Published online Oct 21, 2008. doi: 10.3748/wjg.14.5996
Revised: July 13, 2008
Accepted: July 20, 2008
Published online: October 21, 2008
AIM: To investigate the effects of (dietary) glycine against oxidant-induced injury caused by bile duct ligation (BDL).
METHODS: Either a diet containing 5% glycine or a standard diet was fed to male Sprague-Dawley (SD) rats. Three days later, BDL or sham-operation was performed. Rats were sacrificed 1 to 3 d after BDL. The influence of deoxycholic acid (DCA) in the presence or absence of glycine on liver cells was determined by measurement of calcium and chloride influx in cultivated Kupffer cells and lactate dehydrogenase (LDH) activity was determined in the supernatant of cultivated hepatocytes.
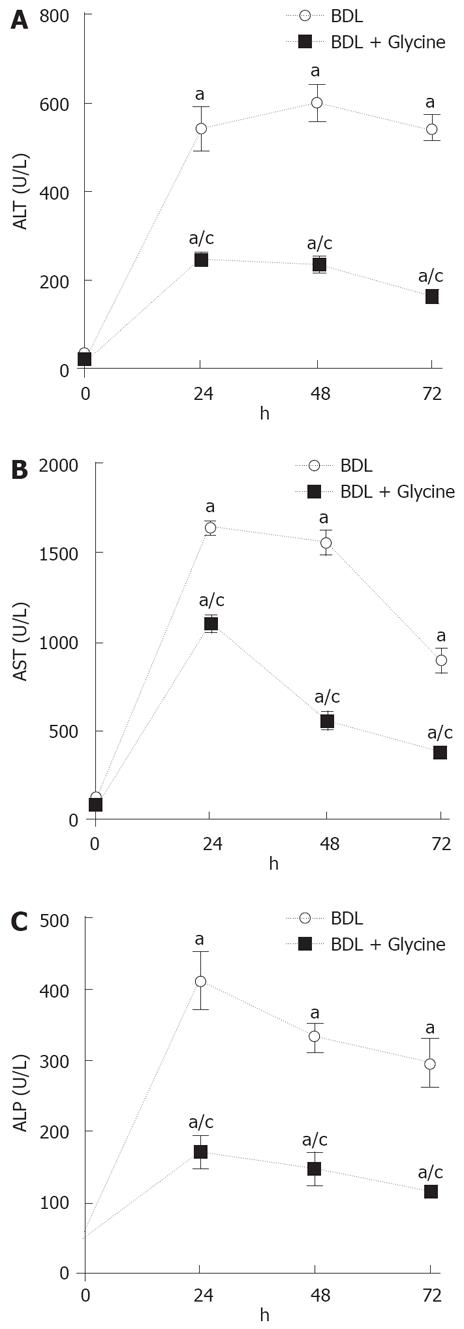
RESULTS: Serum alanine transaminase levels increased to about 600 U/L 1 d after BDL. However, enzyme release was blunted by about two third in rats receiving glycine. Release of the alkaline phosphatase and aspartate aminotransferase was also blocked significantly in the group fed glycine. Focal necrosis was observed 2 d after BDL. Glycine partially blocked the histopathological changes. Incubation of Kupffer cells with DCA led to increased intracellular calcium that could be blocked by incubation with glycine. However, systemic blockage of Kupffer cells with gadolinium chloride had no effects on transaminase release. Incubation of isolated hepatocytes with DCA led to a significant release of LDH after 4 h. This release was largely blocked when incubation with glycine was performed.
CONCLUSION: These data indicate that glycine significantly decreased liver injury, most likely by a direct effect on hepatocytes. Kupffer cells do not appear to play an important role in the pathological changes caused by cholestasis.
- Citation: Froh M, Zhong Z, Walbrun P, Lehnert M, Netter S, Wiest R, Conzelmann L, Gäbele E, Hellerbrand C, Schölmerich J, Thurman RG. Dietary glycine blunts liver injury after bile duct ligation in rats. World J Gastroenterol 2008; 14(39): 5996-6003
- URL: https://www.wjgnet.com/1007-9327/full/v14/i39/5996.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5996
Chronic cholestasis liver diseases lead to liver injury and ultimately progress to portal fibrosis, cirrhosis, and end-stage liver disease requiring liver transplantation. They include primary sclerosing cholangitis, primary biliary cirrhosis, extrahepatic biliary atresia, idiopathic adulthood ductopenia, idiopathic neonatal hepatitis, Byler’s disease, and arteriohepatic dysplasia[1-4]. Various drugs, total parenteral nutrition, sarcoidosis, chronic liver transplant rejection, and graft-versus-host disease may also cause chronic cholestasis[5-7]. Currently the most promising therapy for chronic cholestatic liver diseases is ursodeoxycholic acid[8], that may delay liver disease progression, but cannot prevent liver injury or fibrosis[9]. The pathophysiology of cholestasis induced liver injury and fibrosis remains unclear. One possible mechanism is that hepatic accumulation of hydrophobic bile acids causes oxidative stress in the liver[10]. Previous studies showed that hepatic mitochondria generate reactive oxygen species when isolated hepatocytes are exposed to hydrophobic bile acids[10,11]. This mitochondrial free radical production may be an important mechanism of cholestatic liver injury. However, the major source of free radicals remains unclear. One possible cell type responsible for the generation of free radicals could be the Kupffer cells, the resident macrophages of the liver. They are involved in disease states, such as endotoxin shock[12], alcoholic liver diseases[13], and other toxicant-induced liver injury by releasing eicosanoids, inflammatory cytokines (IL-1, IL-6, TNF-α), and free radical species[14].
