Published online Oct 28, 2008. doi: 10.3748/wjg.14.6249
Revised: September 19, 2008
Accepted: September 26, 2008
Published online: October 28, 2008
AIM: To investigate the ultrastructural location of midkine (MK) in nucleolus and function corresponding to its location.
METHODS: To investigate the ultrastructural location of MK in nucleolus with immunoelectronic microscopy. To study the role that MK plays in ribosomal biogenesis by real-time PCR. The effect of MK on anti-apoptotic activity of HepG2 cells was studied with FITC-conjugated annexin V and propidium iodide PI double staining through FACS assay.
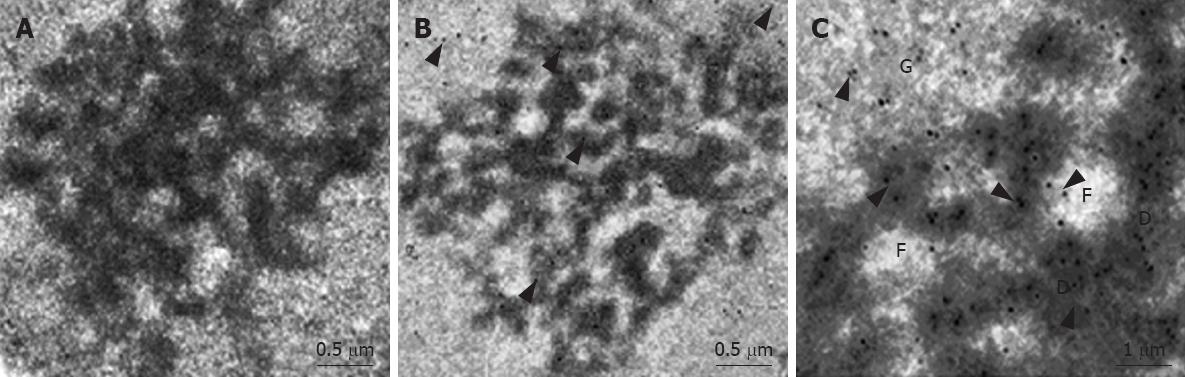
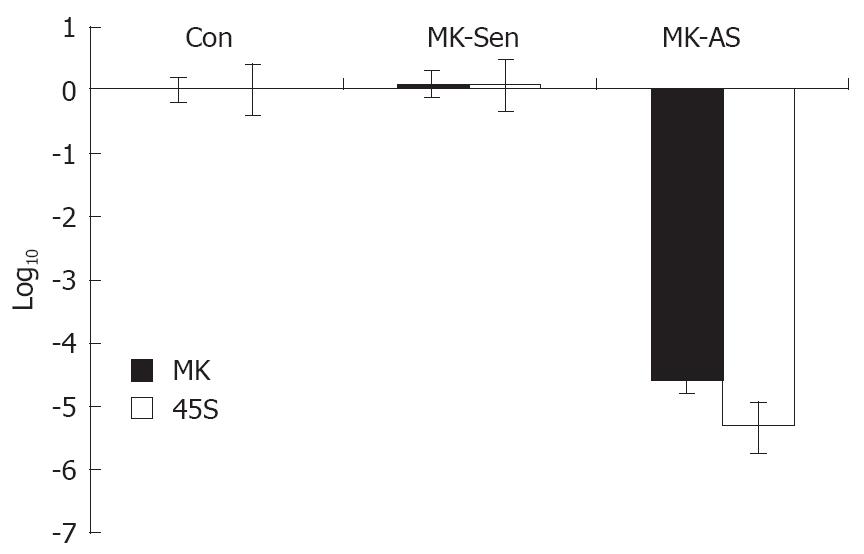
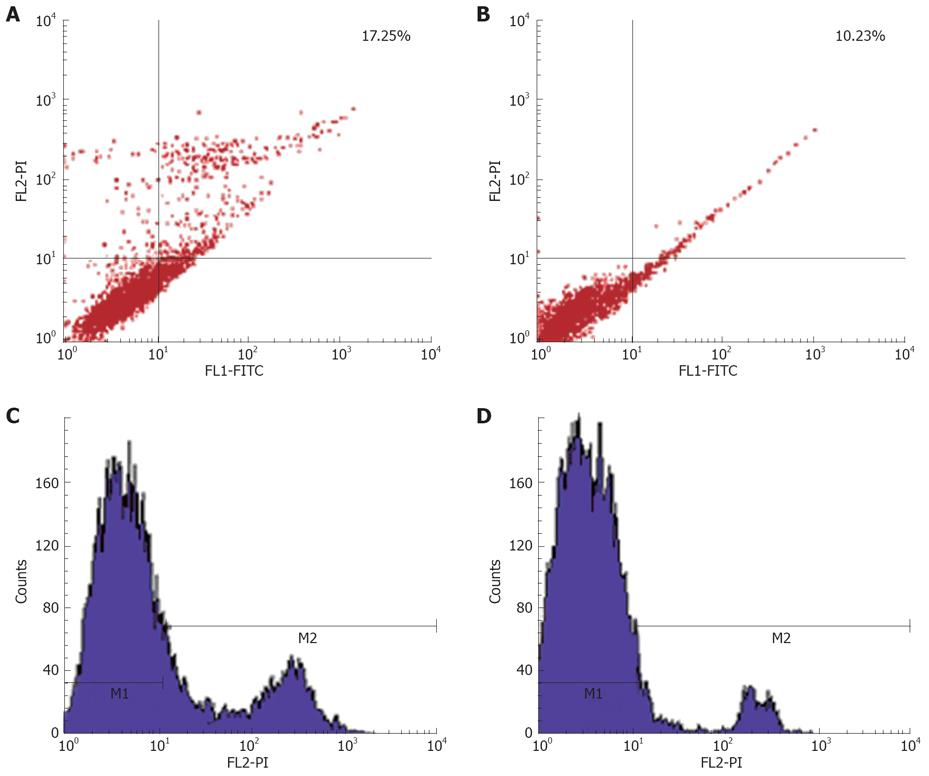
RESULTS: MK mainly localized in the granular component (GC), dense fibrillar component (DFC) and the border between the DFC and fibrillar center (FC). The production of 45S precursor rRNA level was decreased significantly in the presence of MK antisense oligonucleotide in the HepG2 cells. Furthermore, it was found that exogenous MK could protect HepG2 from apoptosis significantly.
CONCLUSION: MK was constitutively translocated to the nucleolus of HepG2 cells, where it accumulated and mostly distributed at DFC, GC components and at the region between FC and DFC, MK played an important role in rRNA transcription, ribosome biogenesis, and cell proliferation in HepG2 cells. MK might serve as a molecular target for therapeutic intervention of human carcinomas.
