Published online Oct 14, 2008. doi: 10.3748/wjg.14.5810
Revised: September 2, 2008
Accepted: September 9, 2008
Published online: October 14, 2008
AIM: To study the inhibitory effect of baculovirus-mediated normal epithelial cell specific-1 (NES1) gene therapy on gastric cancer (GC) in vitro and in vivo.
METHODS: We first constructed recombinant baculovirus vectors and then transfected them into gastric cancer cells (SGC-7901). Efficiency of the baculovirus for gene transfer into SGC-7901 cells and cell growth curves were detected by fluorescence microscopy, Western blot and 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in vitro, respectively. The therapeutic effect of this gene therapy on GC was confirmed in xenografted nude mice. Tumor growth was determined by tumor volume, and expression of NES1 in tumor was analyzed by immunohistochemistry.
RESULTS: Baculovirus vectors were successfully transfected into SGC-7901 cells. SGC-7901 cells transfected with the NES1 gene inhibited cell growth. In the Bac-NES1 treated group, tumor growth was significantly reduced with a high level of NES1 expression
CONCLUSION: Baculovirus-mediated NES1 gene can be used in gene therapy for GC.
- Citation: Huang W, Tian XL, Wu YL, Zhong J, Yu LF, Hu SP, Li B. Suppression of gastric cancer growth by baculovirus vector-mediated transfer of normal epithelial cell specific-1 gene. World J Gastroenterol 2008; 14(38): 5810-5815
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5810.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5810
Gastric cancer (GC) remains one of the most common cancers and a major cause of cancer-related death worldwide. To date, tumor resection has remained the only curative therapy for GC. Since radiotherapy and chemotherapy have no significant therapeutic effect on GC, new therapeutic strategies are required[1]. Gene therapy represents an interesting alternative treatment for cancers, and a number of studies have demonstrated that gene therapy can effectively reduce tumor growth in animal models[2]. Studies aimed at probing the genetic changes that occur in GC have identified several genes as potential targets of gene therapy for GC[3]. Our previous study suggested that normal epithelial cell specific-1 (NES1) gene functions as a tumor suppressor gene in GC and contributes to the malignant progression of GC providing us a promising gene target of gene therapy[4].
The design of vectors for specific gene transfer is a major challenge in medical research. Viral vectors are the most efficient tools for genetic modification of the majority of somatic cells in vitro and in vivo[5]. Recombinant baculoviruses with a mammalian expression promoter have recently been viewed as a new generation of gene therapy vehicles holding a great promise[6,7]. The baculovirus genome is large and thus large transgenes can be accommodated. In addition, they are easy to scale up and obtain high levels of recombinant gene expression[8]. However, despite a good understanding of all these attractive features of baculovirus, gene therapy with the virus is still in its infancy, and no practical application in cancer therapy, even in preclinical animal studies, has been reported[9]. In the present study, we developed a recombinant baculovirus vector encoding the NES1 gene and tested whether intratumoral administration of NES1 gene has anti-tumor activity in GC models of mice. Our results indicate that GC cells (SGC-7901) are permissive to baculovirus infection and baculovirus-mediated NES1 gene can be used in the treatment of GC.
Baculovirus plasmid pFBGFPR was a gift from the Institute of Molecular Biology of Hong Kong University. pCMV-NES1 was a gift from Dr. Vimla Band from Division of Cancer Biology, Department of Radiotherapy, New England Medical Center. Recombinant baculoviruses were generated and propagated in spodoptera frugiperda (Sf-9) insect cells by a Bac-to-Bac system according to the standard manual (Invitrogen). Once the viruses were amplified in Sf-9 insect cells, GFP expression was observed under a fluorescent microscope for the CMV promoter activity in these cells. The viruses were amplified to a high titer by propagation in Sf-9 cells and stored in small aliquots at -80°C. Viral titers were determined by plaque assay on Sf-9 insect cells.
