Published online Oct 7, 2008. doi: 10.3748/wjg.14.5744
Revised: July 22, 2008
Accepted: July 29, 2008
Published online: October 7, 2008
AIM: To study the relationship between MCP-1-2518A/G, IL-8-251A/T polymorphism and acute pancreatitis (AP) in the Han population of Suzhou, China.
METHODS: A case-control study was conducted to compare the distribution of genotype and genetic frequency of MCP-1-2518A/G, IL-8-251A/T gene polymorphism among AP (n = 101), including mild AP (n = 78) and severe AP (n = 23) and control healthy individuals (n = 120) with polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and DNA sequencing, and analyze the relationship between the MCP-1-2518A/G, IL-8-251A/T gene polymorphism and the susceptibility to AP.
RESULTS: Significant differences were found in the distribution of genotype of MCP-1-2518A/G between the healthy control group and mild AP group (χ2 = 32.015, P < 0.001), the same was evident between the healthy control group and severe AP group (χ2 = 12.932, P < 0.05) in Suzhou. However, no difference of genotypic distribution was noted between MAP and SAP (χ2 = 0.006, P = 0.997). The genetic frequencies of G allele in mild AP were 72.4% (113/156) and 76.1% (35/46) in severe AP, both were higher than the controls, 47.1% (113/240) (χ2 = 24.804; P < 0.001, and χ2 = 13.005; P < 0.001), but no difference was found between severe AP and mild AP (χ2 = 0.242; P = 0.623). No difference was found in the distribution of genotype of IL-8-251A/T between the healthy control group and AP group neither in the frequency of A and T allele.
CONCLUSION: The MCP-1-2518 AA genotype of the population in Suzhou may be a protective genotype of AP, while one with higher frequency of G allele is more likely to suffer from pancreatitis. But the genotype of AA and the frequency of G allele could not predict the risk of severe AP. No correlation is found between the IL-8-251 polymorphism and the liability of AP.
-
Citation: Chen WC, Nie JS. Genetic polymorphism of
MCP-1-2518 ,IL-8-251 and susceptibility to acute pancreatitis: A pilot study in population of Suzhou, China. World J Gastroenterol 2008; 14(37): 5744-5748 - URL: https://www.wjgnet.com/1007-9327/full/v14/i37/5744.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5744
| Genotype | Gene frequency | Hardy-Weinberg test | |||||
| AA | AG | GG | Total | A | G | ||
| MCP-1-2518 A/G | |||||||
| Control | 29 (24.2) | 63 (52.5) | 28 (23.3) | 120 | 121 (50.4) | 119 (49.6) | P = 0.583 |
| MAP | 4 (4.5) | 35 (39.8) | 49 (55.7) | 78 | 43 (27.6) | 133 (72.4) | P = 0.469 |
| SAP | 1 (4.3) | 9 (39.1) | 13 (56.5) | 23 | 11 (23.9) | 35 (76.1) | P = 0.718 |
| IL-8-251A/T | |||||||
| Control | 13 (10.8) | 64 (53.4) | 43 (35.8) | 120 | 90 (37.5) | 150 (62.5) | P = 0.131 |
| MAP | 5 (6.4) | 40 (51.3) | 33 (42.3) | 78 | 50 (32.1) | 106 (67. 0) | P = 0.117 |
| SAP | 1 (4.3) | 14 (60.9) | 8 (34.8) | 23 | 16 (34.8) | 30 (65.2) | P = 0.101 |
Chemokines are the cytokines that can activate or chemoattract leukocytes, and provide a stimulus to direct leukocytes to the areas of injury. They play vital roles in inflammatory reaction, infection of causative organism, trauma and renovation, cytotoxic effect, etc[1]. They are 70-90 amino acids in length and approximately 8-10 kDa in molecular weight. They are divided into four subfamilies according to the presence of four cysteine residues in conserved locations of primary structure and the two amino terminal cysteine residues are immediately adjacent or separated by one amino acid. Four subfamilies are CXC, CC, C and CX3C[2]. Up to now, the chemokines we have discovered mainly belong to CXC and CC subgroups, and monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8) are typical examples of those two subgroups.