- Citation: Dai LC, Shao JZ, Min LS, Xiao YT, Xiang LX, Ma ZH. Midkine accumulated in nucleolus of HepG2 cells involved in rRNA transcription. World J Gastroenterol 2008; 14(40): 6249-6253
- URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6249.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6249
Midkine (MK) is a cysteine-rich basic protein with a molecular weight of 13 Ku, which is strongly expressed during mid-gestation embryogenesis[1]. MK can be detected in most carcinoma specimens at a high level in a tissue type-independent manner, including those of esophageal, gastric, gall bladder, pancreas, colorectal, breast, and lung carcinomas, and Wilms’ tumors[2-7]. Furthermore, MK exhibits several cancer-related activities, which include fibrinolytic, anti-apoptotic, mitogenic, transforming, angiogenic, and chemotactic activity[8-10]. Recently, Shibata et al[11] have shown that exogenous MK is endocytosed by cultured mouse L cells and then transported to the nucleus. However, more detailed information on the location and biological mechanism of MK within cells still remains to be elucidated. In a previous study, using green fluorescent protein (GFP) as a tracking molecule we have found that MK is exclusively localized to the nucleus and nucleolus in HepG2 cells[12]. We have also found that MK both with and without signal peptide is exclusively localized to the nucleus and accumulats in the nucleolus of DU145 and MCF7 cell lines[13].