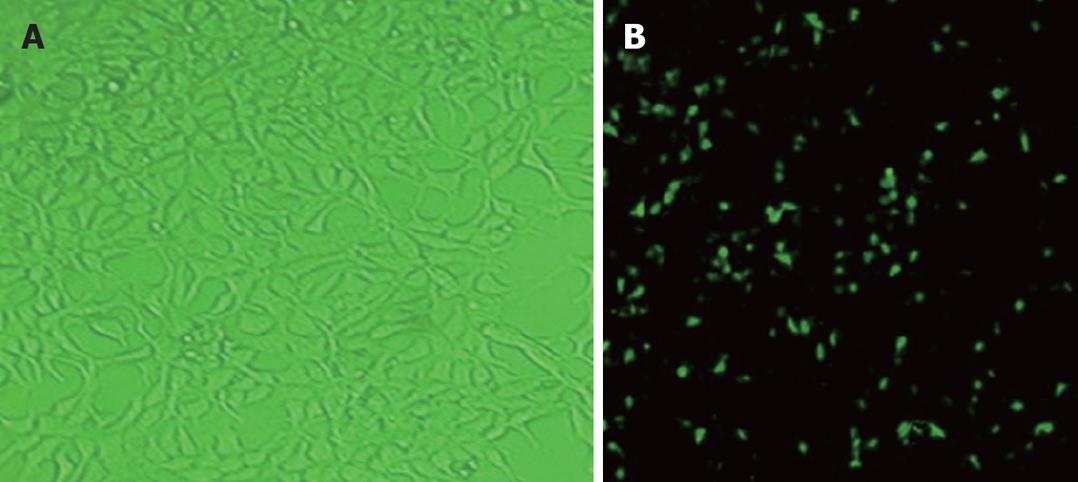
SGC-7901 cells were preserved in our laboratory and maintained in RPMI 1640 with 10% FBS. Sf-9 cells were cultured at 27°C in a spinner culture bottle containing Sf900II (Gibco) supplemented with 10% FBS. SGC-7901 cells were plated at a density of 105 cells per well in 24-well plates. After 1 d, culture was infected with different multiplicities of infection (MOI) of Bac-GFP, which was defined as the number of virus particles per cell for 1 h. The viruses were then removed, and a fresh medium was added to the wells. Twenty-four hours after transduction, the culture was examined for GFP expression by fluorescence microscopy, and photos were taken with a Nikon coolpix990 digital camera. High levels of GFP expression could be detected in SGC-7901 cells transduced with Bac-GFP. The morphological characteristics and growth of SGC-7901 cells were normal during experiment.
Cell lysates were made with standard methods. The protein concentration of each sample was measured using a BCA kit. For SDS-PAGE, 20 μg of protein samples was loaded on 10% polyacrylamide gels. Proteins were transferred to a polyvinylidene difluoride membrane with a tank transfer system (Bio-Rad Laboratory), then blocked with a buffer containing 5% low fat skim milk and 0.1% Tween-20 in Tris-buffered saline (TBST) at room temperature for 1 h. Primary antibodies were diluted in TBST containing 5% skim milk. The membrane was incubated with primary antibodies overnight at 4°C. After washed three times with TBST, the membrane was incubated with a horseradish peroxidase-conjugated secondary antibody (0.02 μg/mL in TBST) for 1 h at room temperature. Chemiluminescence was detected with an ECL Western blot detection kit (Amersham, Little Chalfont, UK) according to its manufacturer’s instructions.
SGC-7901 cells were grown in RPMI 1640 medium containing 10% fetal serum. For cell growth measurement, 2 × 103 cells were reseeded into 96-well plates and incubated at 37°C in 100 mL/L CO2 for 24 h. The media were then replaced with 0.2 mL of RPMI 1640/10% FBS, and the test sample (Bac-NES1, Bac-GFP or PBS) was applied. The number of SGC-7901 cells was quantified by colorimetric 3,-(4,5-dimethylthiazole-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay with a minor modification, as previously described. The proliferation assay was repeated at least three times.
