Published online Oct 14, 2008. doi: 10.3748/wjg.14.5834
Revised: July 14, 2008
Accepted: July 21, 2008
Published online: October 14, 2008
AIM: To investigate the function of NOD2 in colonic epithelial cells (CEC).
METHODS: A combination of in vivo and in vitro analyses of epithelial cell turnover in the presence and absence of a functional NOD2 protein and, in response to enteric Salmonella typhimurium infection, were used. shRNA interference was also used to investigate the consequences of knocking down NOD2 gene expression on the growth and survival of colorectal carcinoma cell lines.
RESULTS: In the colonic mucosa the highest levels of NOD2 expression were in proliferating crypt epithelial cells. Muramyl dipeptide (MDP), that is recognized by NOD2, promoted CEC growth in vitro. By contrast, the growth of NOD2-deficient CECs was impaired. In vivo CEC proliferation was also reduced and apoptosis increased in Nod2-/- mice, which were also evident following enteric Salmonella infection. Furthermore, neutralization of NOD2 mRNA expression in human colonic carcinoma cells by shRNA interference resulted in decreased survival due to increased levels of apoptosis.
CONCLUSION: These findings are consistent with the involvement of NOD2 protein in promoting CEC growth and survival. Defects in proliferation by CECs in cases of CD may contribute to the underlying pathology of disrupted intestinal homeostasis and excessive inflammation.
- Citation: Cruickshank SM, Wakenshaw L, Cardone J, Howdle PD, Murray PJ, Carding SR. Evidence for the involvement of NOD2 in regulating colonic epithelial cell growth and survival. World J Gastroenterol 2008; 14(38): 5834-5841
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5834.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5834
The intestinal epithelium both acts as a physical barrier and senses and responds to commensal bacteria via expression of pattern recognition receptors (PRRs) that recognize microbe associated molecular patterns (MAMPs)[1]. There are two distinct groups of PRRs; the Toll-like receptor family (TLRs) and the NOD-like (nucleotide-binding oligomerisation domain) receptors. The leucine rich repeat sequences of the NOD2 protein are implicated in recognition of fragments of bacterial peptidoglycan (PGN) including muramyl dipeptide (MDP)[2,3].
NOD2 is expressed in the cytosol of professional antigen presenting cells and epithelial cells exposed to microorganisms containing PGN[3-6]. In cell-based models of NOD2 overexpression, MDP stimulation results in NF-κB activation[4,7]. This together with the ability of pro-inflammatory cytokines to influence NOD2 expression[8] suggests NOD2 contributes to the innate immune response to microbial pathogens. As intestinal epithelial cells are generally refractory to TLR signals in the absence of inflammation, NOD2 may have additional functions[9]. In the small intestine NOD2 appears to contribute to Peyer’s patch development[10] and paneth cell production of anti-microbial proteins[11], linking NOD2 and host defense at the epithelial interface.
By contrast, little is known about NOD2 function in the colon. It has been proposed that TLRs control epithelial homeostasis[12]. In considering the cross talk between NOD2 and TLR signaling pathways[13], NOD2 expression in IBD[14] and the central role CARD domain-containing proteins play in regulating apoptosis[15], we determined if activation of NOD2 in CECs is important for promoting CEC turnover and maintaining the integrity of the epithelial barrier. We found that NOD2 contributes to regulating CEC proliferation and survival.
Six to nine wk old C57BL/6-Nod2+/+ and C57BL/6-Nod2-/- (F8)[16] mice bred and maintained in the same animal facility were infected by oral gavage with 106 cfu luciferase-expressing Salmonella enterica serovar typhimurium (SL1344-Tn5lux). Biophotonic imaging (Xenogen Corp. Alameda, CA) was used to determine bacterial cfu in tissue homogenates[17]. All animal experiments were conducted in full accordance with the Animal Scientific Procedures Act 1986 under Home Office approval.
Segments of colon were sequentially incubated three times in dissociation buffer (130 mmol/L NaCl, 10 mmol/L HEPES, pH 7.4, 10% FCS and 1 mmol/L DTT) containing first 1 mmol/L, then 5 mmol/L and finally 10 mmol/L EDTA at 37°C for 15 min[18]. Aliquots of cells were stained with Wright-Giemsa (Baxter, Miami, FL), CD45 (Caltag Labs, Burlingame, CA), cytokeratin (Sigma-Aldrich, Poole, UK) and Ki67 (Dako, Carpinteria, CA) antibodies and incubated with alkaline phosphatase (AlkP) substrate (Vector Labs, Burlingame, CA) to establish CEC purity and identify proliferating (cytokeratin+, Ki67+, CD45-) and differentiated (cytokeratin+, AP+, CD45-) CECs. CEC monolayer cultures, established from dispase-digested fragments of colonic mucosa[19] were incubated with 1-10 mg/mL MDP (Ac-muramyl-Ala-Disoglutamine) for up to 4 d. Cell growth and viability were assessed by trypan blue exclusion. For NF-κB activation, nuclear extracts of CECs cultured for 2 h with MDP (1 mg/mL) or media alone were analyzed by ELISA (BD-Pharmingen) using specific inhibitors to block NF-κB activation as per the manufacturers’ instructions. Recombinant human TNF α (R&D Systems) was added to HT-29 and SW480 human colonic carcinoma cell lines (provided by Prof. Mark Hull, Univ. Leeds) to induce NOD2 expression[8].
