INTRODUCTION
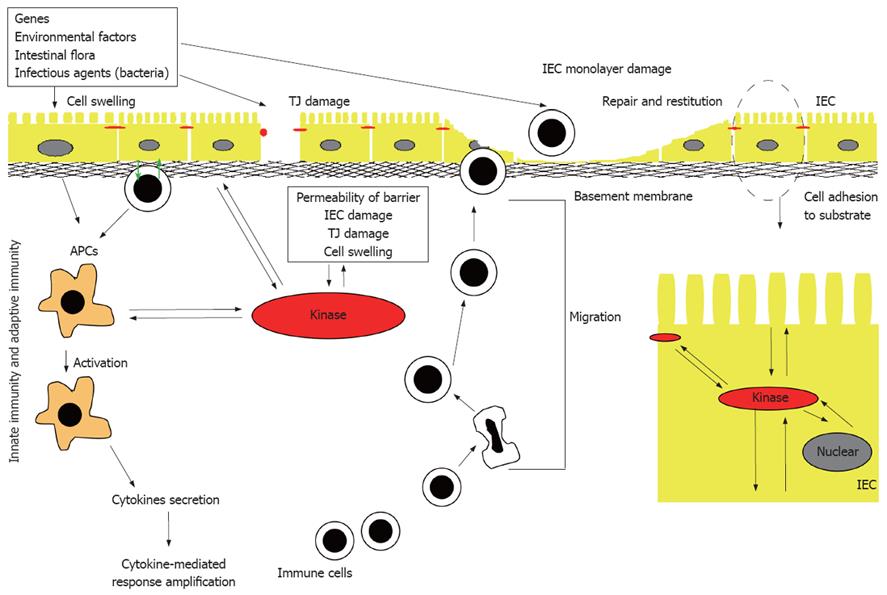
Figure 1 Pathogenesis of IBD.
Many different factors, such as genetic factors, environmental factors, and intestinal non-pathogenic or pathogenic bacteria can damage the mucus, epithelium, or the tight junction, to initiate the inappropriate regulation or deregulation of the immune response, leading to the secretion of pro-inflammatory cytokines, decrease in epithelial barrier function and initiation of the inflammation-related signaling pathways. IEC: Intestinal epithelial cell; APC: Antigen presenting cell; TJ: Tight junction.
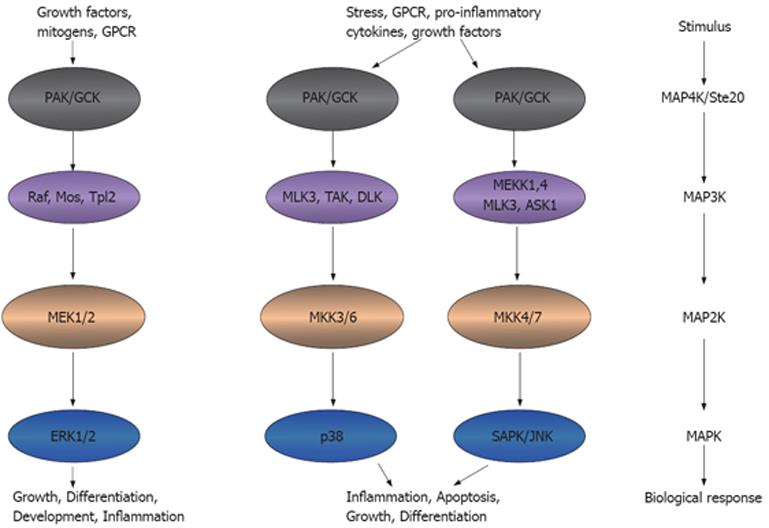
Figure 2 Ste20 kinases participate in inflammation.
Ste20 kinases that function as an MAP4K can activate MAP3K, MAP2K and MAPK, leading to the inflammatory functions. This model adapted from the model presented in http://www.cellsignal.com/pathways/map-kinase.jsp. MAPK: Mitogen-activated protein kinase. GPCR: G-protein coupled receptor; PAK: p21 activated kinase; GCK: Germinal central kinase; MLK: Multiple lineage kinase; TAK: Tat-associated kinase; DLK: Dual leucine zipper-bearing kinase; MEK: MAPK/Erk kinase; MEKK: MEK kinase; ASK: Aspartate kinase; MKK: MAPK kinase; Erk: Extracellular signal-regulated kinase; SAPK: Stress-activated protein kinase; JNK: Jun-amino-terminal kinase.