Recently it was found that chemokines play an important role in the initiation and development of acute pancreatitis (AP), which takes part in systemic inflammatorome reaction syndrome, remote organ complications, and multiple organ dysfunction syndrome[3-6]. MCP-1 is regarded as a mediator of inflammatory reaction in early-stage of AP[7]. Studies carried by Rau et al[8] found that MCP-1 serum concentrations increase dramatically in patients who developed local complications and/or remote organ failure. A close correlation was found between the severity of remote organ failure and MCP-1 elevation. MCP-1 might play a pivotal role in the pathological mechanism of complicated AP. Studies also found that IL-8[9-11] increase in early-stage of acute pancreatitis and the serum concentration is correlated with the severity of pathogenetic condition.
American researchers[12] found that the MCP-1-2518 polymorphism might be related to the severity of AP, and MCP-1-2518G allele is a risk factor for severe AP. Moreover, Hofner[13] drew a conclusion that the frequency of IL-8 polymorphism may predict the risk of SAP. But no similar studies have been done in the ethnic group of China. The polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and DNA sequencing were carried out in our study to assess the possible correlation between the gene polymorphism of MCP-1-2518, IL-8-251 A/T and AP in the Han population of Suzhou, China.
A total of 101 consecutive patients (60 men and 41 women, mean age 51.57 ± 13.39, range 18-80 years) were analyzed in this study, including 78 patients (50 men and 28 women, mean age 52.46 ± 13.04) with mild AP and 23 patients (16 men and 7 women, mean age 56.83 ± 13.14) with severe AP. The patients were treated in the Department of Gastroenterology of the First Affiliated Hospital of Suzhou University. The diagnosis of AP was established based on abdominal pain or abdominal localizing signs and increased amylase levels increased by at least 3 times that of the upper limit of normal, and CT verification of pancreatitis. Pancreatitis was classified as severe when the APACHE II score ≥ 8[14], and the Balthazar CT severity index ≥ D[15]. One hundred and twenty healthy volunteers (71 men and 49 women, mean age 51.05 ± 9.37 years, range 25-69 years) served as a control group. All subjects were Han Chinese people, who came from Suzhou city.
For the examination of MCP-1 and IL-8 polymorphisms, genomic DNA purified from peripheral blood was used. Blood samples were collected in ethylenediaminetetraacetic acid-containing tubes for DNA extraction. Genomic DNA was isolated from peripheral blood leucocytes using a commercial Kit (The Wizard® Genomic DNA Purification Kit, Promega) and the genomic DNA was stored at -20°C for further use.
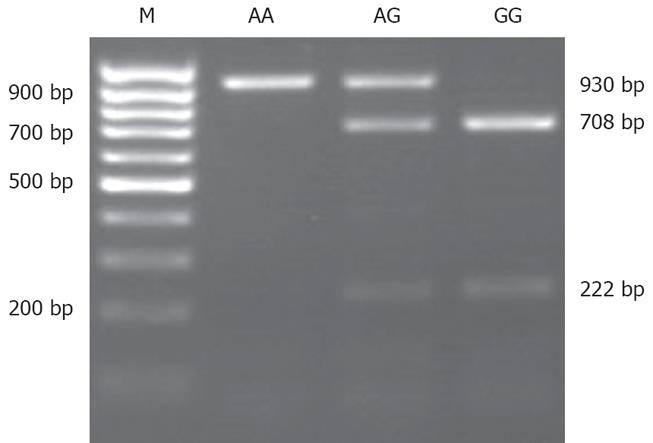
MCP-1-2518 polymorphism was assessed by means of a polymerase chain reaction procedure using the following primers[16]: sense: 5'-CCAGAGTGTTCCCAGCAC AG-3' and antisense: 5'-CTGCTTTGCTTGTGCCTC TT-3'. The PCR reaction mixture (50 μL) contained 100 ng genomic DNA, 5 μL 10 × Buffer, 1 μL of dNTPs (10 mmol/L each), 0.5 μL of Taq polymerase (Fermentas, 5 U/μL). PCR was performed using a 9700 Gene Amp PCR System Thermal Cycler (Applied Biosystems) under the following conditions: 95°C for 5 min, followed by 30 cycles of 95°C for 40 s, 57°C for 40 s, and 72°C for 60 s, with a final extension at 72°C for 10 min. After that the products of PCR were genotyped by restriction fragment length polymorphism (RFLP). Ten μL PCR products were digested with 10 U of Pvu II in 10 × buffer and H2O up to a final volume of 20 μL at 37°C for 16 h. 930 bp PCR product was cut into two bands of 708 bp and 222 bp. These digestion products were visualized in 2% agarose gels, stained with ethidium bromide. Samples showing only a 930 bp band were type AA. Samples showing two bands of 708 bp and 222 bp were considered GG and those showing three bands at 930 bp, 708 bp and 222 bp were designated AG (Figure 1).