Glycine, a simple nonessential amino acid, is a well-known inhibitory neurotransmitter in the central nervous system that acts via a glycine-gated chloride channel and has been shown to be protective against hypoxia, ischemia, and various cytotoxic substances[15-17]. Furthermore, it was demonstrated that dietary glycine protected both, the lung and the liver against lethal doses of endotoxin in the rat[18] and improved graft survival after liver transplantation[19].
Based on pharmacological data[15-17], a glycine-gated chloride channel was detected in Kupffer cells and other macrophages[20] that influenced the activation process of these cells. Glycine binds to and opens a chloride channel at the cell membrane, causing cell hyperpolarization that subsequently blocks calcium influx[20]. Thus it prevents the activation of intracellular signaling cascades.
Accordingly, we hypothesized in this study that dietary glycine has a protective effect in liver injury after bile duct ligation (BDL) by preventing activation of Kupffer cells.
Adult male Sprague-Dawley (SD) rats (200-250 g) were housed four to a cage in a facility approved by the Association for the Accreditation and Assessment of Laboratory Animal Care International. Three days before surgery, rats were randomly assigned to two experimental groups and fed either a semisynthetic powdered diet (Teklad test diets, Madison, WI, USA) containing 5% glycine and 15% casein (glycine group) or 20% casein (control group). After surgery, each rat continued to receive its assigned diet throughout the entire experimental period. All animals received humane care in compliance with guidelines approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Rats underwent BDL and transection or sham operation under ether anesthesia, as described elsewhere[21]. Briefly, the common bile duct was located through a midline abdominal incision, double ligated near the liver, and transected between ligatures. Control rats received sham operation underwent the same procedure except that the bile duct was only gently manipulated, but not ligated or sectioned. Some rats were given gadolinium chloride (GdCl3; 20 mg/kg body weight iv 24 h before BDL) to selectively deplete Kupffer cells. Rats were sacrificed 1 to 3 d after BDL or sham operation for further investigations (n = 5-6 per group).
Blood samples were collected from the tail veins at times indicated. Serum alkaline phosphatase (ALP), alanine aminotransaminase (ALT), aspartate aminotransaminase (AST), and bilirubin were measured using analytic kits from Sigma (St. Louis, MO, USA). On the day of death, each rat was anesthetized with pentobarbital sodium (75 mg/kg ip), the abdomen was opened, and the portal vein was cannulated with a 20-gauge cannula. The liver was rinsed using a syringe containing 10 mL physiological saline, followed by slow infusion of 5 mL 10% buffered formaldehyde (VWR International, West Chester, PA, USA). After 48 h in fixative, paraffin sections were prepared and stained with hematoxylin-eosin. Liver pathology was scored in a blinded manner based on a scoring system described by Nanji et al[22] (inflammation and necrosis: 1 focus per low-power field: 1+; 2 or more foci: 2+).
Kupffer cells were isolated by collagenase digestion and differential centrifugation using Percoll (Sigma, Taufkirchen, Germany) as described elsewhere[23] with slight modifications[24]. Briefly, the liver was perfused through the portal vein with Ca2+- and Mg2+-free HBSS at 37°C for 10 min at a flow rate of 20 mL/min. Subsequently, perfusion was with HBSS containing 0.02% collagenase IV (Sigma) at 37°C for 10 min. After the liver was digested, it was excised and cut into small pieces in collagenase buffer. The suspension was filtered through nylon gauze and the filtrate was centrifuged two times at 70 g for 3 min at 4°C to remove parenchymal cells. The nonparenchymal cell fraction (mostly Kupffer cells) in the supernatant was washed with buffer and centrifuged at 650 g for 7 min at 4°C. Cell pellets were suspended in buffer and centrifuged on a density cushion of Percoll (25% and 50%) at 1800 g for 15 min at 4°C. The Kupffer cell fraction was collected, centrifuged at 650 g for 7 min and suspended again in buffer. Viability of cells was determined by Trypan blue exclusion. Purity (> 90%) of Kupffer cell cultures was evaluated by morphological observation and by phagocytic uptake of FITC-labeled 1 μm latex-beads. Kupffer cells were cultured in RPMI-1640 medium (Sigma) supplemented with 10% FCS and antibiotics/antimycotics (100 U/mL of penicillin G, 100 μg/mL of streptomycin sulfate, and 0.25 μg/mL amphotericin B; Sigma) at 37°C in a 10% CO2-containing atmosphere. Nonadherent cells were removed after 30 min by replacing the culture medium. The parenchymal cell fraction (mostly hepatocytes) was also isolated by Percoll (50%) centrifugation as described previously[25] and cultured at 1 × 106 cells/well in RPMI 1640 medium (Sigma) containing 10% heat-inactivated fetal bovine serum and antibiotics as described above. Cells were cultured for 24 h before used for further experiments.