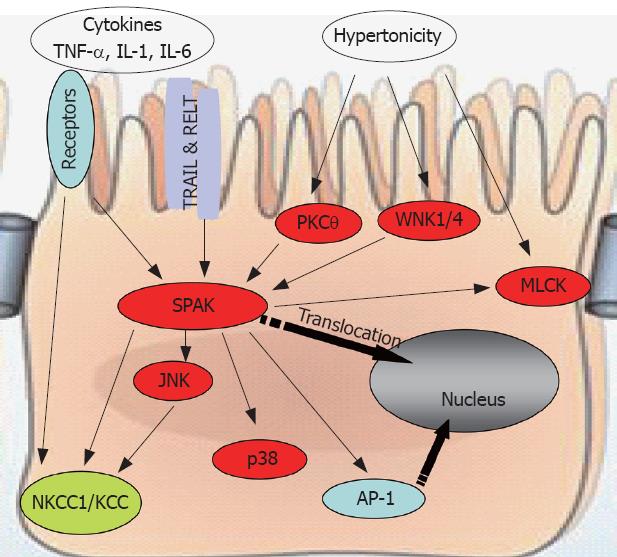
Figure 3 SPAK interacts with other molecules to maintain cellular homeostasis.
SPAK can be a substrate, indirectly or directly, for pro-inflammatory cytokines, environmental stress including hypertonicity, some other kinases such as PKCtheta;, WNK1/4, or other receptors, for example TRAIL & RELT. Also SPAK can function as upstream kinase to JNK, p38, or ion transport NKCC1/KCC, transcription factor AP-1, as well as MLCK. WNK: With no lysine kinase 1/4; TRIL: TNF-related apoptosis-inducing ligand; RELT: Receptor expressed in lymphoid tissues; MLCK: Myosin II regulatory light chain kinase; NKCC1: Sodium potassium chloride chloride transporter 1; KCC: Potassium chloride chloride transporter; AP-1: Activating protein 1.
Inflammatory bowel diseases (IBD), primarily ulcerative colitis (UC) and Crohn’s disease (CD), are chronic idiopathic inflammatory disorders of the gastrointestinal tract that are thought to arise as a result of an interplay of genetic and environmental factors. The mechanisms implicated in the pathogenesis of IBD (Figure 1) include: (1) inappropriate regulation of the innate immune response at the level of the intestinal mucosa; (2) deregulation of the adaptive immune system stemming from an imbalance between regulatory and effector-cell immune responses to luminal antigens; and (3) increased permeability across the mucosal epithelial barrier due to loss of structural integrity and/or abnormal transepithelial transport[1,2]. The loss of barrier function, in particular, has gained increasing support as an IBD pathogenic mechanism because the epithelium represents a potential intersection of both genetic and environmental influences. The intestinal mucosa is composed of a single layer of polarized intestinal epithelial cells (IECs) that protects against direct contact with enteric antigens, bacteria and other pathogens (Figure 1). The integrity of the epithelium is maintained primarily through a combination of intercellular adhesion structures and specialized junctions. In addition, other factors such as the presence of mucins, rapid turnover of epithelial cells, and peristaltic movement of the gastrointestinal tract, all help to protect against colonization and invasion of the intestinal mucosa by pathogens[3]. Moreover, epidemiological and genetic linkage studies have confirmed a strong link between modulation of the barrier function and IBD; these include, for example, the loci IBD1-9, corresponding to regions on chromosomes 16, 12, 6, 14, 5, 19, 1, 16 and 3, respectively[4-13], and a new IBD locus on chromosome 2[14].
MITOGEN-ACTIVATED PROTEIN KINASES (MAPKs) ARE INVOLVED IN INTESTINAL INFLAMMATION
Intracellular signaling cascades are the main route of communication between the plasma membrane and regulatory targets in various intracellular compartments. The evolutionarily conserved MAPK signaling pathway plays an important role in transducing signals from diverse extra-cellular stimuli (including growth factors, cytokines and environmental stresses) to the nucleus in order to affect a wide range of cellular processes, such as proliferation, differentiation, development, stress responses and apoptosis. MAPK signaling cascades, which comprise up to five levels of protein kinases that are sequentially activated by phosphorylation, are also involved in intestinal inflammation[15-17] (Figure 2).
