Published online Jan 28, 2008. doi: 10.3748/wjg.14.590
Revised: September 10, 2007
Published online: January 28, 2008
AIM: To investigate the protective effect of target suppression of uncoupling protein-2 (UCP-2) on ischemia/reperfusion (I/R) injury in fatty liver in ob/ob mice.
METHODS: Plasmids suppressing UCP-2 expression were constructed, and transfected into fatty liver cells cultured in vitro and the ob/ob mouse I/R injury model. Serum tumor necrosis factor (TNF)-α levels, UCP-2 mRNA expression, alanine aminotransferase (ALT) levels in ob/ob mice were tested, and the pathological changes in fatty liver were observed in experimental and control groups.
RESULTS: In ob/ob mouse I/R models, serum TNF-α levels were significantly higher than in normal controls. After the plasmids were transfected into the cultured cells and animal models, expression of UCP-2 mRNA was significantly reduced as compared with that in the control group (21.56 ± 0.15vs 2-0.45 ± 0.15, P < 0.05). In ob/ob mouse models, in which expression of UCP-2 was suppressed, serum ALT levels were significantly lower than those of other groups, and pathological analysis revealed that injury of liver tissues was significantly alleviated.
CONCLUSION: The target suppression of UCP-2 expression in fatty liver can alleviate the I/R injury in the ob/ob mice.
- Citation: Wan CD, Wang CY, Liu T, Cheng R, Wang HB. Alleviation of ischemia/reperfusion injury in ob/ob mice by inhibiting UCP-2 expression in fatty liver. World J Gastroenterol 2008; 14(4): 590-594
- URL: https://www.wjgnet.com/1007-9327/full/v14/i4/590.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.590
It has been reported that primary graft non-function (PNF) occurs in over 25% of donor liver grafts with a lipid volume of > 30%[12]. Previous studies have discovered that PNF of fatty liver after liver transplantation is related to liver ischemia/reperfusion (I/R) injury during transplantation[3–5]. Recently, it has been discovered that one kind of mitochondrial protein, uncoupling protein-2 (UCP-2), may play an important role in I/R injury of liver transplantation[67]. UCP is a protein of the inner mitochondrial membrane and acts as a proton carrier to allow the dissipation of the proton electrochemical gradient, without phosphorylation of ADP to ATP, which results in the uncoupling of oxidation and ATP synthesis[8–10]. The increased expression of UCP-2 leads to a decrease in ATP in liver mitochondria, therefore, the fatty liver is more susceptible to injury than the normal liver under stress, e.g. transplantation[11]. Previous studies have indicated that polyunsaturated fatty acids can stimulate UCP-2 expression in fatty hepatocytes via a peroxisome proliferation activated receptor-α (PPAR-α) mediated pathway[1213], and reactive oxygen species (ROS) generated intracellularly during lipid metabolism participate in UCP-2 induction[1415], In addition, tumor necrosis factor (TNF)-α also increases mitochondrial oxidant production and induces expression of UCP-2 in fatty hepatocytes[1617]. In the previous study by Wan et al, caerulomycin (a specific inhibitor of fatty acid synthetase) preconditioning suppressed the expression of UCP-2 in the fatty liver of ob/ob mice, which suggests that preconditioning alleviates the injury of liver cells following transplantation[18]. Caerulomycin cannot be widely used in clinical practice because it leads to metabolic disorders[1920]. In this study, an antisense technique was used to directly suppress the expression of UCP-2 in fatty liver tissues, and the protective effects of UCP-2 expression inhibition on I/R injury in ob/ob mice was evaluated.
Female 4-6-wk-old obesity mice (C57BL ob/ob) and inbred 10-12-wk-old normal mice (C57BL lean) were bought from the Model Animal Research Center of Nanjing University (China). Other reagents and equipment were from the following sources: collagenase IV (Gibco, USA), Percoll (Second Factory of Shanghai Biochemical Reagents, China), HEPES (Sigma, USA), EGTA (Sigma, USA), Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, USA), TNF-α (Sigma, USA), fetal bovine serum (FBS, Shanghai Huamei Bio-engineering Co., China), Ca2+-free Hank’s solution and PBS (self prepared), ELISA kit (R&D, USA), TRIzol (Gibco, USA), SYBR Green I fluorescent probe, FTC-200 type real-time fluorescent quantitative PCR instrument and fluorescent quantitative PCR analysis software (Shanghai Fengling Bio-tech, China), CO2 incubator (Heraeus, USA), UCP-2 primer (Shanghai Bio-engineering), RibocloneR M-MLV(H-) cDNA synthesis system (Promega, USA), eukaryotic expression carrier pEGFP2-C2 (Clontech, USA), restriction endonucleases XhoI and SalI, T4 DNA lygase (Huamei Bio-engineering Co), Taq enzyme (Takara, Japan), plasmid extraction purification kit, PCR recycling kit and gel DNA recovery kit (Shanghua Shunhua Bio-engineering, China).