Intracellular Ca2+ concentration ([Ca2+]i) was measured fluorometrically using the fluorescent calcium indicator dye fura-2. KC (1 × 106 cells/plate) were incubated in modified HBSS (mHBSS; in mmol/L): 110 NaCl, 5 KCl, 0.3 Na2HPO4, 0.4 KH2PO4, 5.6 glucose, 0.8 MgSO4·7H2O, 4 NaHCO3, 1.26 CaCl2, 15 HEPES, pH 7.4 containing 5 μmol/L fura-2 AM (Molecular Probes, Eugene, OR, USA) at room temperature for 45 min. Coverslips plated with Kupffer cells were rinsed and placed in chambers with mHBSS at room temperature. Changes in fluorescence intensity of fura-2 at excitation wavelengths of 340 and 380 nm and emission at 510 nm were monitored in individual Kupffer cells. A Nikon inverted fluorescent microscope interfaced with dual-wavelength fluorescent photometer (Intracellular Imaging, Cincinnati, OH, USA) was used to ratiometrically determine [Ca2+]i. Data were collected and analyzed using InCyt software (Intracellular Imaging).
Twenty-four hours after isolation, hepatocytes were stimulated with deoxycholic acid (DCA; 0.1 mmol/L; Sigma) or normal saline in the presence or absence of glycine (1 mmol/L; Sigma). After 4 h of culture, supernatant was collected and LDH assays were performed via standard enzymatic techniques as described elsewhere[26].
Assays for uptake of 36Cl used an adaptation of a method described for neurons by Schwartz et al[27] and modified by Morrow and Paul[28]. Briefly, 2 × 106 Kupffer cells were plated on coverslips in 60 mm2 culture dishes and incubated as described above. After 24 h, media was replaced with HEPES buffer (20 mmol/L HEPES, 118 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgSO4, and 2.5 mmol/L CaCl2, pH 7.4) and allowed to equilibrate for 10 min at room temperature. Coverslips were gently blotted dry and incubated in a petri dish with 2 mL of buffer containing 2 mCi/mL 36Cl in the presence of glycine (1 mmol/L) and/or DCA (0.01 and 0.1 mmol/L) for 5 s. Chloride influx was linear between 2-10 s; thus, a 5 s incubation time was chosen for all experiments. Chloride influx was terminated by washing the coverslip with ice-cold buffer for 3 s followed by a second wash for 7 s[28]. Coverslips were placed in scintillation vials, and protein was solubilized by adding 1.6 mL NaOH (0.2 mol/L) for 2 h. An aliquot (0.16 mL) was collected for determination of protein by the method of Lowry et al[29]. Ecolume (10 mL) was added and radioactivity was determined by standard scintillation spectroscopy.
Data are presented as mean ± SD. ANOVA and the Student-Newman-Keuls post hoc tests were used for the determination of statistical significance between treatment groups, and P < 0.05 was selected before the study as the level of significance.
Boxplots illustrate median values and interquartile distance. The error bars represent the 5th and 95th percentiles.
In untreated rats fed a standard chow diet, serum alanine transaminase (ALT) levels average 40 U/L and were not significantly altered by sham operation (data not shown). One day after BDL, ALT increased to 541 U/L (Figure 1A), and remained elevated at day 2 and 3 after BDL with 599 U/L and 543 U/L, respectively. When rats were treated with dietary glycine, ALT levels increased to 248 U/L one day after BDL (Figure 1A). On day 2 and 3, ALT levels were also significantly decreased compared to bile duct-ligated rats fed a control diet (232 U/L on day 2 and 161 U/L on day 3). Serum aspartate aminotransferase (AST) and alkaline phosphatase (AP) levels, which were also measured at day 1, 2, and 3 after BDL, revealed similar results as ALT (Figure 1B and C).
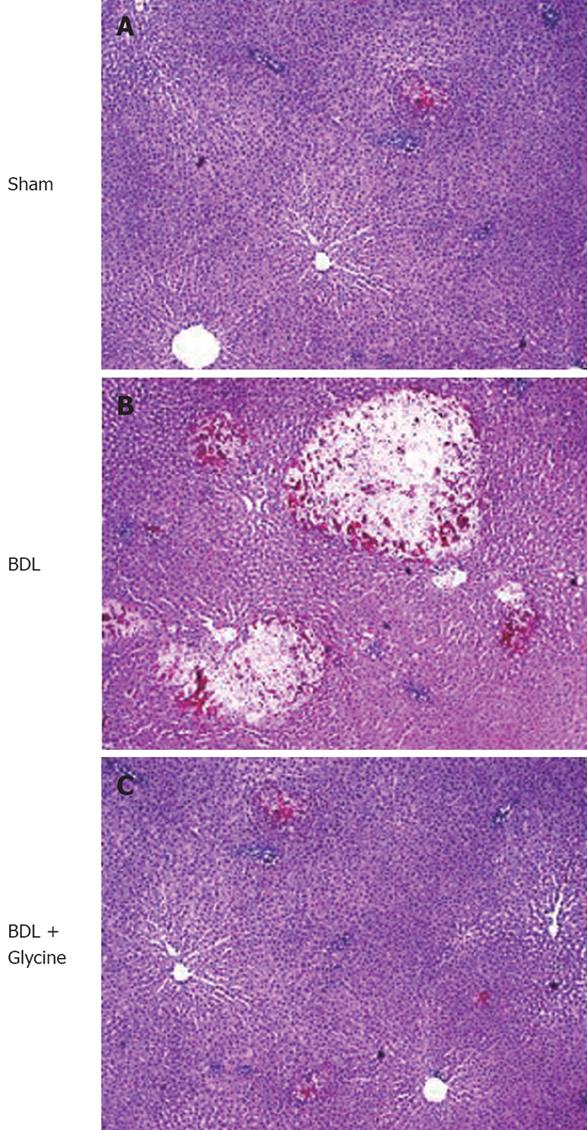
Normal liver architecture was observed in rats on a control and a glycine diet after sham operation (Figure 2A). Two days after BDL focal necrosis and white blood cell infiltration were detected in livers of rats receiving a standard diet (Figure 2B). These pathological changes were partially blocked in rats receiving dietary glycine (Figure 2C).