DNA was extracted from peripheral blood leucocytes, then the IL-8 promoter polymorphism at -251 was amplified with forward primer 5'-ATTGGCTGGCTT ATCTTC-3' and reverse primer 5'-TTCCTGGCTCTTGTCCTA-3', based on GenBank accession No. AF385628. PCR was carried out with the following protocol: 5 min 94°C, 35 cycles of 30 s 94°C, 1 min 52°C, 45 s 72°C, followed by 5 min 72°C, and 10 min 4°C. The product was 485 bp. The sequencing of the PCR products was performed using the M13RP primer and the DNA Big Dye Terminator Sequencing Kit (ABI) on an ABI 377 automated sequencer, following the manufacturers’ protocols.
A comparison of the genotypic and allelic frequencies between the groups was performed using the Fisher exact test or χ2 test when appropriate. Description data of continuous variables were tested by Student’s t test. Statistical significance was established at P < 0.05. There was relationship between the genotypes or carriage of individual alleles and disease severity if presented as odds ratio (OR), with a 95% confidence interval (CI) of odds ratio (95% CI). All statistical calculations were performed with the SPSS 13.0 statistical program. Hardy-Weinberg equilibrium of the allele distribution was tested.
Three genotypes (AA, AG and GG) of MCP-1-2518 A/G polymorphisms were found in Han people of the Suzhou region. Control group AA 24.2% (29/120), AG 52.5% (63/120) and GG 23.3% (28/120); MAP group AA 4.5% (4/78), AG 39.8% (35/78) and GG 55.7% (49/78); and SAP group AA 4.3% (1/23), AG 39.1% (9/23) and GG 56.5% (13/23) (Table 1). There were significant differences in the distribution of genotype of MCP-1-2518 A/G between the healthy control group and MAP group (χ2 = 32.015, P < 0.001), the same was evident between the healthy control group and SAP group (χ2 = 12.932, P < 0.05) in Suzhou. However, no difference of genotypic distribution was noted between MAP and SAP (χ2 = 0.006, P = 0.997). The genetic frequencies of G allele in MAP 72.4% (113/156) and in SAP 76.1% (35/46) were all higher than the controls 49.6% (119/240) (χ2 = 24.804; P < 0.001, and χ2 = 13.005; P < 0.001), but we have found no difference between SAP and MAP (χ2 = 0.242; P = 0.623).
The distribution of the IL-8-251 A and T alleles was as follows: MAP: AA 6.41%, AT 51.28% and TT 42.31%; SAP: AA 4.3%, AT 60.87% and TT 34.78%; Control: AA 10.83%, AT 53.33% and TT 35.83% (Table 1). There were no significant differences in the distribution of genotype of IL-8-251 A/T between the healthy control group and AP group (P > 0.05). There was also no difference in the frequency of A and T allele between the healthy control group and AP group (P > 0.05).
In two polymorphisms, the genotype distributions were studied in the Suzhou population (both AP and control subjects) according to Hardy-Weinberg equilibrium (Table 1).