MAPK signaling pathways are involved in regulating crucial inflammatory mediators and could thus serve as molecular targets for anti-inflammatory therapy. At least six distinct MAPK pathways have been identified in multicellular organisms, of which three, the extra-cellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK) and p38 cascades, are significantly activated and directly involved in inflammatory diseases such as IBD (Figure 2). In this context, cross-talk between these pathways and other inflammatory signaling pathways, including the NF-κB and Janus kinase/signal transducers, and activation of transcription (STAT) cascades[18-20], is also relevant to the action of MAPK pathways.
The involvement of some MAPK members in IBD is suggested by linkage studies. For example, the ERK1 gene is located in a major IBD susceptibility region on chromosome 16[4], and the p38α gene is located in a major IBD susceptibility region on chromosome 6[9]. Activation of p38 MAPK is also known to induce the production and secretion of pro-inflammatory cytokines, such as interleukin (IL)-1β and tumor necrosis factor-α (TNF-α)[21], and increased activity of p38 MAPK has been observed in patients with IBD[18,22]. Inhibition of p38 has been well documented to suppress IBD[17], and the guanylhydrazone compound, CNI-1493, which inhibits both JNK and p38, strongly reduces clinical disease activity in CD patients. In addition, inhibition of either ERK or p38 kinase pathway decreases lipopolysaccharide (LPS)-induced production of the cytokines, IL-6 and TNF-α[23]. The involvement of JNK pathways in intestinal inflammation has been intensively studied both in patients with IBD and in an experimental colitis model[18,24,25]. JNK inhibitors, which affect either JNK signaling pathway indirectly (e.g. CEP1347) or block the catalytic domain of JNK (e.g. SP 600125), have been tested for their potential value in treating IBD. Collectively, these observations demonstrate a very important role for MAPK pathways in the control and therapy of IBD.
STE20-LIKE KINASES ACT UPSTREAM OF MAPK PATHWAYS
The various MAPK pathways share a common family of upstream mediators: the Ste20 kinases. Ste20 was originally identified as a component of the pheromone-response pathway in budding yeast, and has also been shown to participate in the signaling pathways that regulate osmotic responses, including those to high osmolarity glycerol (HOG)[26]. Several mammalian Ste20 homologs have been identified. The Ste20 family includes two subfamilies that share basic structural and functional properties. The first subfamily includes the p21-activated kinases (PAKs), which are characterized by a C-terminal catalytic domain and an N-terminal binding site for the small G proteins, Rac1 and Cdc42. The second family comprises of the germinal center kinases (GCKs), which contain an N-terminal kinase domain and a C-terminal regulatory domain.
Ste20-like kinases function as MAP4Ks, triggering activation of MAPK cascade[27-29] and transmitting signals from extra-cellular stimuli that activate transcription factors (Figure 2). The resulting changes in gene expression, in turn, regulate cellular functions[27-31] that are important in the maintenance of epithelial barrier function, apoptosis, growth, morphogenesis, cell permeability, and rearrangements of the cytoskeleton that lead to changes in cell shape and motility. For example, members of the PAK subfamily of Ste20 kinases have been shown to increase endothelial permeability[32,33]. The pro-inflammatory cytokine, TNF-α, stimulates expression of the yeast Ste20 homolog, Map4k4, through TNF-α-receptor-1-mediated signaling to c-Jun[34], the chemokine CXCL12 and the complement factor C5a. The resulting increase in Map4k4 activity triggers cell migration via a PAK1/2-p38α MAPK-MAPKAP-K2-HSP27 pathway[35]. Other relevant examples include: (1) Ste20-like kinase (SLK)[36], Ste20-like oxidant stress-activated kinase (SOK)[37] and prostate-derived Ste20-like kinase 1-α (PSK1-α)[38], which induce apoptosis by activating the JNK pathway; (2) lymphocyte-oriented kinase (LOK)[39] and SLK[40], which regulate Rac1-mediated actin reorganization during cell adhesion and spreading; (3) mixed lineage kinase-3 (MLK-3)[41], which activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6; and (4) hematopoietic progenitor kinase 1 (HPK1), which is activated by prostaglandin E2 (PGE2) through a G-protein coupled receptor (GPCR) pathway, and negatively regulates transcription of the fos gene[42].