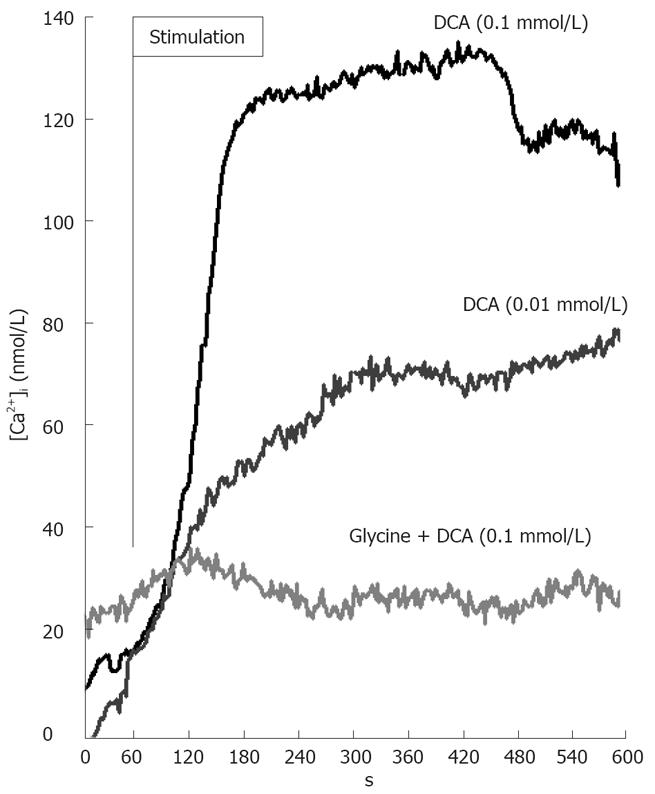
Intracellular calcium concentration ([Ca2+]i) in cultured Kupffer cells was determined fluorometrically with the calcium indicator fura-2 as described in MATERIALS AND METHODS. After the addition of 0.01 or 0.1 nmol/L DCA, [Ca2+]i levels increased as expected over the investigated time period of 10 min (Figure 3). Glycine (1 mmol/L) added 3 min before DCA inhibited this increase/induction in [Ca2+]i. Glycine alone had no detectable effect on [Ca2+]i (data not shown).
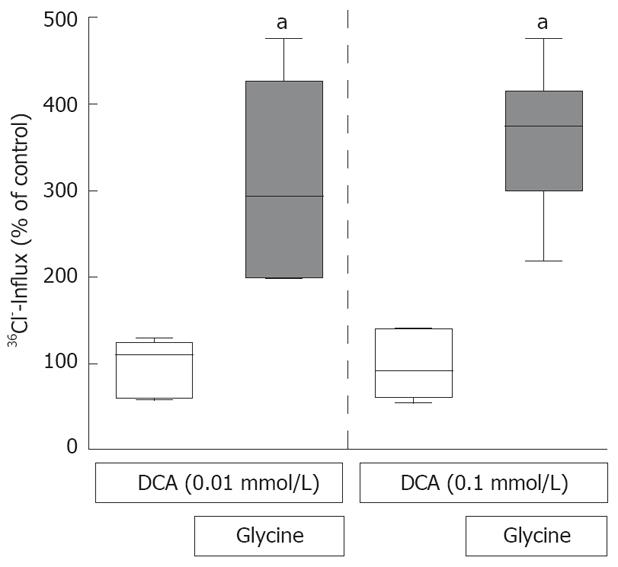
The glycine-gated chloride channel mediates the influx of chloride and hyperpolarizes the cells[30] thereby preventing DCA-induced increases of [Ca2+]i (Figure 3). Indeed, glycine (1 mmol/L) caused a significant, about 4-fold influx of radiolabeled chloride in the presence of DCA (0.01 or 0.1 nmol/L) (Figure 4). This effect of glycine was significantly reduced by the classical glycine-gated chloride channel antagonist strychnine (data not shown).