MCP-1 and IL-8 are known as the members of the family of chemokines. CXC chemokines represented by IL-8 exhibit a potent chemotactic activity for neutrophils while CC chemokines represented by MCP-1 are implicated in the activation of monocytes, macrophages, and lymphocytes[17]. MCP-1 and IL-8 play an important role in the cause and development of AP. Ishibashi[18] found that blocking of MCP-1 activity attenuates the severity of AP in rats, which manifests that MCP-1 may be involved in the progression of severe AP. Grady T[19] found that the level of MCP-1/JE mRNA elevated in caerulin-induced experimental pancreatitis. In human AP, MCP-1 expression was found to up-regulate in pancreatic tissues, and monocytic exudation was considered to be caused by this chemokine[20]. IL-8 as one of the earliest cytokines appearing in the serum of patients with AP remains persistently elevated and therefore it might be of help in assessing the severity of AP after admission[21]. In severe AP patients, IL-8 could concentrate and activate neutrophilic leukocyte, which could play a vital role in the development of adult respiratory distress syndrome[22]. Experiments in rat models suggested that inhibiting the release of IL-8 could lower the severity level and case-fatality rate than inhibiting the other inflammatory factors[23]. All these results provide evidences that MPC-1 and IL-8 play a key role in the occurrence and development of AP.
In recent years, so many studies found that there is a relationship between the gene polymorphism and AP. Balog et al[24] found that high frequencies of the HSP70-2 G and the TNF-α-308 A alleles were associated wit the risk of severe AP. Genotype assessments may be important prognostic tools to predict disease severity and the course of AP. He considered that genotype assessments may also be used to guide treatment or to identify risk populations for severe AP. Rahman et al[25] found that the functional GSTT-1*A genotype was associated with severe attacks of pancreatitis. Intensified oxidative stress characterized by glutathione depletion may be of importance in mediating the progression from mild to severe pancreatitis. All these studies note that variations in the DNA sequence might predispose individuals to AP.
Our study is designed to illustrate the relationship between MCP-1-2518A/G and IL-8-251A/T polymorphism and the liability of AP. The result showed that not only in MAP group but also in SAP group, the proportion of AA genotype was obviously lower in MCP-1-2518 than in the control group. However, the frequency of G allele of MAP and SAP group was both higher than the control group. We therefore can conclude that the patients with the GA and GG genotype in MCP-1-2518 might have higher risks for AP, but the patients with AA genotype in MCP-1-2518 might have less liability to AP. It is possible that the AA genotype has a function of protecting the organism from AP. Nevertheless, our study found no difference between the MAP group and the SAP group of the AA genotype in MCP-1-2518. The study by Papachristou et al[12] using the method of PCR showed that the G allele in MCP-1-2518A/G of SAP group is obviously higher than the control group and MAP group, while the frequency of homozygote AA in the SAP group is lower than the MAP group and the control group. Therefore, it was suggested that the obvious increase of the frequency of G allele of the AP patients is possibly related with the high risk of developing SAP, moreover, the AP patients with the AA genotype are less likely to suffer from pancreatitis. Both our group and Papachristou GI’s group discovered that there is a significant deviation between the AP group and the control group of the genotype and the frequency of allele in MCP-1-2518, but we found no difference between the AA genotype of SAP and MAP groups and the frequency of allele in MCP-1-2518.
At the same time, we did not discover the relationship between genetic polymorphism of MCP-1-251 and the occurrence of AP. And this result is completely diverse from the research by Hofner et al[13]. They found that the IL-8-251A/T had significantly higher frequency in the patients with severe pancreatitis than in the healthy blood donors, while the frequency of the TT genotype was higher in the patients with mild pancreatitis than in the group with severe pancreatitis. We therefore, conclude that the IL-8 polymorphism may identify populations at risk of severe AP. In their study, the distribution of the IL-8 genotypes in control population of Hungary was: TT 82/200 (41%), AA 34/200 (17%) and AT 84/200 (42%), which is similar to ours (χ2 = 4.537, P = 0.103). The distribution of the IL-8 genotypes in our control subjects is also similar to other studies in Beijing and Taiwan[26,27]. But the genetic polymorphisms of IL-8-251 in healthy populations in our and Honfer’s studies were different from that in Denmark[28], German[29], and Spain[30].
All those data indicated that the ethnic difference could be found in the genetic polymorphisms of IL-8-251. Although we and Hofner P gained similar results in the distribution of the IL-8 genotypes, it is possible that various results could be obtained in the polymorphisms of IL-8-251 among different countries. It is well known that only one genetic polymorphism cannot determine the final outcome of the diseases. Since all these studies are lack of larger cohorts, further studies are needed to confirm that the polymorphisms of IL-8-251 could affect the course of AP.