Ste20-like kinases has been reported to be activated by at least three pathogen-associated molecular patterns (PAMPs)-lipopolysaccharide, peptidoglycan, and flagellin-produced by invading microbial pathogens, and has been shown to initiate innate immune responses by binding to pattern recognition receptors (PRRs)[43]. PAMPs activate GCKs (Ste-20 family of kinases), which signal through MLK-2 and -3 to recruit JNK, p38 and their effectors[43]. These findings indicate an important role for GCKs and MLKs in PAMP-stimulated MAPK pathway activation, and therefore in stimulating the expression of pro-inflammatory genes involved in intestinal inflammation.
STE20-RELATED PROLINE/ALANINE-RICH KINASE (SPAK) IS A STE20-LIKE KINASES INVOLVED IN INTESTINAL INFLAMMATION
The GCKs may be divided into eight subfamilies based on homologies in their C-terminal domains (GCKI-VII). The Ste20-like kinase SPAK[44], PASK (the rat SPAK homolog)[45,46] and OSR1[47] share GCK VI homologies. Among these, SPAK and OSR1 are ubiquitously expressed. PASK is also expressed in most rat tissues, but its expression is particularly notable in cells with high ion-transport activity[45,48]. Both SPAK and PASK are highly expressed in epithelia and neurons[49]. On the other hand, PASK is found only in negligible levels in the liver and skeletal muscle[50]. SPAK, OSR1 and PASK contain a series of proline and alanine repeats (PAPA box) at the extreme N-terminus, followed by a serine/threonine kinase domain, a nuclear localization signal, a consensus caspase cleavage recognition motif, and a C-terminal regulatory region. However, the colonic SPAK isoform is unique in that it lacks the PAPA box and N-terminal F-alpha helix loop, due to the presence of a 5' splice junction-like sequence within exon-1[51]. Given its ubiquitous expression and diverse functional domains, the SPAK protein may be associated with diverse biological roles. It has been shown that under hyper-osmotic (but not hypo-osmotic) stress conditions, SPAK translocates from the cytosolic pool to a Triton X-100-insoluble fraction; although present in both fractions, SPAK associated with the Triton X-100-insoluble pool is dephosphorylated[52]. Our laboratory has observed that upon SPAK over-expression[51] or under TNF-α stress conditions (unpublished data), SPAK is cleaved and the N-terminal fragment is translocated to the nucleus.
The Na+-K+-2Cl- cotransporter 1 (NKCC1), a member of the Slc12 family of solute carriers and target of SPAK, plays a crucial role in cell volume regulation, cell proliferation and survival, and epithelial transport[53]. The activity and expression of NKCC1 can be regulated by cell volume[53] and intracellular chloride concentration[54], which act through NKCC1’s N-terminal (R/K) FX (V/I) binding motif. The pro-inflammatory cytokines IL-1β, TNF-α[55] and IL-6[56] also regulate NKCC1 activity. In addition, NKCC1 can be activated by α- and β-adrenergic stimulation via the cAMP/PKA-dependent pathway[57-59] and can be stimulated by PKC in a cell-specific manner[60,61]. Notably, NKCC1 can be phosphorylated by hyperosmolarity and, in vitro, by JNK, which can also be activated by hyperosmolarity[62,63]. As an upstream kinase to NKCC1, SPAK can associate through its conserved C-terminal domain with the (R/K) FX (V/I) motif of NKCC1 and phosphorylate Thr203, Thr207, and Thr212 residues on NKCC1, thereby playing an important role in inflammation[45,64,65]. However, SPAK alone is unable to activate NKCC1. SPAK is a substrate of WNK1/4, which are serine threonine kinases lacking a lysine in subdomain I of the catalytic domain[66]. SPAK physically associates through its conserved C-terminal domain with the C-terminus of WNK, resulting in phosphorylation and activation of SPAK by WNK. WNK is also unable to activate NKCC1 in the absence of SPAK, indicating that this association of SPAK with WNK is required for SPAK-dependent phosphorylation and activation of NKCC1. A mutation of WNK1 is involved in the pathogenesis of pseudohypoaldosteronism type II (PHAII), characterized by hypertension and hyperkalemia[67].