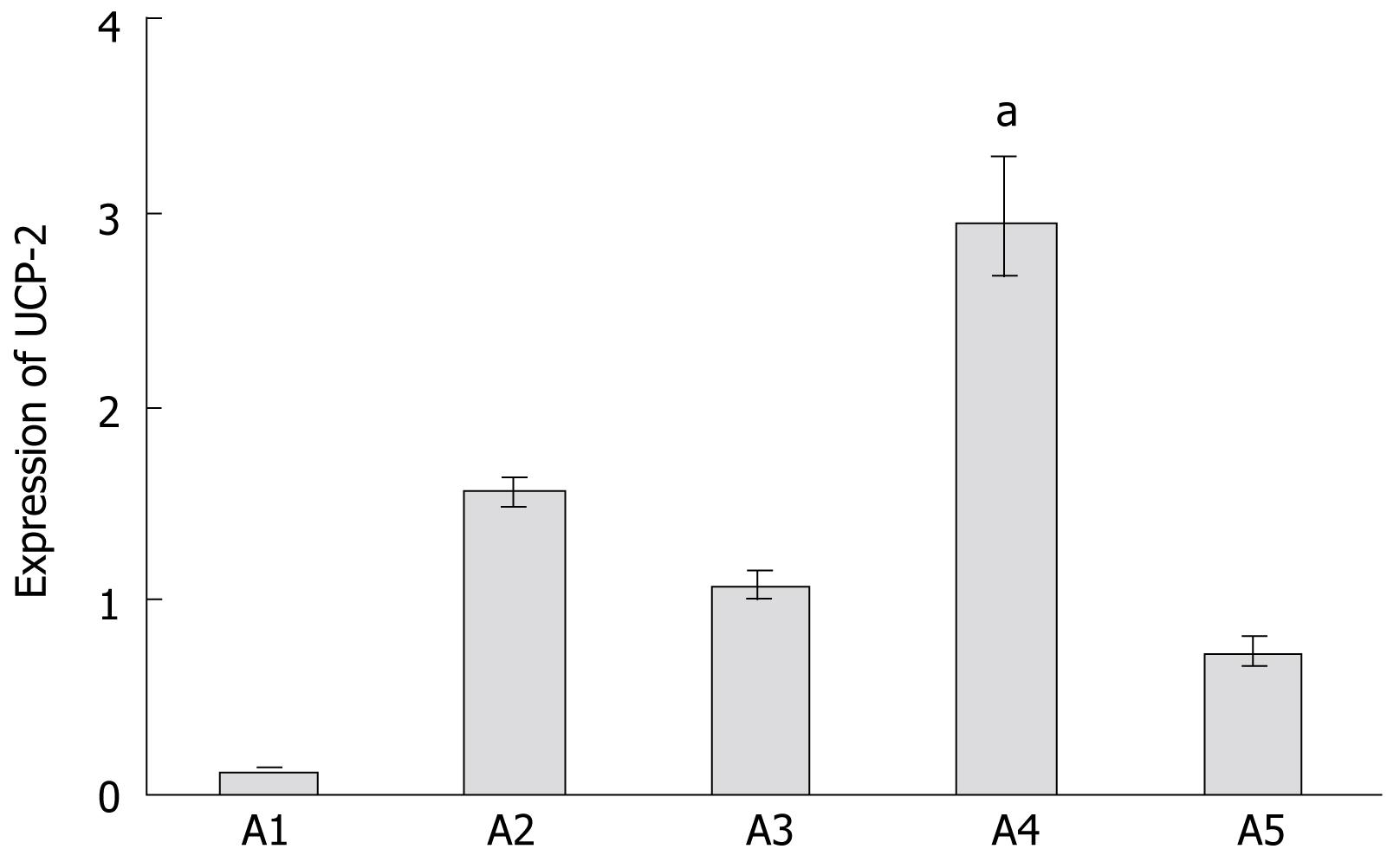
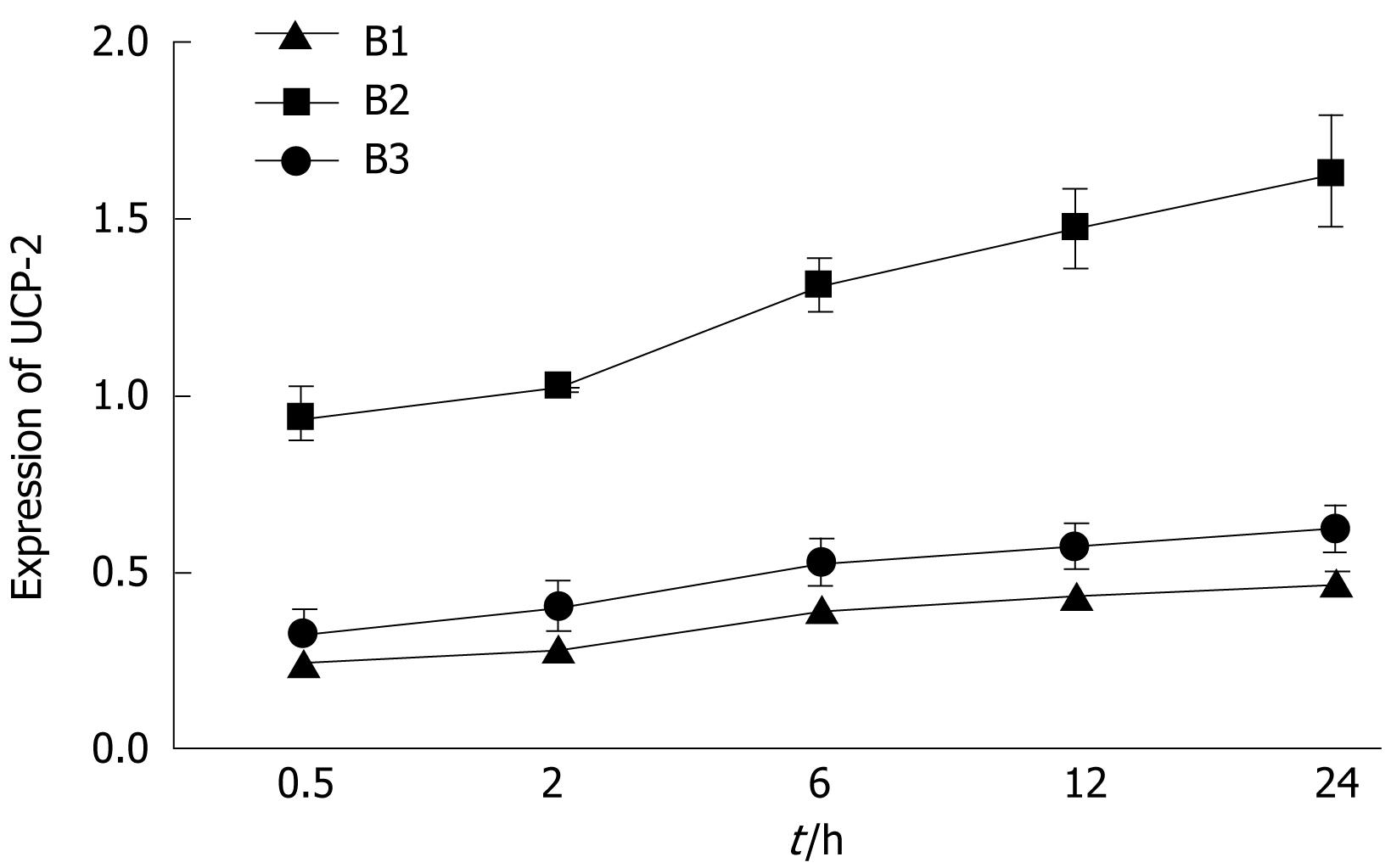
There were five in vitro experimental groups: normal hepatocyte culture (lean liver cells, A1 group); normal hepatocyte culture + TNF-α (lean liver cells + TNF-α, A2 group); fatty liver cell culture (A3 group); fatty liver cell culture + TNF-α treatment (A4 group); and fatty liver cell culture + TNF-α treatment + UCP-2 inhibition (A5 group). There were three in vivo experimental groups (n = 20 in each group): normal mouse I/R group (lean mice, B1 group); ob/ob mouse I/R group (obesity mice, B2 group); and ob/ob mouse I/R + UCP-2 inhibition (B3 group).