Inhibitory effect of the NES1 gene on tumor growth was observed in xenografted nude mice with GC. Male athymic nude mice (Balb/c nude mice) at the age of five weeks were obtained from Shanghai Experimental Animals Centre of Chinese Academy of Sciences and had free access to sterilized food and autoclaved water. A suspension of SGC-7901 cells (1 × 107 cells in 0.2 mL PBS) was injected subcutaneously into the dorsal flank of each mouse. As the tumor grew larger, it was cut into small even pieces and replanted into the dorsal flanks of each mouse. Tumors were grown in mice to more than passage three. When the tumors were palpable, the mice were randomly divided into three groups (10 in each group). Mice in the two experimental groups were injected with 4 × 108 pfu baculoviruses containing the NES1 gene (Bac-NES1) and PBS gene, respectively, directly into the tumors every three days. Mice in the control group were injected with 4 × 108 pfu baculoviruses containing the GFP (Bac-GFP) directly into the tumors every three days. Body weight and volume of xenografts were measured in a blinded fashion using callipers every three days during the 3 wk treatment period. Tumor size was calculated according to the formula AB2/2, where A is the longest diameter and B is the shortest diameter of the tumor. After therapy, the mice were sacrificed, and tumor tissue was fixed in 10% formalin for subsequent immunohistochemistry examination.
Formalin-fixed, paraffin-embedded tissue sections (4 μm) were deparaffinized, rehydrated, and washed with PBS. Tests were performed according to the two-step procedure. After incubated with 3% H2O2 for 10 min at room temperature, unmasked antigens were heated. The sections were covered with animal serum for 20 min, incubated with rabbit polyclonal NES1 antibody (1:200) at 4°C overnight, and further treated with an EnVision kit for 30 min at room temperature. The sections were visualized by diaminobenzidine (DAB) and counterstained with hematoxylin. TBS primary antibodies were replaced with TBS as a negative control. The sections were observed under a microscope after mounted. At least five thin sections of tumor tissue were used for quantitative immunohistochemistry. The results of staining were analyzed and evaluated with American Image-Pro Plus software.
Data were analyzed using the SPSS 10.0 software (Chicago, USA). Each experiment was done in triplicate. The data were presented as mean ± SD. Comparison among experimental groups was performed using ANOVA test. P < 0.05 was considered statistically significant. Nonparametric Kruskal-Wallis test and Spearman’s correlation test were conducted to compare NES1 staining scores.
We developed a baculovirus-derived vector, containing the NES1 gene under control of the CMV promoter. Propagation of Bac-NES1 and Bac-GFP viruses in Sf-9 cells yielded viral stocks with a titer of 6 × 1012 PFU/mL, respectively. Bac-NES1 virus was purified and tittered as 2 × 1013 PFU/mL by standard end point dilution assay.
The efficiency of baculovirus gene transfer into SGC-7901 cells was assessed by fluorescence microscopy after infection with Bac-GFP. SGC-7901 cells transduced with Bac-GFP (MOI = 200) 24 h after transfection were examined by fluorescence microscopy, showing that SGC-7901 cells could be infected with baculovirus (Figure 1).
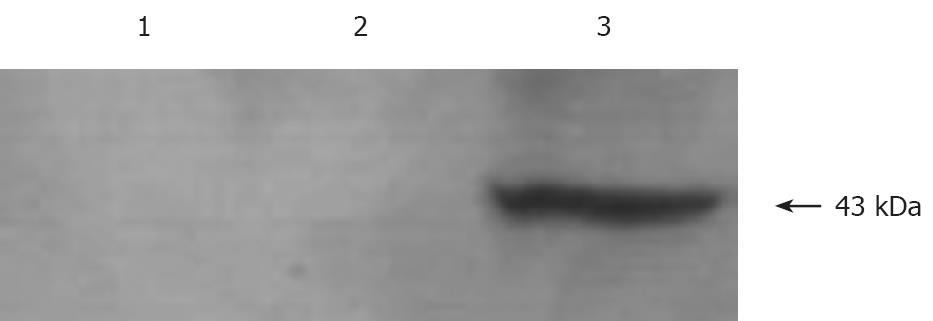
Western blot analysis was performed using antibodies to NES1 was performed fro the expression of NES1 in SGC-7901 cells infected with recombinant baculoviruses (Bac-NES1). A polyclonal antibody to NES1 detected a single protein band at 43 kDa in the SGC-7901 cells + Bac-NES1 group (Figure 2).