In the present study, we demonstrated the ultrastructural location of MK in the nucleolus with immunoelectron microscopy. As is known, the nucleolus is a region of the nucleus that is known to be the locus for ribosomal biogenesis. This prompted us to hypothesize that MK may be involved in RNA transcription and processing. We studied the effect of MK on rRNA synthesis and found 45S rRNA production level decreased in response to down-regulation of MK expression. Since cell proliferation and cancer survival require continuous protein synthesis that depends on a constant supply of ribosomes[14], 45S rRNA production may be affected by endogenous MK level, suggesting that cell proliferation is directly related to MK level. Investigating this possibility, we demonstrated that cell proliferation was inhibited by down-regulation of MK. Moreover, we reported that exogenous human MK is involved in anti-apoptotic activity of HepG2 cells.
HepG2 cells were fixed with 3% paraformaldehyde and 1% glutaraldehyde at 4 degree for 2 h, and then sequentially dehydrated with 30%, 50% and 100% ethanol and embedded in Lowicryl K4M. Sections of 50 nm were cut and mounted on nickel grids. Non-specific binding was blocked with 1% BL (50 mmol/L PBS, pH 7.0, 1% BSA,0.02% PEG20 000,100 mmol/L NaCl,1% NaN3) for 30 min at room temperature. Then, sections were incubated for 1 h at room temperature with anti-MK primary antibody (rabbit polyclonal to human MK, Abcam, UK) at a dilution of 1:100. After another treatment with BL, sections were incubated with 15 nm colloidal gold-labeled second antibody (goat polyclonal antibody to rabbit IgG, 15nm gold; Abcam, UK). Finally, sections were stained sequentially with uranyl acetate for 15 min and lead nitrate for 10 min. The ultrastructural distribution of MK was examined and photographed with a Hitachi H-800 transmission electron microscopy.
The sequence of MK morpholino antisense oligomer was as follows: MK-As (5'-AGGAAGCCTCGGTGCTGCATCTCGC-3'). The sequence for MK-Sen was as follows: (5'-CGCTCTACGTCGTGGCTCCG AAGGA-3'). MK-Sen is a control oligonucleotide that has the same base composition as MK-As, but in the reverse sequence, and thus does not hybridize with MK mRNA. MK-As and MK-Sen were transfected into HepG2 cells in the presence of Lipofectamine-Plus (Life Technologies, Inc) in accordance with the manufacturer’s instructions.
The real-time PCR was performed with an RT-PCR kit (Takara, Japan) according to the manufacturer’s instructions using GAPDH primers (5'-AACGACCCCTTCATTGAC-3' and 5'-TCCACGACA TACTCAGCAC-3'), MK primers (5'-AAACCGAACTCCAGGACCAGAGAC-3' and 5'-AACACTCGCTGCCCTTCTTCAC-3') and 45S primers (5'-CGCCGCTAGAGGTGAAATTC-3' and 5'-CATTCTTGGCAAATGCTTTCG-3'). Samples were amplified in a 7500 Real Time PCR system for 40 cycles using the following PCR parameters: 95°C for 30 s, 58°Cfor 1 min, and 72°C for 1 min.
A total of 1.0 × 104 HepG2 cells were added to each well of a 96-well microtiter plate and allowed to attach overnight. Oligonucleotides at concentrations of 0.2, 0.4 and 0.6 μmol/L were transfected into HepG2 cells with Lipofectamine Plus (Life Technologies, Inc) following the manufacturer’s instructions. The effects of antisense oligodeoxynucleotide on cellular viability were measured using an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay.
1.0 × 105 HepG2 cells were seeded in six-well plates. Three wells were pre-treated with 500 ng/mL MK and three wells were treated with PBS as control for 3 h. Cells then were treated with 10-6 mol/L adriamycin, harvested 20 h later through trypsinization, and washed twice with cold PBS. The cells were centrifuged at 3 000 r/min for 5 min, then the supernatant was discarded and the pellet was resuspended and incubated with FITC-conjugated annexin V and propidium iodide (Pharmingen) for 15 min at room temperature in the dark, and then analyzed by FACS.