Villous crypt height was determined by measuring the distances from the base of the crypt to the villous tip of at least 20 villi from 3 HE-stained sections of colon from 5 mice of each strain prior to and following infection. Axiovision software (Imaging Associates Ltd, Bicester, UK) was used for scaling and measurements.
Antibody staining (cytokeratin and CD45) and flow cytometry was used to assess CEC purity. Apoptotic cells were quantified by Annexin V and propidium iodide (PI) or 7AAD staining[20,21]. Levels of caspase 3 activity in cultured CECs were determined using the NucViewTM 488 substrate (Biotium, Hayward, CA) according to the manufacturers’ recommendation. Stained cells were analyzed using a FACSCalibur and CellQuest software (BD).
Paraffin (5 μm) sections were incubated with Ki67 (Dako), caspase 3 (BD-Pharmingen), BrdU (Oxford Biotechnology Ltd, Oxford, UK) or isotype matched control antibodies followed by biotinylated secondary antibodies (Vector Labs) and streptavidin-horseradish peroxidase plus DAB (Vector Labs) or anti-rabbit EnVisionTM labeled polymer (Dako). For BrdU detection sections were pre-treated with 2 mol/L HCl for 30 min followed by neutralization in 0.1 mol/L Na2B4O7 for 5 min to denature DNA. Stained cells in sections were enumerated using a Zeiss Axiovert 200 M microscope (Zeiss, Welwyn Garden City, UK) equipped with Axiovision software.
NOD2 mRNA was quantified in freshly isolated primary CECs and in HT-29 and SW480 cells using pre-optimized primer sets (Applied Biosystems, Foster City, CA) and an ABI prism 7900HT Sequence Detection System (Applied Biosystems). Threshold cycle (Ct) numbers were determined with Sequence Detection Software (Applied Biosystems) and analysed using the delta Ct comparative method. β-actin was used as a reference gene.