SPAK can also activate p38 pathways in different cell types[51,68,69] to play a role in cell differentiation; an observation that may be relevant in the context of the known relationship between the p38 pathway and inflammation[17,70-74]. Interestingly, p38 activation has been noted in damaged corneal epithelial tissue and in an in vitro intestinal epithelial restitution model[75-78], suggesting that under some circumstances p38 may be involved in regulating cell motility and wound healing. Protein kinase C θ (PKCθ) is known to be an intestinal inflammation-related kinase[79]. By associating with Rho GTPases, PKCθ migrates from the cytosol to the membrane and the actin cytoskeleton[80], where SPAK may act as both a substrate and target of PKCθ in a TCR/CD28-induced signaling pathway that leads selectively to AP-1 activation, T-cell transformation and proliferation, and IL-2 production[81]. SPAK is also known to associate with F-actin under conditions of stress, which, along with the activation and phosphorylation of myosin light chain kinase (MLCK), leads to cytoskeleton rearrangement[47,52]. Fray, the Drosophila orthologue of mammalian SPAK, has been shown to participate in the activation of the JNK pathway by sorbitol[47]. Fray probably functions by activating MAP3K, leading to activation of MAP2K/MEK4 and MEK7, and ultimately, JNK activation.
Accumulating evidence points to the important role that SPAK plays in the physiology and pathogenesis of intestinal inflammation (Figure 3). First, by activating and phosphorylating p38, Ap-1, NKCC1, as well as p21-activated protein kinase 1 (PAK1, another Ste20 line kinase), SPAK induces the transcription of inflammation-related genes or modulates the function of inflammation-related proteins. Second, SPAK is activated and phosphorylated by WNK1/4, PKCtheta; and MLCK. In addition, SPAK has been reported to associate with the heat shock protein HSP105, the cytoskeleton protein gelsolin, and the apoptosis-associated tyrosine kinase AATYK. We have observed that SPAK can increase the permeability of Caco2-BBE cells (unpublished observations). Additional unpublished data indicate that colonic epithelial SPAK expression is increased in IBD patients and in mice with experimentally induced colitis. Importantly, we have also found that the pro-inflammatory cytokine, TNF-α, increases colonic SPAK expression, an observation that underscores the importance of SPAK in the pathogenesis of intestinal inflammation.
PERSPECTIVE
Increased permeability across the mucosal epithelial barrier resulting from loss of structural integrity and/or abnormal transepithelial transport is thought to be one of the main functional changes that lead to IBD. Numerous studies have focused on epithelial barrier function, measuring transepithelial electrical resistance (TER), which is known to be decreased in intestinal epithelium by over-expression of SPAK[51]. Other studies have assessed cell adhesion and migration, providing a measure of wound healing. The pro-inflammatory cytokine TNF-α is both necessary and sufficient to trigger the onset of IBD. In fact, nearly half of the drugs used for the treatment of IBD target TNF-α. In in vitro studies, we have found that TNF-α increases SPAK expression in intestinal epithelial cells in a dose- and time- dependent manner (unpublished data). It is therefore reasonable to speculate that the regulation of SPAK by TNF-α could account for TNF-α-mediated alterations of barrier function and inflammation in intestinal epithelial cells. Additional studies on the role of SPAK in intestinal barrier function would likely substantially advance the field of IBD.
Intestinal inflammation is usually associated with hyper-osmotic status in the lumen. The WNK1/4-SPAK-NKCC1 pathway has been highlighted in this context as a molecular mechanism that may contribute to ion transport and cell volume changes. This pathway, together with its interactions with other related molecules, such as MLCK, claudin and zo-1, may play an important role in maintaining cell shape, since the epithelial cell tight junctions that play a dominant role in TER would collapse in IBD. In short, more attention should be paid to tight junction and cell volume regulation as important contributing factors in IBD.
It should be evident from this review that SPAK occupies an important intracellular position, integrating extra-cellular pro-inflammatory signals and converting them into pro-inflammatory cellular responses. Given its unique position at the crossroads of multiple pathways, SPAK appears to represent an attractive target for developing effective and efficient strategies to treat IBD. Continuing work along the lines suggested above could make important contributions to the effort to realize the potential of this therapeutic approach.
Peer reviewer: Alessandro Fichera, MD, FACS, FASCRS, Assistant Professor, Department of Surgery-University of Chicago, 5841 S. Maryland Ave, MC 5031, Chicago, IL 60637, United States
S- Editor Tian L L- Editor Anand BS E- Editor Lin YP