Under anesthesia, mice were laparotomized, the portal and hepatic veins were ligated by non-invasive vessel clamp for 15 min, then re-opened to establish the I/R model. Hepatocytes were isolated using the collagenase perfusion method[21]. Mice were anesthetized and laparotomized to explore the liver hilus. After intubation of the portal vein, HEPES-buffered Hank’s solution (pH 7.4) containing EGTA was perfused into the portal vein at 37°C, and subsequently, the tissues were perfused with collagenase IV buffer (0.5%) containing Ca2+ for digestion. Digested hepatocytes were harvested, re-suspended in culture medium, filtered through a sieve, centrifuged at low speed, and washed with PBS. Non-parenchymal cells were removed with Percoll by centrifugation. Hepatocytes were washed with PBS, centrifuged and stained with Trypan blue to assess cell viability. Hepatocytes were prepared into single cell suspensions and added to a six-well culture plate (1.2 × 106 cells/well) with addition of 2 mL DMEM containing 5% FBS incubated in a 5% CO2 incubator at 37°C. Eight hours later, the solution was changed, and then changed every 48 h.
Mouse UCP-2 mRNA was extracted from adipose tissue and a cDNA library. Mouse full-length UCP-2 cDNA was synthesized by the Riboclone M-MLV(H-) cDNA synthesis technique and PCR. The primer was designed according to the Green fluorescent protein (GFP) reading frame of eukaryotic expression carrier pEGFP2-C2, which allowed the coding region reading frame of UCP-2 consistent with that of GFP. Antisense plasmid was constructed with a pair of primers with SalI restriction sites at the 5’ end and XhoI restriction sites at the 3’ end. Sequences of primers used for construction of UCP-2 fluorescent eukaryotic expression carrier, which was synthesized by Shanghai Bio-engineering was as follows:
P1 SalI5’-3’: 5’-ATGTCGACGTCGGAGATACCAGAGCACT-3’;
P2 XhoI5’-3’: 5’-TTCTCGAGGTGACCTGC GCTGTG GTACT-3’.
PCR products were cut by XhoI and SalI restriction enzymes and linked to the enkaryotic expression carrier pEGFP-C2 (pEGFP-C2-UCP2-antisense). After the construction was identified to be successful, the transfected competent Escherichia coli were cultured and the extracted plasmids were recombined with reverse transcriptional viruses. In B3 group, the mice were transfected with UCP-2 plasmids via the portal vein at the same time as the vessel opening.
In all cultured cell groups, cells was added with different concentration of TNF-α (0, 2.5, 5, 10 ng/mL) at 48 h. In A5 group, fatty liver cells were transfected with UCP-2 plasmids after 8 h of culture when the medium were exchanged. Twenty-four hours after TNF-α stimulation, i.e. after 72 h culture, the cells and supernatants were harvested, centrifuged, and stored at -80°C for later use. In the I/R models, four animals in each group were killed at the time points of reperfusion, at 0.5, 2, 6, 12 and 25 h. Blood was taken from the heart, centrifuged at 3200 r/min for 5 min at room temperature, and the serum was separated, frozen and stored at -80°C for later detection. Simultaneously, some liver tissues were frozen and stored in liquid nitrogen, and other liver tissues were fixed with 4% paraformaldehyde, embedded with paraffin, stained with HE, and observed pathologically under a light microscope.
The TNF-α levels in the supernatants and serum were determined by ELISA, and each sample was duplicated. The serum levels of alanine aminotransferase (ALT) were tested by the velocity method.
Expression of UCP-2 in the cells and liver tissues was detected by using RT-PCR[22]. In brief, total RNA of each sample was extracted, the purity and concentration were assayed by DNA/Protein Analyzer, and the same total RNA concentration in each group was adjusted. cDNA was prepared by reverse transcription. The primer sequences of UCP-2 for PCR amplification were as follows: F, 5’-ATGGTTGGTTCAAGGCCAC-3’; R, 5’-TCATGAGGTTGGCTTTCAGG-3’[23]. The reaction conditions included: 94°C for 3 min; 94°C for 30 s, and 60°C for 1 min (45 cycles). The products were electrophoresed with 1.5% agarose gel. The sample expression of mRNA was calculated according to the following formula: Relative value of the detected samples = 2(ΔCtdetected sample-ΔCtβ-actin); ΔCt = Ctnegative control - Ctdetected sample.