NOD2 and scrambled NOD2 shRNA sequences (Dharamacon, Lafayette, CO) were cloned into GFP-expressing lentiviral vector, pLL 3.7. shRNA expressing lentiviruses were prepared using the ViraPower Expression System (Invitrogen). HT-29 and SW480 human colonic carcinoma cells were infected with lentiviruses and 10 mg/mL polybrene (Sigma) for 16 h. Quantitative (Taqman) RT-PCR was used to assess CARD15 mRNA knockdown. Viability and growth of infected (GFP+) and non-infected (GFP-) cells was assessed by flow cytometry as described above.
All data were assessed for normal distribution using a Shapiro-Wilk test. For parametric and non-parametric data, analysis was performed using Student t-test and Mann Whitney test, respectively using the Student Package for the Social Sciences software (SPSS). P values < 0.05 were considered significant.
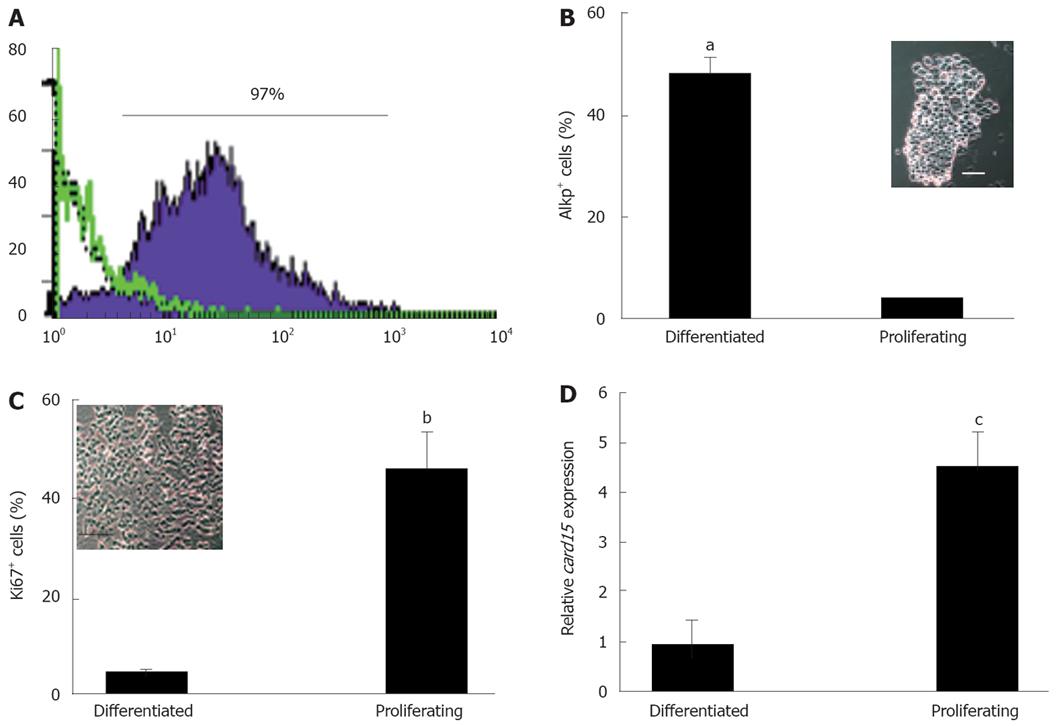
In view of previous reports of NOD2 expression in human colonic epithelial cell lines, isolated colonic crypts and among individual CECs[5,8,14] we sought evidence of NOD2 expression in primary murine colonic crypt epithelia. Initial immunohistochemical studies identified weak immunoreactivity among epithelial cells at the base of colonic crypts in wild type mice with a NOD2 antiserum (data not shown). To verify this finding, quantitative RT-PCR was used to measure NOD2 mRNA levels in CECs from different regions of the crypt. Sequential incubations in EDTA-containing buffers provided two morphologically distinct populations of epithelial cells of high purity (> 97% cytokeratin+) enriched for cells expressing markers of proliferation (Ki67) or differentiation (alkaline phosphatase, AlkP; Figure 1A-C). Preparations enriched for proliferating cells (about 50% Ki67+, < 5% AP+) contained significantly higher (0.95 ± 0.5 vs 4.5 ± 0.7, P < 0.05.) levels of NOD2 mRNA than fractions enriched for differentiated CECs (about 50% AP+, < 5% Ki67+; Figure 1D).
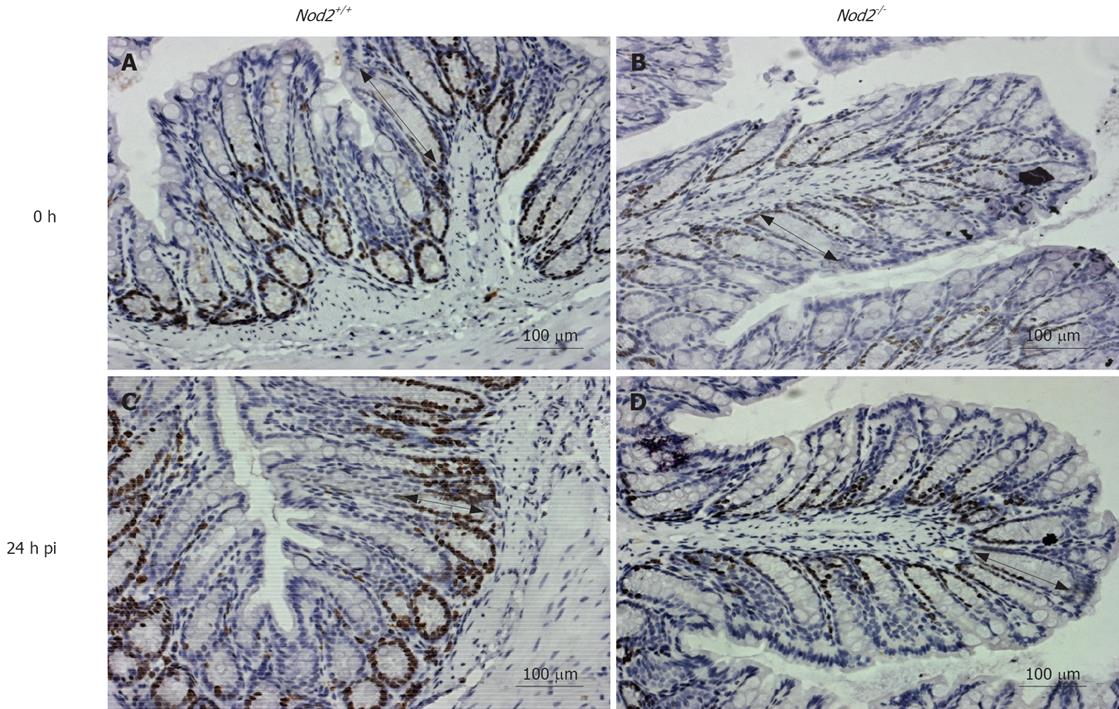