In summary, our study suggested that genetic polymorphism possibly determines the occurrence and development of AP. Moreover, ethnic difference in genetic polymorphisms may determine the functions in different populations. Thus, the relationship between genetic polymorphism and AP will need more thorough researches.
Chemokines play an important role in the pathogenesis of acute pancreatitis (AP). Monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8) are typical chemokines. Many researchers have explored the relationship between gene polymorphism and the occurrence and development of AP to reveal whether polymorphisms of MCP-1 and IL-8 genes relate with the severity of AP.
Recently the polymorphism of gene correlated with the occurrence and development of diseases is a hotspot. New methods, for example, gene chip techniques, for the research of polymorphism and new findings have been found.
The polymorphism of MCP-1-2518 might affect the course of AP, but the genotype of AA and the frequency of G allele could not predict the risk of SAP, which is different from Papachristou GI’s research. And no correlation has been found between the IL-8-251 polymorphism and the liability of AP. The result is obviously diverse from Hofner P’s research.
This study is aimed to find a new way to monitor and evaluate the course of AP, and guide therapy or to identify populations at risk of severe AP.
PCR-RFLP: polymerase chain reaction-restriction fragment length polymorphism.
The authors have studied MCP-1 and IL-8 polymorphism in MAP and SAP. The recent environmental and genetic researches have added more knowledge in the field of pancreatitis pathophysiology and prognosis. This is an interesting study.
Peer reviewer: Ibrahim A Al Mofleh, Professor, Department of Medicine, College of Medicine, King Saud University, PO Box 2925, Riyadh 11461, Saudi Arabia
S- Editor Li DL L- Editor Ma JY E- Editor Yin DH
| 2. | Laing KJ, Secombes CJ. Chemokines. Dev Comp Immunol. 2004;28:443-460. |
| 3. | Hotz HG, Reber HA. Acute pancreatitis. Curr Opin Gastroenterol. 1999;15:392-397. |
| 4. | Sun J, Bhatia M. Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2007;292:G143-G153. |
| 5. | Sugita H, Yamaguchi Y, Ikei S, Yamada S, Ogawa M. Enhanced expression of cytokine-induced neutrophil chemoattractant (CINC) by bronchoalveolar macrophages in cerulein-induced pancreatitis rats. Dig Dis Sci. 1997;42:154-160. |
| 6. | Makhija R, Kingsnorth AN. Levels of the chemokines growth-related oncogene alpha and epithelial neutrophil-activating protein 78 are raised in patients with severe acute pancreatitis (Br J Surg 2002; 89: 566-572). Br J Surg. 2002;89:1194. |
| 7. | Brady M, Bhatia M, Christmas S, Boyd MT, Neoptolemos JP, Slavin J. Expression of the chemokines MCP-1/JE and cytokine-induced neutrophil chemoattractant in early acute pancreatitis. Pancreas. 2002;25:260-269. |
| 8. | Rau B, Baumgart K, Krüger CM, Schilling M, Beger HG. CC-chemokine activation in acute pancreatitis: enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med. 2003;29:622-629. |
| 9. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. |
| 10. | Gross V, Andreesen R, Leser HG, Ceska M, Liehl E, Lausen M, Farthmann EH, Schölmerich J. Interleukin-8 and neutrophil activation in acute pancreatitis. Eur J Clin Invest. 1992;22:200-203. |
| 11. | Berney T, Gasche Y, Robert J, Jenny A, Mensi N, Grau G, Vermeulen B, Morel P. Serum profiles of interleukin-6, interleukin-8, and interleukin-10 in patients with severe and mild acute pancreatitis. Pancreas. 1999;18:371-377. |
| 12. | Papachristou GI, Sass DA, Avula H, Lamb J, Lokshin A, Barmada MM, Slivka A, Whitcomb DC. Is the monocyte chemotactic protein-1 -2518 G allele a risk factor for severe acute pancreatitis? Clin Gastroenterol Hepatol. 2005;3:475-481. |