UCP-2 mRNA, TNF-α and ALT levels were expressed as the mean ± SD and analyzed by using SPSS10.0 software. Comparison of the same index in various groups was performed by using ANOVA and the t test. P < 0.05 was considered statistically significant.
TNF-α levels in A2, A4 and A5 groups differed significantly. TNF-α levels at the five time points after reperfusion in the B1, B2 and B3 groups are shown in Table 1. Results indicated that TNF-α levels in the B2 and B3 groups were significantly higher than that in the B1 group (P < 0.05), but there was no significant difference between the B2 and B3 groups.
| Group | Serum TNF-αlevels | ||||
| 0.5 h | 2 h | 6 h | 12 h | 24 h | |
| B1 | 59 ± 7 | 81 ± 11 | 153 ± 23 | 196 ± 35 | 278 ± 43 |
| B2 | 106 ± 16a | 142 ± 24a | 231 ± 31a | 316 ± 47a | 394 ± 52a |
| B3 | 97 ± 17a | 151 ± 21a | 244 ± 27a | 332 ± 45a | 401 ± 48a |
Fluorescent quantitative PCR analysis revealed that in the A1, A2, A3, A4 and A5 groups, expression levels of UCP-2 were 0.125 (0.117-0.134), 1.558 (1.548-1.636), 1.079 (1.007-1.157), 2.949 (2.670-3.278) and 0.732 (0.660-0.812), respectively. It was indicated that in the A2 and A4 groups, the expression levels of UCP-2 were significantly higher than in the corresponding non-stimulated groups (P < 0.05). The expression level in the A4 group was significantly higher than in any other group, and in the A5 group, UCP-2 expression was significantly reduced as compared with that in the A4 group (P < 0.05). In the B1 group, expression levels of UCP-2 in liver tissues at the five time points after reperfusion were 0.252 (0.248-0.255), 0.287 (0.281-0.293), 0.401 (0.389-0.412), 0.432 (0.427-0.438) and 0.470 (0.438-0.503), respectively; those in the B2 group were 0.940 (0.883-1.000), 1.020 (1.016-1.024), 1.309 (1.241-1.382), 1.469 (1.360-1.587) and 1.629 (1.478-1.796), respectively; and those in the B3 group were 0.320 (0.255-0.402), 0.400 (0.333-0.481), 0.530 (0.467-0.602), 0.570 (0.510-0.638) and 0.621 (0.564-0.682), respectively. The expression level in B3 was significantly lower than in B2, which suggests that the expression of UCP-2 was inhibited (Figures 1 and 2).
In all I/R model mice, serum ALT levels were increased. ALT levels in the B1, B2 and B3 groups were 296 ± 65, 952 ± 147 and 301 ± 74 U/L, respectively. Statistical analysis revealed that ALT levels in the B2 group were significantly higher than those in the other two groups. Among the three groups, B3 had a significantly decreased ALT level after transfection of the plasmids that inhibited expression of UCP-2, which suggests that inhibition of UCP-2 expression can alleviate liver injury.