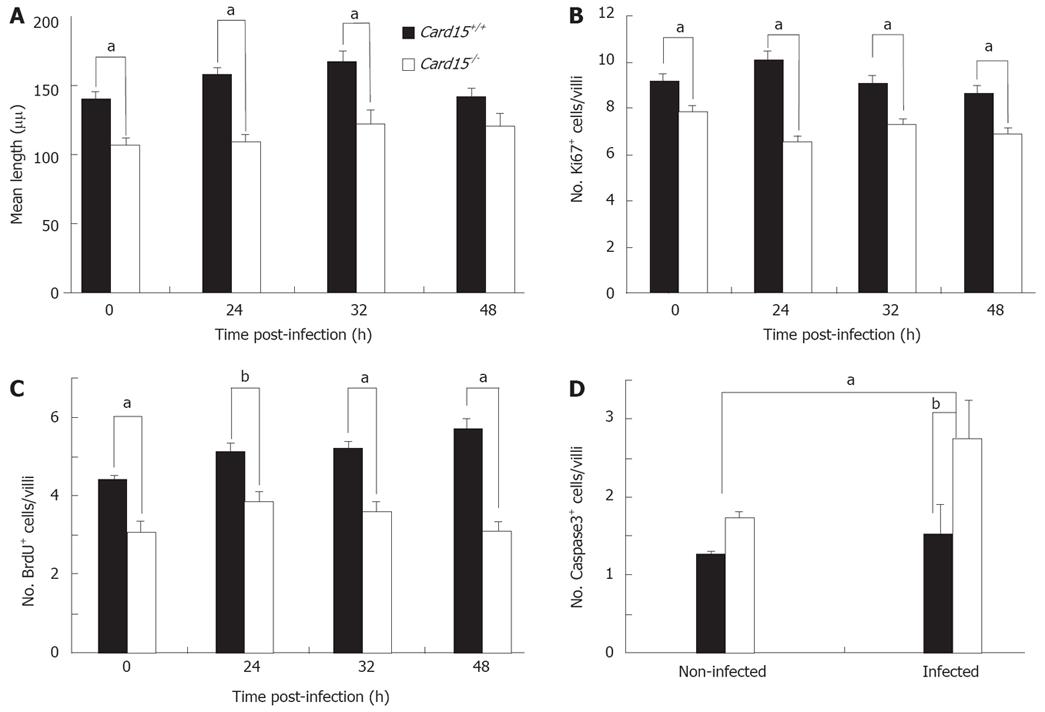
Consistent with NOD2 involvement in epithelial homeostasis, the length of colonic crypts in adult Nod2-/- mice was significantly shorter (143 ± 10 vs 93 ± 5, P < 0.05) compared to that in Nod2+/+ mice (Figure 2 and 3A). These differences were also evident after oral infection with a non-lethal dose of Salmonella enterica serovar typhimurium. The average length in Nod2-/- mice was increased upon infection, but was still significantly shorter (134.74 ± 4.22 vs 170.97 ± 6.55, P < 0.05) than in infected Nod2+/+ mice at 32 h.
Nod2-/- mice were more susceptible than wild type mice to invasion by Salmonella. During the first 24 h of Salmonella infection the colonic bacterial burden of Nod2-/- mice (cfu at 4 h: 840 ± 161; at 8 h: 1123 ± 187, at 24 h: 631 ± 202) was significantly higher (P < 0.0005) than in Nod2+/+ mice (cfu at 4 h: 78 ± 52; at 8 h: 88 ± 55; at 24 h: 101 ± 33). Nod2-/- animals also manifested severe diarrhea compared to Nod2+/+ mice, which persisted for 36 h, although they fully recovered after 3 d.
CEC turnover in vivo was investigated further by BrdU uptake and Ki67 expression. Significantly fewer Ki67+ and BrdU+ CECs were present in Nod2-/- mice compared to Nod2+/+ mice, both prior to and after Salmonella infection (Figures 2, 3B and 3C). Proliferating (BrdU+) epithelial cells also migrated shorter distances from the crypts in Nod2-/- mice compared to Nod2+/+ mice (unpublished observations), consistent with reduced CEC proliferation. Nod2-/- mice also had significantly higher numbers of apoptotic (caspase3+) CECs compared to Nod2+/+ mice after Salmonella infection (1.52 ± 0.37 vs 2.75 ± 0.48, P < 0.001, Figure 3D). These in vivo studies do not, however, exclude the possibility that the effects of NOD2 deficiency on CEC growth and apoptosis are secondary to changes in the activity of mucosal immunocompetent cells.