| 13. | Hofner P, Balog A, Gyulai Z, Farkas G, Rakonczay Z, Takács T, Mándi Y. Polymorphism in the IL-8 gene, but not in the TLR4 gene, increases the severity of acute pancreatitis. Pancreatology. 2006;6:542-548. |
| 14. | Domínguez-Muñoz JE, Carballo F, García MJ, de Diego JM, Campos R, Yangüela J, de la Morena J. Evaluation of the clinical usefulness of APACHE II and SAPS systems in the initial prognostic classification of acute pancreatitis: a multicenter study. Pancreas. 1993;8:682-686. |
| 15. | Balthazar EJ, Robinson DL, Megibow AJ, Ranson JH. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174:331-336. |
| 16. | Mühlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Schölmerich J, Hellerbrand C. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085-93. |
| 17. | Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9-23. |
| 18. | Ishibashi T, Zhao H, Kawabe K, Oono T, Egashira K, Suzuki K, Nawata H, Takayanagi R, Ito T. Blocking of monocyte chemoattractant protein-1 (MCP-1) activity attenuates the severity of acute pancreatitis in rats. J Gastroenterol. 2008;43:79-85. |
| 19. | Grady T, Liang P, Ernst SA, Logsdon CD. Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology. 1997;113:1966-1975. |
| 20. | Saurer L, Reber P, Schaffner T, Büchler MW, Buri C, Kappeler A, Walz A, Friess H, Mueller C. Differential expression of chemokines in normal pancreas and in chronic pancreatitis. Gastroenterology. 2000;118:356-67. |
| 21. | Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6, IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol. 2003;37:263-266. |
| 22. | Donnelly SC, Strieter RM, Kunkel SL, Walz A, Robertson CR, Carter DC, Grant IS, Pollok AJ, Haslett C. Interleukin-8 and development of adult respiratory distress syndrome in at-risk patient groups. Lancet. 1993;341:643-647. |
| 23. | Abe R, Shimosegawa T, Kimura K, Abe T, Kashimura J, Koizumi M, Toyota T. The role of endogenous glucocorticoids in rat experimental models of acute pancreatitis. Gastroenterology. 1995;109:933-943. |
| 24. | Balog A, Gyulai Z, Boros LG, Farkas G, Takács T, Lonovics J, Mándi Y. Polymorphism of the TNF-alpha, HSP70-2, and CD14 genes increases susceptibility to severe acute pancreatitis. Pancreas. 2005;30:e46-e50. |
| 25. | Rahman SH, Ibrahim K, Larvin M, Kingsnorth A, McMahon MJ. Association of antioxidant enzyme gene polymorphisms and glutathione status with severe acute pancreatitis. Gastroenterology. 2004;126:1312-1322. |
| 26. | Lee WP, Tai DI, Lan KH, Li AF, Hsu HC, Lin EJ, Lin YP, Sheu ML, Li CP, Chang FY. The -251T allele of the interleukin-8 promoter is associated with increased risk of gastric carcinoma featuring diffuse-type histopathology in Chinese population. Clin Cancer Res. 2005;11:6431-6441. |
| 27. | Lu W, Pan K, Zhang L, Lin D, Miao X, You W. Genetic polymorphisms of interleukin (IL)-1B, IL-1RN, IL-8, IL-10 and tumor necrosis factor {alpha} and risk of gastric cancer in a Chinese population. Carcinogenesis. 2005;26:631-636. |
| 28. | Vogel U, Christensen J, Wallin H, Friis S, Nexø BA, Tjønneland A. Polymorphisms in COX-2, NSAID use and risk of basal cell carcinoma in a prospective study of Danes. Mutat Res. 2007;617:138-146. |
| 29. | Arinir U, Klein W, Rohde G, Stemmler S, Epplen JT, Schultze-Werninghaus G. Polymorphisms in the interleukin-8 gene in patients with chronic obstructive pulmonary disease. Electrophoresis. 2005;26:2888-2891. |
| 30. | Infante J, Llorca J, Berciano J, Combarros O. Interleukin-8, intercellular adhesion molecule-1 and tumour necrosis factor-alpha gene polymorphisms and the risk for multiple system atrophy. J Neurol Sci. 2005;228:11-13. |