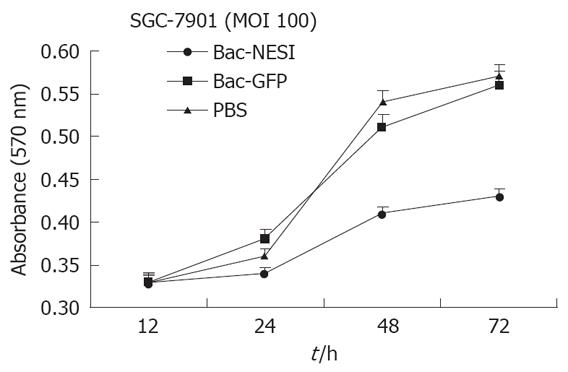
SGC-7901 cells (2 × 103/well) were planted in 24 well plates and infected with Bac-NES1 or Bac-GFP at a multiplicity of infection (MOI) of 100 or treated with PBS. Proliferation of cells was quantified at 12 h, 24 h, 48 h, and 72 h, respectively by the modified MTT assay. Data are presented as mean ± SD of six wells. The proliferation of SGC-7901 cells infected with Bac-NES1 was significantly suppressed at MOI of 100 compared with PBS- and Bac-GFP- treated groups (P < 0.05), but there was no difference between the two groups at MOI = 100 (Figure 3). The data indicate that NES1 induced by baculovirus vectors had a significant in vitro inhibitory effect on proliferation of SGC-7901 cells.
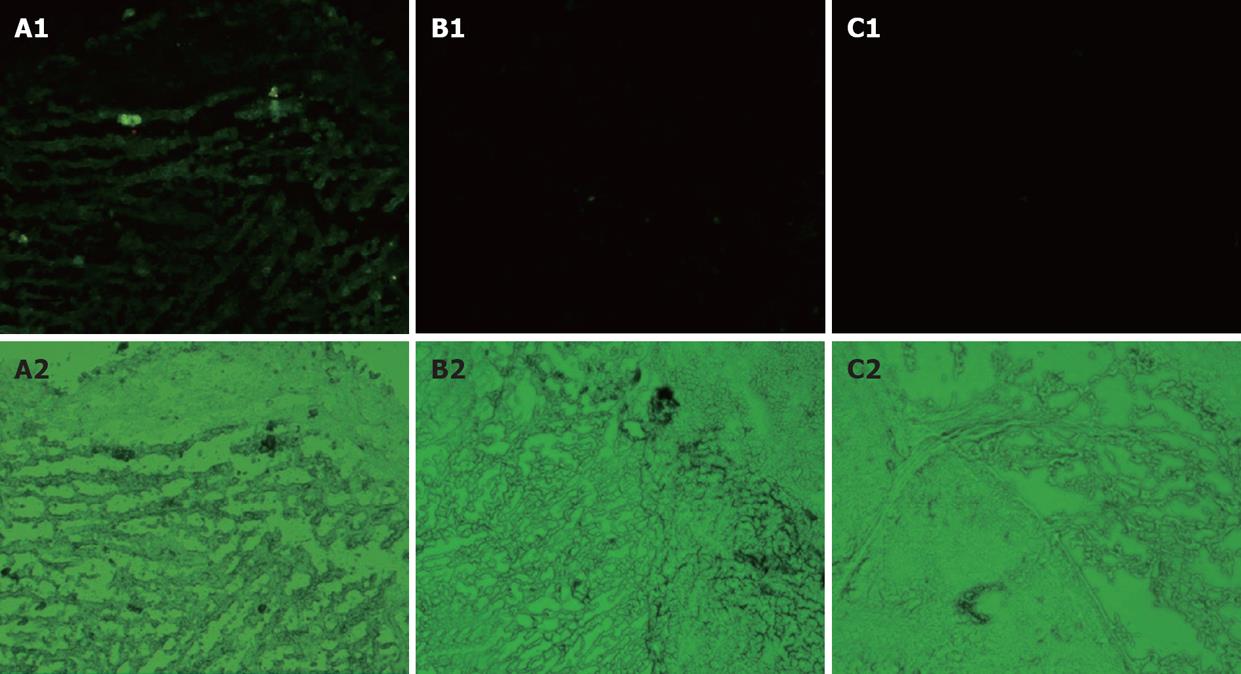
To investigate the possibility of using baculoviruses for in vivo GC gene therapy, we tested its expression in an animal GC xenograft model. The GFP expression could be visually detected under a fluorescent microscope in the Bac-GFP-treated group (Figure 4). We did not observe any GFP positive cells in the Bac-NES1- and PBS- treated groups. The data indicate that gastric tumor xenografts could be infected with baculovirus.