In a previous study, we demonstrated that MK exclusively localizes to the nucleus and nucleolus in HepG2 cells, using GFP as a tracking molecule[12]. At the ultrastructural level, the nucleolus includes three components: the fibrillar center (FC), the dense fibrillar component (DFC), and the granular component (GC)[15]. Although we have found that MK accumulats in the nucleolus, the exact actions that MK performed on the nucleolus are still unclear. Therefore, we employed immunogold-labeling electron microscopy to investigate the ultrastructural location of MK, and found MK mainly localized in the GC, DFC and the border between the DFC and FC (Figure 1).
In order to make clear that the function of MK corresponds to its ultrastructural location in the nucleolus, we investigated the role that MK plays in ribosomal biogenesis by real-time PCR. We found the production of 45S precursor rRNA in the HepG2 cells was decreased significantly in the presence of MK antisense oligonucleotide (Figure 2). This suggests that endogenous MK plays an important role in cancer cell proliferation.
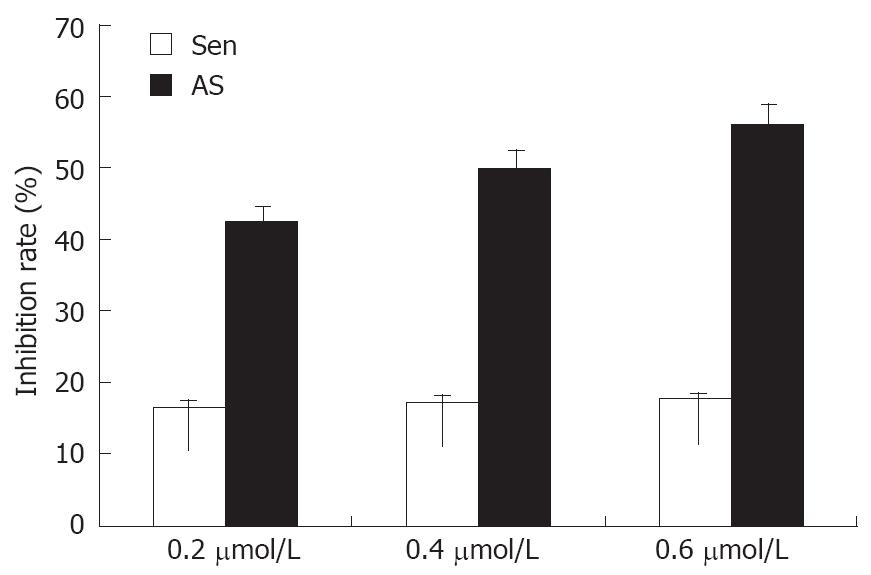
Due to the central importance of rRNA transcription in cell growth, decreased rRNA transcription will slow cell proliferation. In this study, we showed that MK-As reduced cell proliferation rates by 41%, 48%, 58% after transfection of 0.2, 0.4 and 0.6 μmol/L MK antisense oligonucleotide into 104 HepG2 cells. The control MK-Sen did not inhibit cell proliferation significantly (Figure 3).
In order to investigate whether MK plays a role in the apoptosis of cancer cells, we added exogenous MK to HepG2 cells in the presence of adriamycin, inducing apoptosis. The results showed that exogenous MK could protect HepG2 from apoptosis significantly (Figure 4). This led us to suggest that MK may be a drug target for curing cancer.