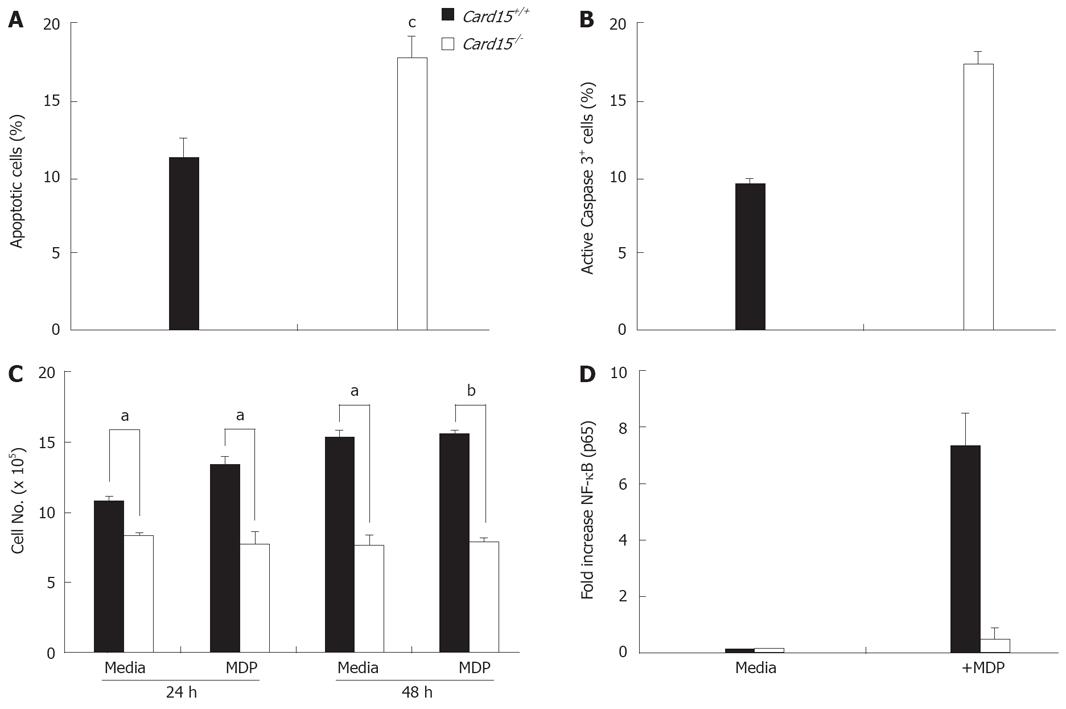
Growth and apoptosis of Nod2-/- CECs in culture following isolation was analyzed. Primary cultures of Nod2-/- CECs (see Methods section) contained significantly higher (11.5 ± 1.1 vs 17.7 ± 1.4, P < 0.0105) numbers of apoptotic cells compared to Nod2+/+ CECs (Figure 4A) with consistently more cells expressing caspase3 (Figure 4B). The growth of Nod2-/- CEC in vitro was also significantly less than Nod2+/+ CECs at 24 h (10.7 ± 0.4 vs 8.3 ± 0.2, P < 0.026, Figure 4C). Of note, the addition of MDP to cultures of Nod2+/+ CEC increased cell numbers, whereas no increase in Nod2-/- CEC number was seen in response to MDP (Figure 4C). The defect in Nod2-/- CEC growth in vitro was correlated with defective MDP-mediated NF-κB activation (Figure 4D).
Further evidence for the direct involvement of NOD2 in regulating epithelial cell proliferation was sought using RNA interference (RNAi) to knockdown expression of NOD2 in HT-29 and SW480 human colonic carcinoma cells.
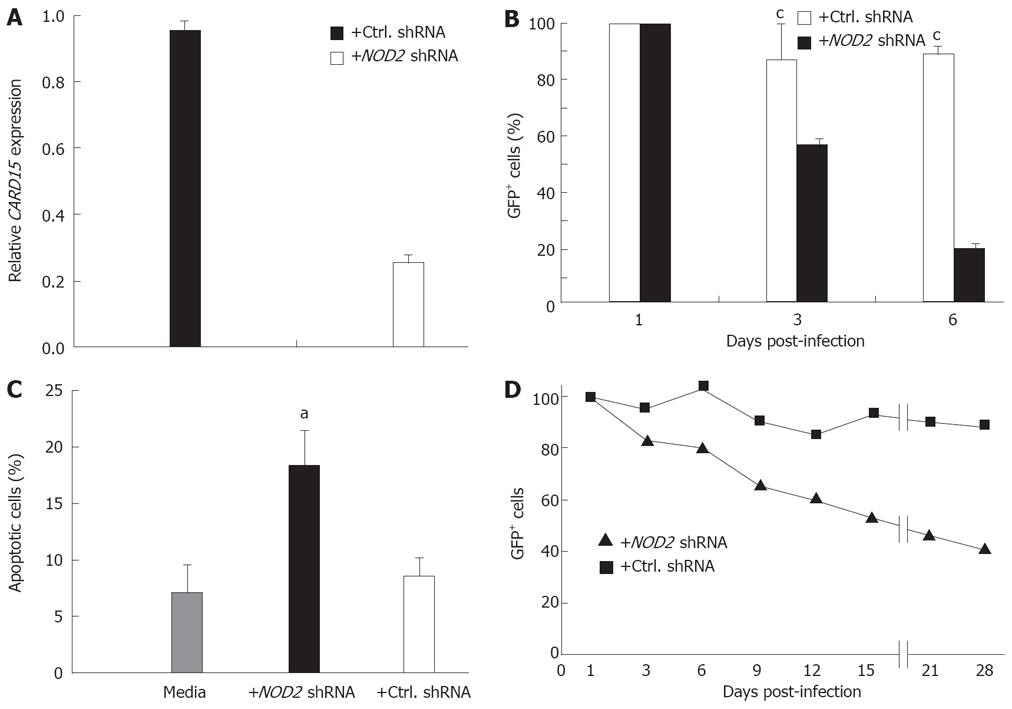
After transfection with lentiviral vectors expressing short hairpin RNA (shRNA) NOD2 sequences, the level of CARD15 mRNA in both HT-29 and SW480 cells, as determined by quantitative RT-PCR, was reduced by 75%-80%, compared to both non-treated cells and cells infected with control lentivirus expressing scrambled NOD2 sequences (Figure 5A and data not shown). NOD2 shRNA treatment led to a significant decline in HT-29 cell survival with < 20% of GFP+ (virus infected) cells surviving beyond 6 days (Figure 5B). The decline in GFP+ cells was explained by decreased cell viability and increased apoptosis (Figure 5C). Similar to primary Nod2+/+ and Nod2-/- CEC, knockdown of NOD2 mRNA had no effect on cell cycle progression, although the number of cycling cells was reduced (unpublished observations) reflecting the increased levels of cell death. By comparison, the growth and survival of HT-29 cells infected with lentiviral vectors expressing scrambled NOD2 shRNA sequences was unaffected (Figure 5B). A similar outcome of NOD2 shRNA on epithelial cell growth was seen in SW480 cells (Figure 5D), although the loss of GFP+ SW480 cells occurred over a longer time period with about 60% reduction in GFP+ cells seen after 4 wk (Figure 5D).
The integrity of the colonic epithelium is maintained by the continual renewal of epithelial cells as a result of the accelerated division of crypt cells that migrate upwards from the base of the crypts. Little is known about the origin and nature of the factors that regulate these processes. Here, we provide evidence for the involvement of NOD2 in regulating murine CEC turnover.