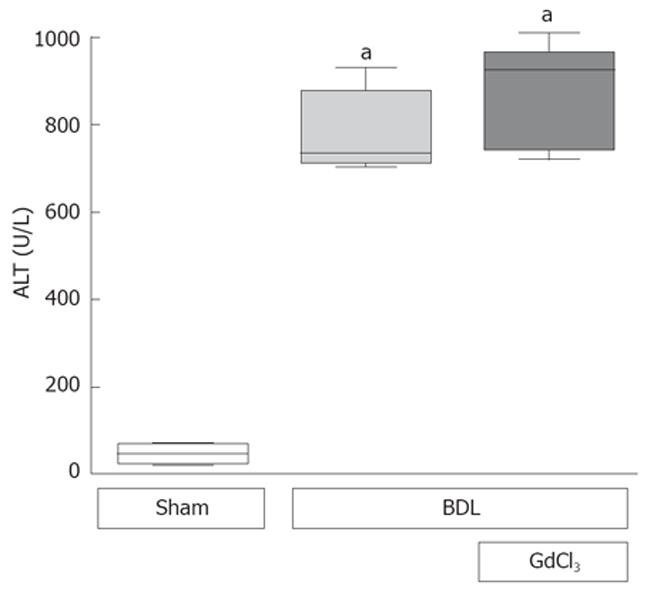
To investigate whether Kupffer cells play an important role in cholestatic liver injury, rats were treated with GdCl3 that selectively depletes Kupffer cells, before BDL. Suppression of Kupffer cells with GdCl3 neither blunted ALT release (Figure 5) nor attenuated focal necrosis after BDL (data not shown), confirming our previously published data[31]. In detail, ALT levels increased up to 779 U/L (± 53) 24 h after BDL. Pretreatment with GdCl3 one day before BDL had no effect on this transaminase release after BDL (870 ± 78 U/L).
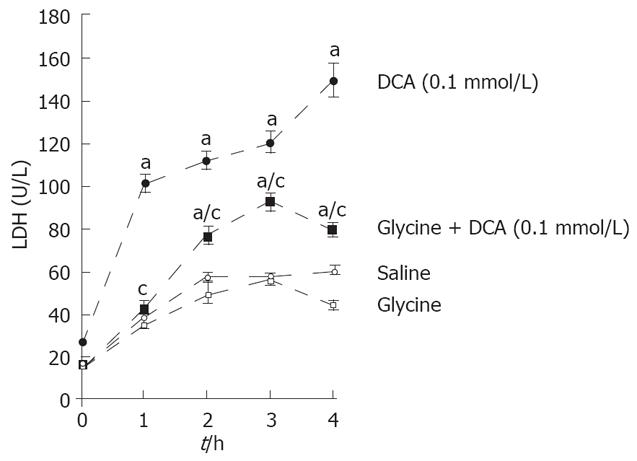
Incubation of isolated hepatocytes with DCA (0.1 mmol/L) led to a specific release of LDH (Figure 6) over the investigated time period (1 h incubation time: 101 ± 8 U/L; 2 h incubation time: 112 ± 7 U/L; 3 h incubation time: 119 ± 8 U/L; 4 h incubation time: 149 ± 18 U/L). This release was significantly blocked when glycine was simultaneously added (1 h incubation time: 42 ± 7 U/L; 2 h incubation time: 77 ± 7 U/L; 3 h incubation time: 92 ± 8 U/L; 4 h incubation time: 79 ± 6 U/L). Incubation with glycine or saline alone had almost no effect on the release of LDH (Figure 6).
Chronic cholestatic liver diseases are one of the leading indications for liver transplantation in children and adults[1,2]. Therefore, new strategies to reduce the pathological changes caused by (chronic) cholestasis are needed, because current therapies, such as ursodeoxycholic acid[9], do not prevent liver injury. Oxidative stress and activation of Kupffer cells are probably involved in the pathogenesis of liver injury caused by cholestasis. Glycine has been shown to be an anti-inflammatory amino acid acting via inhibitory effects on several white blood cells, including Kupffer cells[15-19]. Glycine activates a chloride channel, leading to cell hyperpolarization and a concomitant blocking of calcium influx via a voltage dependent calcium channel[20]. Accordingly, we hypothesized that the activation of Kupffer cells and the associated free radical formation after BDL could be blocked by glycine, thus leading to a decreased liver injury.
In confirmation of previous work from our and several other laboratories[21,31-35], BDL caused hepatic enzyme release (Figure 1) and focal cell necrosis (Figure 2), as expected. However, hepatic enzyme release (ALT, AST, AP) was significantly blunted and histopathological changes were partially blocked in the group receiving dietary glycine. Incubation of Kupffer cells with DCA led to increased intracellular calcium that was inhibited by incubation with glycine (Figure 3), most likely thru a glycine stimulated influx of chloride (Figure 4). However, systemic blockage of Kupffer cells with gadolinium chloride had no effect on transaminase release (Figure 5), indicating a minor, if any role of Kupffer cells in the pathophysiology of experimental cholestasis. Incubation of hepatocytes with DCA in vitro led to a significant release of LDH that was reduced by glycine (Figure 6).
The protective effects of glycine are probably due to its direct effect on target cells or mediated by inhibition of inflammatory cell activation. Glycine appears to exert several protective effects, including anti-inflammatory, immunomodulatory and direct cytoprotective actions. The underlying mechanisms are not completely understood. Glycine decreases oxidative stress[15-19] by different and partly indirect mechanisms that prevent reactive oxygen species formation. Furthermore, glycine protects renal tubular cells, hepatocytes and endothelial cells against injury from hypoxia, ischemia-reperfusion and ATP depletion[16,36-39]. Most studies show that glycine protects plasma membrane integrity, but does not restore ATP levels or affect intracellular pH[16,38-40].