In this study, we showed that MK was constitutively translocated to the nucleus of HepG2 cells, where it accumulated (Figure 1) and mostly distributed at the region between the FC and DFC, and GC components of the nucleolus (Figure 2). In a previous study, it has been demonstrated that each component of the nucleolus corresponds to a special biological function: the nascent transcripts appear in the junction region between the FC and DFC and accumulate in the DFC, and continue during the intranucleolar migration of the rRNA towards the GC. This implies that the role of the MK in the nucleolus is possibly related to control of rRNA gene transcription, pre-rRNA processing, and nascent ribosome subunit assembly, which could be the downstream elements of controlling cell proliferation. The finding that MK is involved in rRNA transcription in HepG2 cells in our study is significant for understanding cancer biology. One of the hallmarks of cancer is sustained cell growth and this can only be achieved by increased protein synthesis. To accommodate this need, there must be an increase in ribosome biogenesis. The role MK plays in rRNA transcription in cancer cells suggests that up-regulation of MK expression in various cancer cells not only induces tumor growth, but also directly contributes to cell proliferation. Thus, inhibitors targeting MK will be more effective than those that inhibit cancer cell proliferation alone. rRNA transcription regulates ribosome production and consequently, the translation potential of a cell, and increasingly expressed ribosomal proteins and rRNA transcription is an important factor in cancer transformation[16]. It is conceivable that deregulation of rRNA transcription may be an important determinant in transformation of cancer cells. Continuous nuclear translocation of MK in cancer cells can be possible one of the contributing factors in determinant of transformation. Indeed, inhibiting MK expression reduced tumorigenicity and reversed the malignant phenotype of cancer cells, by antisense oligodeoxynucleotide targeted to MK. It is therefore possible that other effects such as repression of rRNA transcription of cancer cells also contributed to the marked anticancer activity. In conclusion, the results showed that MK played an important role in rRNA transcription, ribosome biogenesis, and cell proliferation in HepG2 cells. However, the mechanism by which MK stimulates rRNA transcription is still unclear at present. More extensive work is needed to understand how MK is translocated to the nucleus, whether it interacts with the RNA polymerase I machinery or binds to DNA.
Midkine (MK) has been found to play important roles in carcinogenesis, including mitogenic, anti-apoptotic, transforming, fibrinolytic, chemotactic, and angiogenic cancer-related activities. In a previous study, it has been demonstrated that each component of the nucleolus corresponds to a special biological function: the nascent transcripts appear in the junction region between the fibrillar center (FC) and dense fibrillar component (DFC) and accumulate, in the DFC, and continues during the intranucleolar migration of the rRNA towards the granular component (GC). This implies that the role of the MK in the nucleolus is possibly related to control of rRNA gene transcription, pre-rRNA processing, and nascent ribosome subunit assembly, which could be the downstream elements of controlling cell proliferation. In a previous study, we found that MK exclusively localized to the nucleus and nucleolus in HepG2 cells. However, it is unclear what is the function of MK in the nucleus and nucleolus.
MK expression is increased in many human carcinomas, such as esophageal, stomach, colon, pancreatic, thyroid, lung, breast, urinary bladder, uterine, ovarian, prostate and hepatocellular carcinomas, osteosarcoma, neuroblastoma and glioblastoma. This phenomenon is observed in about 80% of cases in many types of carcinomas. Furthermore, MK exhibits several cancer-related activities, which include fibrinolytic, anti-apoptotic, mitogenic, transforming, angiogenic, and chemotactic activity.
In our study, we demonstrated that MK may be involved in 45S rRNA transcription. Since rRNA transcription is essential for tumor growth and proliferation, inhibition of MK-stimulated transcription of rRNA may be developed into a novel method to inhibit the growth of tumors.
The results may provide valuable evidence for the further study on the functions of MK in the nuclus and its mechanisms, in which rRNA transcription and ribosome assembly are involved. MK might serve as a molecular target for therapeutic intervention in human carcinomas.
FC, DFC and GC are three components of the nucleolus. The nucleolus can be observed with the light microscope and its structure has more recently been clarified using the electron microscope.
In this manuscript, authors report that the oncogenic function of MK could be associated with its role in 45S rRNA transcription. This finding is interesting.