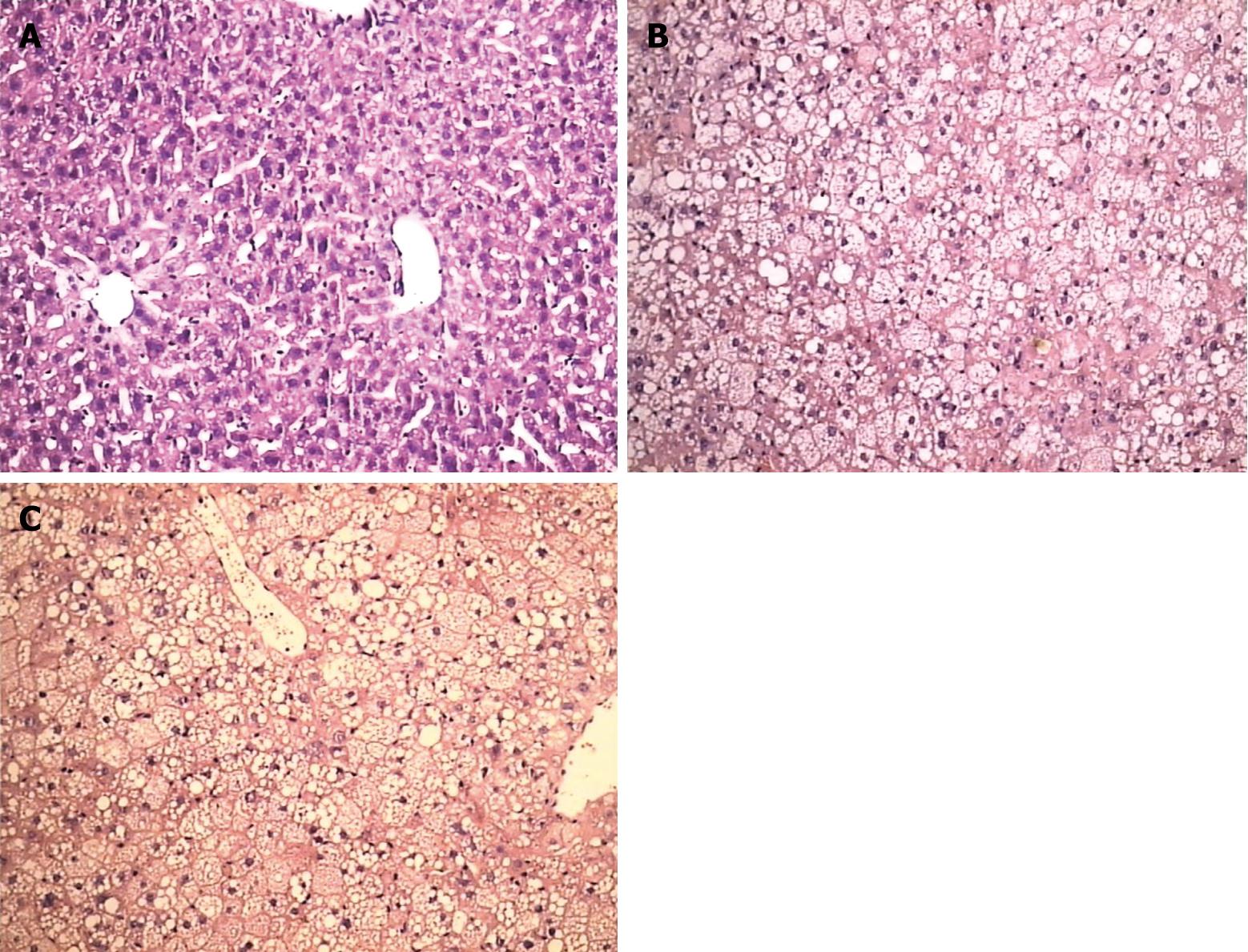
In the B1 group, swelling and hydropic degeneration of hepatocytes and hepatic sinusoid endothelial cells, stasis of hepatic sinusoids, and disorder of sinus funiculus were seen. There was exfoliation of partial endothelial cells in the central vein, vacuolar degeneration of hepatocytes on the peripheral zone, and local necrosis in hepatic lobules and at the region adjacent to the portal area. In the B2 group, the above pathological changes were aggravated, and showed multiple local necrosis. However, in the B3 group, the pathological changes in liver tissues were significantly alleviated as compared with those in B2 group (Figure 3).
It has been reported that UCP-2 is not expressed in normal liver tissues, but is over-expressed in fatty liver[24]. Moreover, the abnormal over-expression of UCP-2 in fatty liver can result in a decrease of hepatic ATP content[2526]. Studies by Chavin et al have discovered that in ob/ob mice (approximately 70%-80% of liver lipid content) expression of UCP-2 is four to five times higher than in normal liver tissues, and the ATP content is decreased in mitochondria[27]. In normal conditions, over-expression of UCP-2 does not result in abnormal levels of serum ALT, bile acid metabolites and clotting factors, but under stress conditions, e.g. I/R, over-expressed UCP-2 can lead to severe hepatic cell injury because of ATP reserve deficiency[2829]. In this study, it was found that in primary culture of fatty liver cells and ob/ob mouse I/R models, expression of UCP-2 was significantly higher than that in normal liver cells and normal mouse I/R models, and TNF-α levels in ob/ob mice were significantly higher than those in normal mice. However, in fatty liver cells stimulated with TNF-α, expression of UCP-2 was significantly higher than that in other cultured cells, which suggests that increased serum TNF-α levels can further enhance the increased expression of UCP-2 in fatty liver tissues. Previous studies have also revealed that TNF-α can induce the expression of UCP-2 in normal hepatocytes and mouse liver tissues, and it is believed that the increased TNF-α is related to lipopolysaccharide (LPS) stimulation[1730]. Therefore, in I/R, LPS stimulation can result in increased TNF-α levels, which in turn induce higher expression of UCP-2 in fatty liver cells, which causes further decrease of the ATP reserve in hepatocytes, which subsequently aggravates the I/R injury in fatty liver. In this study, the plasmids that inhibited UCP-2 expression were constructed and transfected into hepatocytes and the mouse I/R models, and it was found that expression of UCP-2 mRNA was significantly reduced, which was not related to TNF-α levels. Simultaneously, in the model with transfected mice, serum ALT levels were significantly lower than those in non-transfected mice, and hepatic pathological examination revealed that the I/R injury of liver tissues was alleviated, which suggests that targeting inhibition of UCP-2 expression in fatty liver may protect ob/ob mice from I/R injury. In future studies, the role of UCP-2 expression inhibition in obese mouse liver transplantation will be further investigated.