Expression of NOD2 in preparations of CECs enriched for proliferating cells described here, together with prior accounts of increased epithelial expression under conditions of rapid proliferation in vitro and in the inflamed and infected intestine[6,11,14,22] and, the increased severity of chemical-induced colonic mucosal damage in NOD2-deficient mice[10], suggests NOD2 contributes to regulating epithelial cell turnover and promoting epithelial repair. The low amount of NOD2 expression in the colonic mucosa, under steady-state conditions described here and previously[14], is consistent with this hypothesis and that altered epithelial cell proliferation and death[23] and increased epithelial permeability[24] contribute to the development of IBD. The absence of intestinal inflammation in Nod2-/- mice[10,11,16,25], or in mice with a knock-in mutation corresponding to the predominant disease-associated mutant form of human NOD2 in Crohn’s disease[25], suggests that while NOD2 gene mutations and altered epithelial turnover are a prerequisite for developing Crohn’s disease, they are not sufficient in themselves[26]. Other additional genetic factors or environmental triggers that disrupt the epithelial barrier[10] must also occur for the development of disease.
The absence of NOD2 in CECs does not completely abolish cellular proliferation. NOD2 may, therefore, function as part of a network of interacting and interdependent factors that includes other PRRs and various cytoprotective and repair factors that collectively regulate epithelial homeostasis with other components partially compensating for the absence of NOD2. NOD2 does, however, appear to play a more essential role in promoting the growth and survival of immortalized HT-29 and SW480 colonic carcinoma cells. Differences in the survival curves of these cell lines after NOD2 shRNA treatment may relate to differences in the genetic mutations and their impact on the requirement for NOD2 dependent-survival signals. Additional studies, using primary human CECs and colonic specimens from patients bearing CD-associated NOD2 mutations, are required to substantiate these findings. However, the inability of HT-29 and SW480 cells to survive NOD2 RNAi treatment is consistent with the disrupted growth of Nod2-/- primary CECs in vitro and in vivo and, that the sustained expression of NOD2 is required to maintain high rates of CEC proliferation. Identifying the pathways and mediators of NOD2 signaling in CECs will help establish how NOD2 contributes to CEC and tumor cell survival.