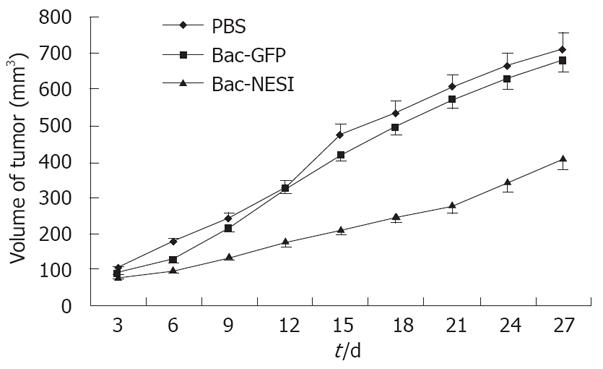
Xenografted GC was induced by injection of SGC-7901 cells into 5-week-old Balb/c nude mice. Treatment consisted of injection with 4 × 108 pfu Bac-NES1, 4 × 108 pfu Bac-GFP, or PBS directly into the tumor every three days. Tumor sizes were measured and presented as a mean. Tumor growth curves are shown in Figure 5. The growth of engrafted tumors was significantly inhibited in the Bac-NES1-treated groups (42.3%) compared with the control group on day 27 (P < 0.01). Significant differences in tumor volume were discovered between the control and Bac-NES1 treatment groups on day 9. However, the reduction rate was not significantly different between the Bac-GFP and PBS-treated groups throughout the treatment.
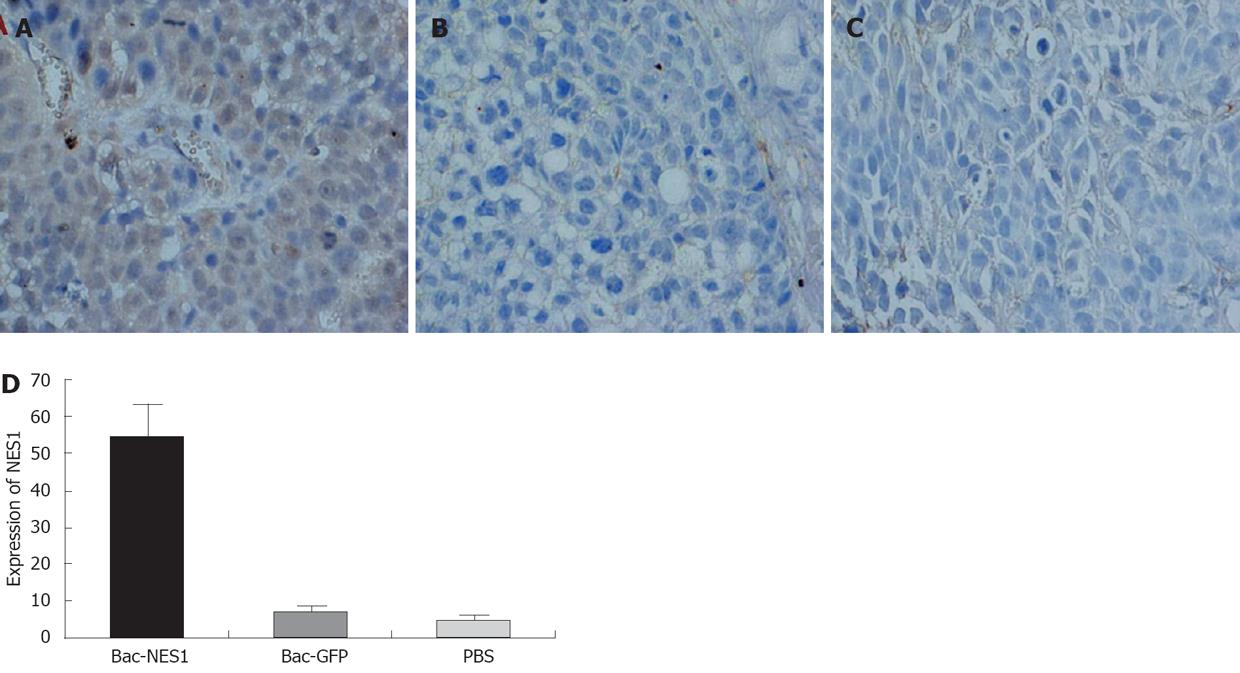
The expression of NES1 in SGC-7901 gastric tumor xenografts was assessed by immunostaining with NES1 antibody. Representative images of the three groups are shown in Figure 6. Tumors treated with Bac-NES1 exhibited a significantly higher NES1 expression (54.2 ± 15.7) than those treated with Bac-GFP (6.9 ± 1.7) and PBS (5.6 ± 1.4) (P < 0.01). The results suggest that reduced tumor growth and size might be associated with increased NES1 gene expression.
GC is one of the most common malignant tumors in the world, especially in Eastern Asia. Although the disease at its early stages is treatable with surgical resection, advanced GC does not generally respond to conventional chemotherapy or radiotherapy. Therefore, gene therapy represents an alternative modality for it[10]. Gene therapy is considered a promising therapeutic modality for cancer and has been successfully applied in animal models using various types of viral vector, gene expression regulation elements, and putative antitumor genes. Autographa californica multiple nucleopolyhedrovirus (AcMNPV)-based vector, traditionally used as a biopesticide to kill infected insects, is recently tested as a new type of delivery vehicle for transgene expression in mammalian cells[11]. These viruses can enter but not replicate in mammalian cells. With mammalian expression promoters, recombinant baculoviruses provide a high transduction efficiency in different cells and tissues, including several tumor cell lines[12]. One of the attractive advantages of using AcMNPV as a cancer gene therapy vector is the large cloning capacity conferred by its 130-kb viral genome, which may