ghrelin effect[17] and more specifically in exhibiting the transient inhibitory property (Figure 1F). Furthermore, the transient property for leptin inhibition of ghrelin-induced [Ca2+]i increases is neither due to excessive concentration of ghrelin, since the ghrelin concentration of 10-10 mol/L used in the present study is close to a maximal, but never super-maximal concentration in activating NPY neurons[16]. Therefore, the transient manner with which leptin counteracts ghrelin action reflects the intrinsic property of interaction between leptin and ghrelin.
The present study clearly indicated that the leptin signaling underlying the inhibition of [Ca2+]i responses to orexin-A in NPY neurons is distinct from that to ghrelin. The transient action of leptin to inhibit ghrelin-induced [Ca2+]i increases in NPY neurons may be mediated by the leptin signaling via PI3-kinase and PDE3, since the inhibitors for these enzymes block the leptin action (Figure 2A and D)[17]. The ghrelin signaling via the cAMP system could be the target for this leptin signaling[17]. On the other hand, the long-lasting action of leptin to counteract orexin-induced [Ca2+]i increases was not affected by inhibitors for PI3-kinase, PDE3, MAP-kinase and STAT3, well known leptin signaling molecules. This result suggests that the long-lasting counteracting action of leptin for orexin is mediated by a yet unidentified leptin signaling, which may long-lastingly inhibit the orexin-stimulated PLC-PKC-IP3 pathway reported previously[18], though further study is definitely needed to elucidate the signaling interaction between leptin and orexin.
The transient nature with which leptin counteracts the ghrelin action on NPY neurons may serve as the neuronal mechanism that allows fasting-associated increases in ghrelin levels to activate the ARC NPY neurons in the continuous presence of leptin[14] and thereby stimulate feeding.
Both ghrelin and orexin have been suggested to be concerned with obesity and type 2 diabetes[26,27]. Based on the present results, leptin resistance could alter the sensitivity to ghrelin and orexin in the ARC NPY neurons, which may alter the energy metabolism and thereby influence the pathogenesis of obesity and type 2 diabetes.
Ghrelin and orexins are potent orexigenic peptides working primarily via activating neuropeptide Y (NPY) neurons in the arcuate nucleus (ARC). NPY neurons in the ARC also serve as a major target for the anorexigenic leptin. Therefore, interactions of leptin with ghrelin and orexin in the ARC NPY neurons may play a key role in physiological regulation of feeding.
The authors study the time course of leptin effects on ghrelin-induced vs orexin-induced [Ca2+]i increases in NPY neurons.
The study clearly indicated that the leptin signaling underlying the inhibition of [Ca2+]i responses to orexin-A in NPY neurons is distinct from that to ghrelin. The transient action of leptin to inhibit ghrelin-induced [Ca2+]i increases in NPY neurons may be mediated by the leptin signaling via PI3-kinase and PDE3, The ghrelin signaling via the cAMP system could be the target for this leptin signaling.
The transient nature with which leptin counteracts the ghrelin action on NPY neurons may serve as the neuronal mechanism that allows fasting-associated increases in ghrelin levels to activate the ARC NPY neurons in the continuous presence of leptin[14] and thereby stimulate feeding.
A well designed, in-depth paper about the time course of leptin effects on ghrelin-induced vs orexin-induced [Ca2+]i increases in NPY neurons.
S- Editor Xiao LL E- Editor Lin YP
| 1. | Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661-671. |
| 2. | Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci. 2005;8:1289-1291. |
| 3. | Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683-685. |
| 4. | Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573-585. |
| 5. | Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748-753. |
| 6. | Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974;247:284-286. |
| 7. | Muroya S, Uramura K, Sakurai T, Takigawa M, Yada T. Lowering glucose concentrations increases cytosolic Ca2+ in orexin neurons of the rat lateral hypothalamus. Neurosci Lett. 2001;309:165-168. |
| 8. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. |
| 9. | Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194-198. |
| 10. | Asakawa A, Inui A, Kaga T, Katsuura G, Fujimiya M, Fujino MA, Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947-952. |
| 11. | Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255-4261. |
| 12. | Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, Kojima M, Kangawa K, Arima T, Matsuo H. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51:124-129. |
| 13. | Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004;53:3142-3151. |
| 14. | Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714-1719. |
| 15. | Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437-451. |
| 16. | Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes. 2003;52:948-956. |
| 17. | Kohno D, Nakata M, Maekawa F, Fujiwara K, Maejima Y, Kuramochi M, Shimazaki T, Okano H, Onaka T, Yada T. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology. 2007;148:2251-2263. |
| 18. | Muroya S, Funahashi H, Yamanaka A, Kohno D, Uramura K, Nambu T, Shibahara M, Kuramochi M, Takigawa M, Yanagisawa M. Orexins (hypocretins) directly interact with neuropeptide Y, POMC and glucose-responsive neurons to regulate Ca 2+ signaling in a reciprocal manner to leptin: orexigenic neuronal pathways in the mediobasal hypothalamus. Eur J Neurosci. 2004;19:1524-1534. |
| 19. | Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17:305-311. |
| 20. | Erickson JC, Hollopeter G, Palmiter RD. Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science. 1996;274:1704-1707. |
| 21. | Muroya S, Yada T, Shioda S, Takigawa M. Glucose-sensitive neurons in the rat arcuate nucleus contain neuropeptide Y. Neurosci Lett. 1999;264:113-116. |
| 22. | Yada T, Vigh S, Arimura A. Pituitary adenylate cyclase activating polypeptide (PACAP) increases cytosolic-free calcium concentration in folliculo-stellate cells and somatotropes of rat pituitary. Peptides. 1993;14:235-239. |
| 23. | Zhao AZ, Huan JN, Gupta S, Pal R, Sahu A. A phosphati-dylinositol 3-kinase phosphodiesterase 3B-cyclic AMP pathway in hypothalamic action of leptin on feeding. Nat Neurosci. 2002;5:727-728. |
| 24. | Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci USA. 2004;101:4661-4666. |
| 25. | Benomar Y, Roy AF, Aubourg A, Djiane J, Taouis M. Cross down-regulation of leptin and insulin receptor expression and signalling in a human neuronal cell line. Biochem J. 2005;388:929-939. |
| 26. | Yada T, Dezaki K, Sone H, Koizumi M, Damdindorj B, Nakata M, Kakei M. Ghrelin regulates insulin release and glycemia: physiological role and therapeutic potential. Curr Diabetes Rev. 2008;4:18-23. |
| 27. | Tsuneki H, Sugihara Y, Honda R, Wada T, Sasaoka T, Kimura I. Reduction of blood glucose level by orexins in fasting normal and streptozotocin-diabetic mice. Eur J Pharmacol. 2002;448:245-252. |