The proposed role of NOD2 in colonic epithelial homeostasis does not necessarily contradict the notion that it is a bacterial sensor and contributes to innate immunity[27,28]. The importance of NOD2 in innate anti-bacterial responses is demonstrated by the increased bacterial burden and inflammation seen here in Nod2-/- mice after enteric Salmonella infection and previously after infection with Listeria[11], both of which disrupt epithelial barrier function. Cell type specific influences and microenvironmental factors may account for contrasting roles for NOD2 in different studies. The importance of environmental influences is perhaps best demonstrated by its divergent role(s) in the very different environment of the small intestine, where it regulates anti-microbial protein production[11] and GALT development[10], and the colon where it regulates epithelial cell turnover. In addition, differences in the expression of NOD2-regulatory proteins[29,30], or endogenous inhibitors of NOD2[31], could also explain different outcomes of NOD2 activation in different cell types. The recent identification of a NOD2 target gene (DMBT1) that is predominantly expressed in epithelial cells[32] is also consistent with cell type specific NOD2 responses.
How NOD2 carries out its diverse array of functions in different regions of the gastrointestinal tract remains to be determined. In the colon, NOD2’s effect on CEC growth could be direct by, for example, regulating expression of genes and/or proteins involved in cell growth. mRNA profiling of wild type and Nod2-/- CECs by microarray analysis has identified securin (pituitary tumor transforming gene-1) that is required for maintaining appropriate cell division[33] as one of the most underrepresented genes in Nod2-/- CECs (unpublished observation). Alternatively, the involvement of PRRs in the production of cytoprotective and reparative cytokines in the colon[12] and reports of altered patterns of cytokine production by NOD2-deficient cells[34-36] suggest an indirect mechanism of action of NOD2. The growth-promoting effect of NOD2 in CECs contrasts with MDP-induced apoptosis of rabbit kidney epithelial cells, which may reflect cell type specific differences in the response to MDP and its interaction with different cellular proteins including NOD2[37]. Further studies aimed at identifying NOD2 signaling pathways in CECs will be important in determining how this protein functions in intestinal epithelial cells.
In summary, we have shown that NOD2 contributes to maintaining epithelial cell homeostasis in the colon. Compromised barrier repair may, therefore, underlie aspects of Crohn’s disease where mutant NOD2 alleles contribute to disease.
Mutations in NOD2 alleles are associated with an increased risk of developing Crohn’s disease (CD), an Inflammatory Bowel Disease (IBD). However, it is unclear how NOD2 mutations contribute to the development of CD and in particular to intestinal epithelial homeostasis.
Our study has identified a novel function for NOD2 in the regulation of colonic epithelial cell growth and in the survival of colonic epithelial tumor cell lines.
This study is the first to provide direct evidence of a role for NOD2 in epithelial cell growth and survival. Compromised epithelial barrier restitution and repair after injury may, therefore, contribute to the pathogenesis of CD in patients with mutant NOD2 alleles.
Further understanding of the function of NOD2 and the role it plays in the pathogenesis of CD will be of benefit in developing new therapies for CD.
This interesting study was to determine the molecular mechanisms regulating NOD2 function in colonic epithelial cells. The authors conducted both in vivo and in vitro studies to show the specific functions of the NOD2 protein. This is an outstanding and clearly written paper that provides strong evidence for a role for NOD2 in murine colonic epithelial survival.
Peer reviewers: Jian-Ying Wang, Professor, University of Maryland School of Medicine, Baltimore VA Medical Center (112), 10N. Greene St, Baltimore, MD 21201, United States; Marc Basson, MD, PhD, MBA, Chief of Surgery, John D. Dingell VA Medical Center, 4646 John R. Street, Detroit, MI 48301, United States
S- Editor Tian L L- Editor Rippe RA E- Editor Yin DH
| 1. | Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953-964. |
| 2. | Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509-5512. |
| 3. | Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869-8872. |
| 4. | Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem. 2001;276:4812-4818. |
| 5. | Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993-1000. |
| 6. | Lala S, Ogura Y, Osborne C, Hor SY, Bromfield A, Davies S, Ogunbiyi O, Nunez G, Keshav S. Crohn's disease and the NOD2 gene: a role for paneth cells. Gastroenterology. 2003;125:47-57. |
| 7. | Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194-199. |
| 8. | Rosenstiel P, Fantini M, Brautigam K, Kuhbacher T, Waetzig GH, Seegert D, Schreiber S. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001-1009. |