be used to deliver a large functional gene or multiple genes from a single vector[13]. Other empirical advantages of baculovirus vectors include easy construction of a recombinant viral vector and simple procedure of purifying large quantities of viruses with high titers. It would be possible to scale up the less labor-intensive process to pharmaceutical levels[14].
Human kallikrein 10 (KLK10)/NES1 is a member of the human tissue kallikrein family of secreted serine proteases, encoded by a family of 15 genes clustered in tandem on chromosome 19q13.3-4[15]. The kallikrein gene family has been under intensive study due to its implications in carcinogenesis[16,17] and many members have been used as biomarkers for the diagnosis and monitoring of certain cancers[18-21]. Our previous study suggested that NES1, as a tumor suppressor gene in GC, contributes to the malignant progression of GC[2]. When the NES1 gene is transfected into tumorigenic breast cancer line MDA-MB-231, its anchorage-independent growth is reduced and when this cell line is inoculated into nude mice, tumor formation is significantly decreased[22]. The mechanism of how NES1 induces suppression of the tumorigenic phenotype is currently unknown. Given that NES1 is a secreted protein, it is likely that it functions extracellularly as a regulator of cell growth and/or differentiation in an autocrine or paracrine manner. In this study, the effect of Bac-NES1 on human GC cell lines was examined both in vitro and in vivo. The extent of baculovirus-mediated gene transfer was evaluated by measuring the expression of the GFP gene under fluorescence microscopy. Baculovirus vectors could transfer the GFP gene into over 90% of GC cell lines examined at MOI 100. Furthermore, Bac-NES1 could successfully deliver and express NES1 protein in Bac-NES1-transfected GC cell lines. These data show that baculovirus can transfer exogenous genes efficiently into GC cell lines. In vitro, GC cells infected with Bac-NES1 were significantly suppressed at MOI 100 compared with PBS treated and Bac-GFP infected cells. In order to observe the inhibitory effect of NES1 on tumor growth, a GC model was established. Baculovirus-mediated NES1 gene significantly inhibited the growth of SGC-7901 xenografted gastric tumors. Immunohistochemistry data showed that the reduced tumor growth and size might contribute to the reexpression of NES1 in xenografted gastric tumors.