Primary graft non-function (PNF) after fatty liver transplantation was related to liver ischemia-reperfusion (I/R) injury during transplantation. Uncoupling protein 2 (UCP-2) has shown a close relationship with I/R injury of fatty liver transplantation by mediating proton leak and interfering with mitochondrial ATP synthesis
The impact of UCP-2 deficiency during acute liver injury has been investigated recently, and it has been found that lack of UCP-2 reduces Fas-mediated liver injury in ob/ob mice. Although ablation of UCP2 may have no significant impact on steatosis-induced changes in ROS production of Kupffer cells, it alleviates the energy compromise in acutely challenged fatty hepatocytes.
UCP-2 has been reported to be closely related to I/R injury of steatotic liver. However, the direct link between UCP-2 expression and susceptibility of fatty liver to I/R injury is lacking. This study considered UCP-2 as a therapeutic target and investigated its role in fatty liver I/R injury in vitro and in vivo. The results showed that suppression of UCP-2 expression alleviated I/R injury of fatty liver in mice. This may provide a new gene therapy target.
The result of this study indicated that UCP-2 could be an effective gene therapy target of fatty liver I/R injury. By suppressing UCP-2 expression, more steatotic liver donors may be used in liver transplantation, and partially alleviate the shortage of donor livers.
UCP-2 is an inner mitochondrial membrane carrier. It mediates proton leak and allows the dissipation of the proton electrochemical gradient, which results in the uncoupling of oxidation and ATP synthesis. ROS include superoxide, hydrogen peroxide, and hydroxyl radicals. At low levels, these species may function in cell signaling processes. At higher levels, these species may damage cellular macromolecules.
This is a well written and interesting manuscript. The authors demonstrated the alleviation of I/R injury in ob/ob mice by inhibiting UCP-2 expression.
| 1. | Ploeg RJ, D’Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. |
| 2. | Markin RS, Wisecarver JL, Radio SJ, Stratta RJ, Langnas AN, Hirst K, Shaw BW Jr. Frozen section evaluation of donor livers before transplantation. Transplantation. 1993;56:1403-1409. |
| 3. | Husberg BS, Genyk YS, Klintmalm GB. A new rat model for studies of the ischemic injury after transplantation of fatty livers: improvement after postoperative administration of prostaglandin. Transplantation. 1994;57:457-458. |
| 4. | Koneru B, Reddy MC, dela Torre AN, Patel D, Ippolito T, Ferrante RJ. Studies of hepatic warm ischemia in the obese Zucker rat. Transplantation. 1995;59:942-946. |
| 5. | Serafin A, Rosello-Catafau J, Prats N, Xaus C, Gelpi E, Peralta C. Ischemic preconditioning increases the tolerance of Fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587-601. |
| 6. | Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet. 1997;15:269-272. |
| 7. | Uchino S, Yamaguchi Y, Furuhashi T, Wang FS, Zhang JL, Okabe K, Kihara S, Yamada S, Mori K, Ogawa M. Steatotic liver allografts up-regulate UCP-2 expression and suffer necrosis in rats. J Surg Res. 2004;120:73-82. |
| 8. | Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, Penicaud L, Casteilla L. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809-815. |
| 9. | Boss O, Muzzin P, Giacobino JP. The uncoupling proteins, a review. Eur J Endocrinol. 1998;139:1-9. |
| 11. | Lemasters JJ, Nieminen AL, Qian T, Trost LC, Herman B. The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol Cell Biochem. 1997;174:159-165. |