Peer reviewer: Xin-Yuan Guan, Professor, Department of Clinical Oncology, The University of Hong Kong, Room L10-56, 10/F, Laboratory Block, 21 Sassoon Road, Pokfulam, Hong Kong, China
S- Editor Xiao LL L- Editor Li M E- Editor Lin YP
| 1. | Muramatsu T. [Midkine (MK): a retinoic acid-responsive, heparin-binding growth factor in relationship with differentiation, development, cancer and neural function]. Seikagaku. 1993;65:1494-1504. |
| 2. | Michikawa M, Kikuchi S, Muramatsu H, Muramatsu T, Kim SU. Retinoic acid responsive gene product, midkine, has neurotrophic functions for mouse spinal cord and dorsal root ganglion neurons in culture. J Neurosci Res. 1993;35:530-539. |
| 3. | Michikawa M, Xu RY, Muramatsu H, Muramatsu T, Kim SU. Midkine is a mediator of retinoic acid induced neuronal differentiation of embryonal carcinoma cells. Biochem Biophys Res Commun. 1993;192:1312-1318. |
| 4. | Garver RI Jr, Radford DM, Donis-Keller H, Wick MR, Milner PG. Midkine and pleiotrophin expression in normal and malignant breast tissue. Cancer. 1994;74:1584-1590. |
| 5. | Take M, Tsutsui J, Obama H, Ozawa M, Nakayama T, Maruyama I, Arima T, Muramatsu T. Identification of nucleolin as a binding protein for midkine (MK) and heparin-binding growth associated molecule (HB-GAM). J Biochem. 1994;116:1063-1068. |
| 6. | Kojima S, Muramatsu H, Amanuma H, Muramatsu T. Midkine enhances fibrinolytic activity of bovine endothelial cells. J Biol Chem. 1995;270:9590-9596. |
| 7. | Adachi Y, Matsubara S, Pedraza C, Ozawa M, Tsutsui J, Takamatsu H, Noguchi H, Akiyama T, Muramatsu T. Midkine as a novel target gene for the Wilms' tumor suppressor gene (WT1). Oncogene. 1996;13:2197-2203. |
| 8. | Kadomatsu K, Muramatsu T. Midkine and pleiotrophin in neural development and cancer. Cancer Lett. 2004;204:127-143. |
| 9. | Kawai H, Sato W, Yuzawa Y, Kosugi T, Matsuo S, Takei Y, Kadomatsu K, Muramatsu T. Lack of the growth factor midkine enhances survival against cisplatin-induced renal damage. Am J Pathol. 2004;165:1603-1612. |
| 10. | Friedrich C, Holtkamp N, Cinatl J Jr, Sakuma S, Mautner VF, Wellman S, Michaelis M, Henze G, Kurtz A, Driever PH. Overexpression of Midkine in malignant peripheral nerve sheath tumor cells inhibits apoptosis and increases angiogenic potency. Int J Oncol. 2005;27:1433-1440. |
| 11. | Shibata Y, Muramatsu T, Hirai M, Inui T, Kimura T, Saito H, McCormick LM, Bu G, Kadomatsu K. Nuclear targeting by the growth factor midkine. Mol Cell Biol. 2002;22:6788-6796. |
| 12. | Dai L, Xu D, Yao X, Lu Y, Xu Z. Conformational determinants of the intracellular localization of midkine. Biochem Biophys Res Commun. 2005;330:310-317. |
| 13. | Dai LC, Xu DY, Yao X, Min LS, Zhao N, Xu BY, Xu ZP, Lu YL. Construction of a fusion protein expression vector MK-EGFP and its subcellular localization in different carcinoma cell lines. World J Gastroenterol. 2006;12:7649-7653. |
| 15. | Cmarko D, Verschure PJ, Rothblum LI, Hernandez-Verdun D, Amalric F, van Driel R, Fakan S. Ultrastructural analysis of nucleolar transcription in cells microinjected with 5-bromo-UTP. Histochem Cell Biol. 2000;113:181-187. |
| 16. | Ruggero D, Pandolfi PP. Does the ribosome translate cancer? Nat Rev Cancer. 2003;3:179-192. |