In conclusion, NES1 gene transfection can inhibit the proliferation of SGC-7901 cells and suppress the transfected GC cells-derived tumor growth in vivo. Although further investigation is required to assess systemic vs regional issues, systemic adverse effects, and immunological response problems, baculovirus-mediated NES1 gene therapy may be a potent strategy for the treatment of GC.
Gastric cancer (GC) is one of the most common malignant tumors in the world, especially in Eastern Asia. Although the disease at its early stages is treatable with surgical resection, advanced GC does not generally respond to conventional chemotherapy or radiotherapy. Therefore, gene therapy represents an alternative modality for GC. Normal epithelial cell specific-1 (NES1) gene functions as a tumor suppressor gene in GC and contributes to the malignant progression of GC, thus providing us a promising gene target of gene therapy.
Gene therapy is considered a promising therapeutic modality for cancer and has been successfully applied in animal models using various types of viral vector, gene expression regulation elements, and putative antitumor genes. Autographa californica multiple nucleopolyhedrovirus (AcMNPV)-based vectors, traditionally used as a biopesticide to kill infected insects, has been recently tested as a new type of delivery vehicle for transgene expression in mammalian cells.
Recombinant baculovirus viruses with a mammalian expression promoter have recently been viewed as a new generation of gene therapy vehicles holding a great promise. The baculovirus genome is large and thus large transgenes can be accommodated. In addition, they are easy to scale up and obtain high levels of recombinant gene expression. Although further investigation is required to assess systemic vs regional issues, systemic adverse effect, and immunological response problems, baculovirus-mediated NES1 gene therapy may be a potent strategy for the treatment of GC.
Baculovirus-mediated expression of NES1 inhibits the growth of xenografted GC by 42.3% compared with controls. Thanks to the highly efficient gene delivery, non-cytotoxicity and ease of scaling up, baculovirus may become a novel gene-delivery system for cancer gene therapy.
Gene therapy is a promising molecular alternative treatment modality for cancer, including replacement of defective tumor suppressor genes, inactivation of oncogenes, introduction of suicide genes, genetic immunotherapy, anti-angiogenetic gene therapy, and virotherapy.
The paper describes that baculovirus-mediated NES1 gene can be used in treatment of GC in a mouse model. The study is well designed and interesting.