| 9. | Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406-1415. |
| 10. | Barreau F, Meinzer U, Chareyre F, Berrebi D, Niwa-Kawakita M, Dussaillant M, Foligne B, Ollendorff V, Heyman M, Bonacorsi S. CARD15/NOD2 is required for Peyer's patches homeostasis in mice. PLoS ONE. 2007;2:e523. |
| 11. | Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731-734. |
| 12. | Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229-241. |
| 13. | Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9-20. |
| 14. | Berrebi D, Maudinas R, Hugot JP, Chamaillard M, Chareyre F, De Lagausie P, Yang C, Desreumaux P, Giovannini M, Cezard JP. Card15 gene overexpression in mononuclear and epithelial cells of the inflamed Crohn's disease colon. Gut. 2003;52:840-846. |
| 15. | Ting JP, Davis BK. Caterpiller: a novel gene family important in immunity, cell death, and diseases. Annu Rev Immunol. 2005;23:387-414. |
| 16. | Pauleau AL, Murray PJ. Role of nod2 in the response of macrophages to toll-like receptor agonists. Mol Cell Biol. 2003;23:7531-7539. |
| 17. | Burns-Guydish SM, Olomu IN, Zhao H, Wong RJ, Stevenson DK, Contag CH. Monitoring age-related susceptibility of young mice to oral Salmonella enterica serovar Typhimurium infection using an in vivo murine model. Pediatr Res. 2005;58:153-158. |
| 18. | Inan MS, Tolmacheva V, Wang QS, Rosenberg DW, Giardina C. Transcription factor NF-kappaB participates in regulation of epithelial cell turnover in the colon. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1282-G1291. |
| 19. | Baumgart DC, Olivier WA, Reya T, Peritt D, Rombeau JL, Carding SR. Mechanisms of intestinal epithelial cell injury and colitis in interleukin 2 (IL2)-deficient mice. Cell Immunol. 1998;187:52-66. |
| 20. | Cruickshank SM, McVay LD, Baumgart DC, Felsburg PJ, Carding SR. Colonic epithelial cell mediated suppression of CD4 T cell activation. Gut. 2004;53:678-684. |
| 21. | Singh JC, Cruickshank SM, Newton DJ, Wakenshaw L, Graham A, Lan J, Lodge JP, Felsburg PJ, Carding SR. Toll-like receptor-mediated responses of primary intestinal epithelial cells during the development of colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G514-G524. |
| 22. | Ogura Y, Lala S, Xin W, Smith E, Dowds TA, Chen FF, Zimmermann E, Tretiakova M, Cho JH, Hart J. Expression of NOD2 in Paneth cells: a possible link to Crohn's ileitis. Gut. 2003;52:1591-1597. |
| 23. | Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7:68-77. |
| 24. | Rask-Madsen J, Hammersgaard EA, Knudsen E. Rectal electrolyte transport and mucosal permeability in ulcerative colitis and Crohn's disease. J Lab Clin Med. 1973;81:342-353. |
| 25. | Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734-738. |
| 26. | Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473-485. |
| 27. | Murray PJ. NOD proteins: an intracellular pathogen-recognition system or signal transduction modifiers? Curr Opin Immunol. 2005;17:352-358. |
| 28. | Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250-1257. |
| 29. | Kufer TA, Kremmer E, Banks DJ, Philpott DJ. Role for erbin in bacterial activation of Nod2. Infect Immun. 2006;74:3115-3124. |
| 30. | McDonald C, Chen FF, Ollendorff V, Ogura Y, Marchetto S, Lecine P, Borg JP, Nunez G. A role for Erbin in the regulation of Nod2-dependent NF-kappaB signaling. J Biol Chem. 2005;280:40301-40309. |
| 31. | Rosenstiel P, Huse K, Till A, Hampe J, Hellmig S, Sina C, Billmann S, von Kampen O, Waetzig GH, Platzer M. A short isoform of NOD2/CARD15, NOD2-S, is an endogenous inhibitor of NOD2/receptor-interacting protein kinase 2-induced signaling pathways. Proc Natl Acad Sci USA. 2006;103:3280-3285. |
| 32. | Rosenstiel P, Sina C, End C, Renner M, Lyer S, Till A, Hellmig S, Nikolaus S, Folsch UR, Helmke B. Regulation of DMBT1 via NOD2 and TLR4 in intestinal epithelial cells modulates bacterial recognition and invasion. J Immunol. 2007;178:8203-8211. |
| 33. | Wang Z, Yu R, Melmed S. Mice lacking pituitary tumor transforming gene show testicular and splenic hypoplasia, thymic hyperplasia, thrombocytopenia, aberrant cell cycle progression, and premature centromere division. Mol Endocrinol. 2001;15:1870-1879. |
| 34. | Netea MG, Kullberg BJ, de Jong DJ, Franke B, Sprong T, Naber TH, Drenth JP, Van der Meer JW. NOD2 mediates anti-inflammatory signals induced by TLR2 ligands: implications for Crohn's disease. Eur J Immunol. 2004;34:2052-2059. |
| 35. | Netea MG, Ferwerda G, de Jong DJ, Jansen T, Jacobs L, Kramer M, Naber TH, Drenth JP, Girardin SE, Kullberg BJ. Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol. 2005;174:6518-6523. |
| 36. | van Heel DA, Ghosh S, Hunt KA, Mathew CG, Forbes A, Jewell DP, Playford RJ. Synergy between TLR9 and NOD2 innate immune responses is lost in genetic Crohn's disease. Gut. 2005;54:1553-1557. |
| 37. | Chen D, Texada DE, Duggan C, Liang C, Reden TB, Kooragayala LM, Langford MP. Surface calreticulin mediates muramyl dipeptide-induced apoptosis in RK13 cells. J Biol Chem. 2005;280:22425-22436. |