Activation of the glycine-gated chloride channel is another widely postulated mechanism for the effects of glycine. The glycine receptor exists in a wide variety of cells, beside its typical occurrence at the postsynaptic neuronal membranes of the spinal cord. Besides endothelial cells and renal proximal tubular cells[17,41], cells involved in inflammatory and immune responses, such as macrophages, monocytes, neutrophils, and T lymphocytes, express a glycine receptor[18,20,42-44]. Glycine acts thru its receptor on inflammatory cells, such as Kupffer cells, to suppress activation of transcription factors and the formation of free radicals and inflammatory cytokines. In the plasma membrane, glycine appears to activate a chloride channel[20,30] that stabilizes or hyperpolarizes the plasma membrane potential (Figure 4). As a consequence, agonist-induced opening of L-type voltage dependent calcium channels and the resulting increases in intracellular calcium ions are suppressed (Figure 3), which may account for the immunomodulatory and anti-inflammatory effects of glycine. By preventing Kupffer cell activation, a decreased formation of inflammatory and fibrogenic mediators may be achieved. However, the role of Kupffer cells in fibrosis is controversial. Destruction of Kupffer cells attenuated liver fibrosis caused by carbon tetrachloride[45]. By contrast, in a rat model of reversible biliary obstruction, inactivation of Kupffer cells impaired collagen metabolism and inhibited the resolution of fibrosis[46]. Kupffer cells release many mediators, like TNF-α, TGF-β, human growth factor, PDGF, and reactive oxygen species[47,48] that activate stellate cells leading to fibrosis. TNF-α production and NF-κB activation increase during cholestasis[49,50]. Activation of NF-κB, probably due to oxidative stress, could lead to expression of TNF-α. However, suppression of Kupffer cell function with GdCl3, a treatment that blocks carbon tetrachloride-induced fibrosis, did not attenuate injury caused by cholestasis (Figure 5) confirming previous work from our laboratory[31]. This finding indicates that Kupffer cells most likely do not play a prominent role in cholestasis-induced fibrosis in vivo and that glycine does not work exclusively by inhibiting Kupffer cell activation.
Recent work suggested that liver parenchymal cells at least contain a glycine dependent receptor. In isolated hepatocytes, glycine blocks the increase in intracellular calcium due to PGE2 and phenylephrine, an a1-type adrenergic receptor agonist[51]. Low-dose strychnine partially reverses the inhibition by glycine. When extracellular chloride is omitted, glycine is much less effective in preventing increases in intracellular calcium due to PGE2. These data suggested that hepatoprotection by glycine is, in part, due to its direct effect on hepatocytes via regulating of intracellular calcium[51]. Consistent with these earlier findings, a direct effect of glycine on LDH release in isolated hepatocytes was observed in the present study after DCA challenge (Figure 6). Nevertheless, the effect of the conjugation of glycine and the used “secondary bile acid” DCA on the ability to lyse cells directly and solubilize cellular and membrane components should be also considered.
In conclusion, we demonstrated that hepatic injury, due to BDL, is significantly reduced by dietary glycine. Moreover, the data indicate that glycine decreases liver injury under the conditions of experimental cholestasis thru a direct effect on hepatocytes. Surprisingly, Kupffer cells do not appear to play a major role in the pathological changes caused by cholestasis.
Chronic cholestasis leads to liver injury and will ultimately progress to portal fibrosis, cirrhosis and end-stage liver disease requiring liver transplantation. Oxidative stress and activation of Kupffer cells are probably involved in liver injury caused by cholestasis. The nonessential amino acid glycine has been shown to be anti-inflammatory in several injury models, acting via inhibitory effects on several white blood cells, including Kupffer cells. Additionally, it activates a chloride channel, leading to cell hyperpolarization and a concomitant blocking of calcium influx into the cell via a voltage dependent calcium channel.
Ursodeoxycholic acid is currently the most promising therapy for chronic cholestatic liver diseases; however, it cannot prevent fibrosis. How cholestasis induces liver injury and fibrosis remains unclear. One possible mechanism is that accumulation of hydrophobic bile acids causes oxidative stress in the liver, leading to tissue injury, fibrosis and finally liver cirrhosis. One possible cell type responsible for the generation of free radicals could be the Kupffer cells, the resident macrophages of the liver. It is known that destruction of Kupffer cells by gadolinium chloride or transduction of Kupffer cells by recombinant adenovirus can protect the liver against injury. However, the role of Kupffer cells in fibrosis is controversial. Destruction of Kupffer cells attenuated liver fibrosis caused by carbon tetrachloride. By contrast, in a rat model of reversible biliary obstruction, inactivation of Kupffer cells impaired collagen metabolism and inhibited the resolution of fibrosis.
Recent studies demonstrated that dietary glycine protected both the lung and liver against lethal doses of endotoxin in the rat and improved graft survival after liver transplantation. Based on pharmacological data a glycine-gated chloride channel could be detected in Kupffer cells and other macrophages that influence the activation process of these cells by preventing the activation of intracellular signaling cascades.
The aim of this study was to investigate the effects of (dietary) glycine against oxidant-induced injury caused by bile duct ligation (BDL). The findings suggested that glycine significantly decreased liver injury, most likely by a direct effect on hepatocytes. Kupffer cells do not appear to play an important role in the pathological changes caused by cholestasis.
Glycine, a simple nonessential amino acid, is a well-known inhibitory neurotransmitter in the central nervous system that acts via a glycine-gated chloride channel and has been shown to be protective against hypoxia, ischemia, and various cytotoxic substances. Kupffer cells, which are derived from monocyte/macrophage cell lineage, are the resident macrophages of the liver. Although they represent about 80% of the total fixed macrophage population, they are less than 5% of the total hepatic cell population. Kupffer cells play a critical role in the pathogenesis of several disease states, including endotoxin shock and alcoholic liver disease, because they release physiologically active substances such as eicosanoids, inflammatory cytokines, and many free radical species leading to localized tissue injury.