Peer reviewer: Yaron Niv, Professor, Department of Gastroenterology, Rabin Medical Center, Beilinson Campus, Tel Aviv University, 2 Hadekel St., Pardesia 42815, Israel
S- Editor Li DL L- Editor Wang XL E- Editor Zhang WB
| 1. | Foukakis T, Lundell L, Gubanski M, Lind PA. Advances in the treatment of patients with gastric adenocarcinoma. Acta Oncol. 2007;46:277-285. |
| 2. | Vogiatzi P, Cassone M, Claudio PP. Personalizing gene therapy in gastric cancer. Drug News Perspect. 2006;19:533-540. |
| 3. | Tamura G. Alterations of tumor suppressor and tumor-related genes in the development and progression of gastric cancer. World J Gastroenterol. 2006;12:192-198. |
| 4. | Huang W, Zhong J, Wu LY, Yu LF, Tian XL, Zhang YF, Li B. Downregulation and CpG island hypermethylation of NES1/hK10 gene in the pathogenesis of human gastric cancer. Cancer Lett. 2007;251:78-85. |
| 5. | Khalighinejad N, Hariri H, Behnamfar O, Yousefi A, Momeni A. Adenoviral gene therapy in gastric cancer: a review. World J Gastroenterol. 2008;14:180-184. |
| 6. | Boyce FM, Bucher NL. Baculovirus-mediated gene transfer into mammalian cells. Proc Natl Acad Sci USA. 1996;93:2348-2352. |
| 7. | Matilainen H, Rinne J, Gilbert L, Marjomaki V, Reunanen H, Oker-Blom C. Baculovirus entry into human hepatoma cells. J Virol. 2005;79:15452-15459. |
| 8. | Jarvis DL, Garcia A Jr. Long-term stability of baculoviruses stored under various conditions. Biotechniques. 1994;16:508-513. |
| 9. | Heideman DA. Gene therapy and virotherapy of gastric cancer: preclinical results and clinical developments. Dig Dis. 2004;22:374-379. |
| 10. | Fumoto S, Nishi J, Nakamura J, Nishida K. Gene therapy for gastric diseases. Curr Gene Ther. 2008;8:187-200. |
| 11. | Condreay JP, Kost TA. Baculovirus expression vectors for insect and mammalian cells. Curr Drug Targets. 2007;8:1126-1131. |
| 12. | Hofmann C, Sandig V, Jennings G, Rudolph M, Schlag P, Strauss M. Efficient gene transfer into human hepatocytes by baculovirus vectors. Proc Natl Acad Sci USA. 1995;92:10099-10103. |
| 13. | Kost TA, Condreay JP. Recombinant baculoviruses as mammalian cell gene-delivery vectors. Trends Biotechnol. 2002;20:173-180. |
| 14. | Ghosh S, Parvez MK, Banerjee K, Sarin SK, Hasnain SE. Baculovirus as mammalian cell expression vector for gene therapy: an emerging strategy. Mol Ther. 2002;6:5-11. |
| 15. | Luo L, Herbrick JA, Scherer SW, Beatty B, Squire J, Diamandis EP. Structural characterization and mapping of the normal epithelial cell-specific 1 gene. Biochem Biophys Res Commun. 1998;247:580-586. |
| 16. | Yousef GM, Luo LY, Diamandis EP. Identification of novel human kallikrein-like genes on chromosome 19q13.3-q13.4. Anticancer Res. 1999;19:2843-2852. |
| 17. | Diamandis EP, Yousef GM, Luo LY, Magklara A, Obiezu CV. The new human kallikrein gene family: implications in carcinogenesis. Trends Endocrinol Metab. 2000;11:54-60. |
| 18. | Zhang Y, Bhat I, Zeng M, Jayal G, Wazer DE, Band H, Band V. Human kallikrein 10, a predictive marker for breast cancer. Biol Chem. 2006;387:715-721. |
| 19. | Yunes MJ, Neuschatz AC, Bornstein LE, Naber SP, Band V, Wazer DE. Loss of expression of the putative tumor suppressor NES1 gene in biopsy-proven ductal carcinoma in situ predicts for invasive carcinoma at definitive surgery. Int J Radiat Oncol Biol Phys. 2003;56:653-657. |
| 20. | Dhar S, Bhargava R, Yunes M, Li B, Goyal J, Naber SP, Wazer DE, Band V. Analysis of normal epithelial cell specific-1 (NES1)/kallikrein 10 mRNA expression by in situ hybridization, a novel marker for breast cancer. Clin Cancer Res. 2001;7:3393-3383. |
| 21. | Luo LY, Rajpert-De Meyts ER, Jung K, Diamandis EP. Expression of the normal epithelial cell-specific 1 (NES1; KLK10) candidate tumour suppressor gene in normal and malignant testicular tissue. Br J Cancer. 2001;85:220-224. |
| 22. | Li B, Goyal J, Dhar S, Dimri G, Evron E, Sukumar S, Wazer DE, Band V. CpG methylation as a basis for breast tumor-specific loss of NES1/kallikrein 10 expression. Cancer Res. 2001;61:8014-8021. |