| 12. | Kelly LJ, Vicario PP, Thompson GM, Candelore MR, Doebber TW, Ventre J, Wu MS, Meurer R, Forrest MJ, Conner MW. Peroxisome proliferator-activated receptors gamma and alpha mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology. 1998;139:4920-4927. |
| 13. | Armstrong MB, Towle HC. Polyunsaturated fatty acids stimulate hepatic UCP-2 expression via a PPARalpha-mediated pathway. Am J Physiol Endocrinol Metab. 2001;281:E1197-E1204. |
| 14. | Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259-268. |
| 15. | Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100-124. |
| 16. | Murakami K, Kobayashi F, Ikegawa R, Koyama M, Shintani N, Yoshida T, Nakamura N, Kondo T. Metalloproteinase inhibitor prevents hepatic injury in endotoxemic mice. Eur J Pharmacol. 1998;341:105-110. |
| 17. | Lee FY, Li Y, Zhu H, Yang S, Lin HZ, Trush M, Diehl AM. Tumor necrosis factor increases mitochondrial oxidant production and induces expression of uncoupling protein-2 in the regenerating mice [correction of rat] liver. Hepatology. 1999;29:677-687. |
| 18. | Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976;40:681-697. |
| 19. | Dridi S, Ververken C, Hillgartner FB, Arckens L, Van der Gucht E, Cnops L, Decuypere E, Buyse J. FAS inhibitor cerulenin reduces food intake and melanocortin receptor gene expression without modulating the other (an)orexigenic neuropeptides in chickens. Am J Physiol Regul Integr Comp Physiol. 2006;291:R138-R147. |
| 20. | De Vos ML, Lawrence DS, Smith CD. Cellular pharmacology of cerulenin analogs that inhibit protein palmitoylation. Biochem Pharmacol. 2001;62:985-995. |
| 21. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. |
| 22. | Goodsaid FM, Palamanda JR, Montgomery D, Mandakas G, Gu C, Li Z, You X, Norton L, Smith R, Chu I. Assessment of temporal biochemical and gene transcription changes in rat liver cytochrome P450: utility of real-time quantitative RT-PCR. Pharm Res. 2003;20:1373-1380. |
| 23. | Damon M, Vincent A, Lombardi A, Herpin P. First evidence of uncoupling protein-2 (UCP-2) and -3 (UCP-3) gene expression in piglet skeletal muscle and adipose tissue. Gene. 2000;246:133-141. |
| 24. | Cortez-Pinto H, Zhi Lin H, Qi Yang S, Odwin Da Costa S, Diehl AM. Lipids up-regulate uncoupling protein 2 expression in rat hepatocytes. Gastroenterology. 1999;116:1184-1193. |
| 25. | Stuart JA, Harper JA, Brindle KM, Jekabsons MB, Brand MD. Physiological levels of mammalian uncoupling protein 2 do not uncouple yeast mitochondria. J Biol Chem. 2001;276:18633-18639. |
| 26. | Chan CB, De Leo D, Joseph JW, McQuaid TS, Ha XF, Xu F, Tsushima RG, Pennefather PS, Salapatek AM, Wheeler MB. Increased uncoupling protein-2 levels in beta-cells are associated with impaired glucose-stimulated insulin secretion: mechanism of action. Diabetes. 2001;50:1302-1310. |
| 27. | Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, Walajtys-Rode E, Rashid A, Chen CH, Huang CC. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692-5700. |
| 28. | Clavien PA, Selzner M. Hepatic steatosis and transplantation. Liver Transpl. 2002;8:980. |
| 29. | Rashid A, Wu TC, Huang CC, Chen CH, Lin HZ, Yang SQ, Lee FY, Diehl AM. Mitochondrial proteins that regulate apoptosis and necrosis are induced in mouse fatty liver. Hepatology. 1999;29:1131-1138. |
| 30. | Cortez-Pinto H, Yang SQ, Lin HZ, Costa S, Hwang CS, Lane MD, Bagby G, Diehl AM. Bacterial lipopolysaccharide induces uncoupling protein-2 expression in hepatocytes by a tumor necrosis factor-alpha-dependent mechanism. Biochem Biophys Res Commun. 1998;251:313-319. |