This is an interesting study. It investigated the effects of (dietary) glycine against oxidant-induced injury caused by BDL.
Peer reviewer: Tom H Karlsen, MD, Institute of Immunology, Rikshospitalet University Hospital, Oslo N-0027, Norway
S- Editor Li DL L- Editor Rippe RA E- Editor Zhang WB
| 1. | Starzl TE, Demetris AJ, Van Thiel D. Liver transplantation (1). N Engl J Med. 1989;321:1014-1022. |
| 2. | Whitington PF, Balistreri WF. Liver transplantation in pediatrics: indications, contraindications, and pretransplant management. J Pediatr. 1991;118:169-177. |
| 4. | Kim WR, Ludwig J, Lindor KD. Variant forms of cholestatic diseases involving small bile ducts in adults. Am J Gastroenterol. 2000;95:1130-1138. |
| 5. | Engelhardt JF, Ye X, Doranz B, Wilson JM. Ablation of E2A in recombinant adenoviruses improves transgene persistence and decreases inflammatory response in mouse liver. Proc Natl Acad Sci USA. 1994;91:6196-6200. |
| 6. | Tyler DD. Polarographic assay and intracellular distribution of superoxide dismutase in rat liver. Biochem J. 1975;147:493-504. |
| 7. | Qureshi WA. Intrahepatic cholestatic syndromes: pathogenesis, clinical features and management. Dig Dis. 1999;17:49-59. |
| 8. | Stiehl A, Rudolph G, Raedsch R, Moller B, Hopf U, Lotterer E, Bircher J, Folsch U, Klaus J, Endele R. Ursodeoxycholic acid-induced changes of plasma and urinary bile acids in patients with primary biliary cirrhosis. Hepatology. 1990;12:492-497. |
| 9. | Skulina D, Owczarek D, Ciecko-Michalska I, Szczepanski W, Tetnowski J, Garlicka M, Janas-Skulina U. [The influence of ursodeoxycholic acid on some biochemical, immunologic and histopathologic parameters in patients with primary biliary cirrhosis]. Przegl Lek. 1999;56:201-204. |
| 10. | Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM Jr. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109:1249-1256. |
| 11. | Pastor A, Collado PS, Almar M, Gonzalez-Gallego J. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J Hepatol. 1997;27:363-370. |
| 12. | Chensue SW, Terebuh PD, Remick DG, Scales WE, Kunkel SL. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991;138:395-402. |
| 13. | Nolan JP, Leibowitz A, Vladatin AL. Influence of alcohol on Kupffer cell function and possible significance in liver injury. The reticuloendothelial system and pathogenesis of liver disease. Amsterdam: Elsevier 1980; 125-136. |
| 14. | Ogle CK, Wu JZ, Mao X, Szczur K, Alexander JW, Ogle JD. Heterogeneity of Kupffer cells and splenic, alveolar, and peritoneal macrophages for the production of TNF, IL-1, and IL-6. Inflammation. 1994;18:511-523. |
| 15. | Zhong Z, Connor HD, Yin M, Moss N, Mason RP, Bunzendahl H, Forman DT, Thurman RG. Dietary glycine and renal denervation prevents cyclosporin A-induced hydroxyl radical production in rat kidney. Mol Pharmacol. 1999;56:455-463. |
| 16. | Venkatachalam MA, Weinberg JM, Patel Y, Saikumar P, Dong Z. Cytoprotection of kidney epithelial cells by compounds that target amino acid gated chloride channels. Kidney Int. 1996;49:449-460. |
| 17. | Miller GW, Schnellmann RG. A putative cytoprotective receptor in the kidney: relation to the neuronal strychnine-sensitive glycine receptor. Life Sci. 1994;55:27-34. |
| 18. | Wheeler MD, Rose ML, Yamashima S, Enomoto N, Seabra V, Madren J, Thurman RG. Dietary glycine blunts lung inflammatory cell influx following acute endotoxin. Am J Physiol Lung Cell Mol Physiol. 2000;279:L390-L398. |
| 19. | Bachmann S, Caldwell-Kenkel JC, Currin RT, Tanaka Y, Takei Y, Marzi I, Thurman RG, Lemasters JJ. Ultrastructural correlates of liver graft failure from storage injury: studies of graft protection by Carolina rinse solution and pentoxifylline. Transplant Proc. 1993;25:1620-1624. |
| 20. | Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G856-G863. |
| 21. | Symeonidis A, Trams EG. Morphologic and functional changes in the livers of rats after ligation or excision of the common bile duct. Am J Pathol. 1957;33:13-27. |
| 22. | Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994;205:243-247. |
| 23. | Pertoft H, Smedsrod B. Separation and characterization of liver cells. Cell separation: methods and selected applications. New York: Academic Press 1987; 1-24. |
| 24. | Froh M, Kono A, Thruman RG. Isolation of liver Kupffer cells. Current Protocols in Toxicology. New York: John Wiley & Sons 2002; 14.04.01-14.04.12. |
| 25. | Garcia-Ruiz C, Morales A, Ballesta A, Rodes J, Kaplowitz N, Fernandez-Checa JC. Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. J Clin Invest. 1994;94:193-201. |
| 26. | Bergmeyer HU, Bernt E. Lactate dehydrogenase UV assay with pyruvate and NADPH. Methods of Enzymatic Analysis. New York: Academic Press 1974; 574-579. |
| 27. | Schwartz RD, Paul SM, Majewska MD. Factors modulating the sensitivity of the GABA receptor-gated chloride ion channel. Clin Neuropharmacol. 1986;9 Suppl 4:389-391. |
| 28. | Morrow AL, Paul SM. Benzodiazepine enhancement of gamma-aminobutyric acid-mediated chloride ion flux in rat brain synaptoneurosomes. J Neurochem. 1988;50:302-306. |
| 29. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. |
| 30. | Ikejima K, Qu W, Stachlewitz RF, Thurman RG. Kupffer cells contain a glycine-gated chloride channel. Am J Physiol. 1997;272:G1581-G1586. |
| 31. | Zhong Z, Froh M, Lehnert M, Schoonhoven R, Yang L, Lind H, Lemasters JJ, Thurman RG. Polyphenols from Camellia sinenesis attenuate experimental cholestasis-induced liver fibrosis in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1004-G1013. |
| 32. | Froh M, Conzelmann L, Walbrun P, Netter S, Wiest R, Wheeler MD, Lehnert M, Uesugi T, Scholmerich J, Thurman RG. Heme oxygenase-1 overexpression increases liver injury after bile duct ligation in rats. World J Gastroenterol. 2007;13:3478-3486. |
| 33. | Parola M, Leonarduzzi G, Robino G, Albano E, Poli G, Dianzani MU. On the role of lipid peroxidation in the pathogenesis of liver damage induced by long-standing cholestasis. Free Radic Biol Med. 1996;20:351-359. |
| 34. | Sokol RJ, Devereaux M, Khandwala RA. Effect of dietary lipid and vitamin E on mitochondrial lipid peroxidation and hepatic injury in the bile duct-ligated rat. J Lipid Res. 1991;32:1349-1357. |
| 35. | Zhong Z, Froh M, Wheeler MD, Smutney O, Lehmann TG, Thurman RG. Viral gene delivery of superoxide dismutase attenuates experimental cholestasis-induced liver fibrosis in the rat. Gene Ther. 2002;9:183-191. |
| 36. | Marsh DC, Vreugdenhil PK, Mack VE, Belzer FO, Southard JH. Glycine protects hepatocytes from injury caused by anoxia, cold ischemia and mitochondrial inhibitors, but not injury caused by calcium ionophores or oxidative stress. Hepatology. 1993;17:91-98. |
| 37. | Nichols JC, Bronk SF, Mellgren RL, Gores GJ. Inhibition of nonlysosomal calcium-dependent proteolysis by glycine during anoxic injury of rat hepatocytes. Gastroenterology. 1994;106:168-176. |
| 38. | Qian T, Nieminen AL, Herman B, Lemasters JJ. Mitochondrial permeability transition in pH-dependent reperfusion injury to rat hepatocytes. Am J Physiol. 1997;273:C1783-C1792. |
| 39. | Weinberg JM, Davis JA, Abarzua M, Kiani T. Relationship between cell adenosine triphosphate and glutathione content and protection by glycine against hypoxic proximal tubule cell injury. J Lab Clin Med. 1989;113:612-622. |
| 40. | Frank A, Rauen U, de Groot H. Protection by glycine against hypoxic injury of rat hepatocytes: inhibition of ion fluxes through nonspecific leaks. J Hepatol. 2000;32:58-66. |
| 41. | Yamashina S, Konno A, Wheeler MD, Rusyn I, Rusyn EV, Cox AD, Thurman RG. Endothelial cells contain a glycine-gated chloride channel. Nutr Cancer. 2001;40:197-204. |
| 42. | Ikejima K, Iimuro Y, Forman DT, Thurman RG. A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol. 1996;271:G97-103. |
| 43. | Li X, Bradford BU, Wheeler MD, Stimpson SA, Pink HM, Brodie TA, Schwab JH, Thurman RG. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: role for glycine-gated chloride channel. Infect Immun. 2001;69:5883-5891. |
| 44. | Wheeler M, Stachlewitz RF, Yamashina S, Ikejima K, Morrow AL, Thurman RG. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000;14:476-484. |
| 45. | Rivera CA, Bradford BU, Hunt KJ, Adachi Y, Schrum LW, Koop DR, Burchardt ER, Rippe RA, Thurman RG. Attenuation of CCl(4)-induced hepatic fibrosis by GdCl(3) treatment or dietary glycine. Am J Physiol Gastrointest Liver Physiol. 2001;281:G200-G207. |
| 46. | Roggin KK, Papa EF, Kurkchubasche AG, Tracy TF Jr. Kupffer cell inactivation delays repair in a rat model of reversible biliary obstruction. J Surg Res. 2000;90:166-173. |
| 47. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. |
| 48. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. |
| 49. | Fox ES, Kim JC, Tracy TF. NF-kappaB activation and modulation in hepatic macrophages during cholestatic injury. J Surg Res. 1997;72:129-134. |
| 50. | Bemelmans MH, Gouma DJ, Greve JW, Buurman WA. Cytokines tumor necrosis factor and interleukin-6 in experimental biliary obstruction in mice. Hepatology. 1992;15:1132-1136. |
| 51. | Qu W, Ikejima K, Zhong Z, Waalkes MP, Thurman RG. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic parenchymal cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1249-G1256. |