Published online Oct 7, 2008. doi: 10.3748/wjg.14.5738
Revised: August 26, 2008
Accepted: September 2, 2008
Published online: October 7, 2008
AIM: To prospectively assess the changes in parameters of computed tomography (CT) perfusion pre- and post-transarterial chemoembolization (TACE) of hepatocellular carcinoma (HCC) in different treatment response groups, and to correlate the changes with various responses of HCC to TACE.
METHODS: Thirty-nine HCC patients underwent CT perfusion examinations pre-(1 d before TACE) and post-treatment (4 wk after TACE). The response evaluation criteria for solid tumors (RECIST) were referred to when treatment responses were distributed. Wilcoxon-signed ranks test was used to compare the differences in CT perfusion parameters pre- and post-TACE for different response groups.
RESULTS: Only one case had treatment response to CR and the CT perfusion maps of post-treatment lesion displayed complete absence of signals. In the PR treatment response group, hepatic artery perfusion (HAP), hepatic arterial fracture (HAF) and hepatic blood volume (HBV) of viable tumors post-TACE were reduced compared with pre-TACE (P = 0.001, 0.030 and 0.001, respectively). In the SD group, all CT perfusion parameters were not significantly different pre- and post-TACE. In the PD group, HAP, HAF, portal vein perfusion (PVP) and hepatic blood flow (HBF) of viable tumors post-TACE were significantly increased compared with pre-TACE (P = 0.005, 0.012, 0.035 and 0.005, respectively).
CONCLUSION: Changes in CT perfusion parameters of viable tumors are correlated with different responses of HCC to TACE. Therefore, CT perfusion imaging is a feasible technique for monitoring response of HCC to TACE.
- Citation: Chen G, Ma DQ, He W, Zhang BF, Zhao LQ. Computed tomography perfusion in evaluating the therapeutic effect of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2008; 14(37): 5738-5743
- URL: https://www.wjgnet.com/1007-9327/full/v14/i37/5738.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5738
| PR | SD | |||||
| n = 14 | Pre-treatment | Post-treatment | P value | Pre-treatment | Post-treatment | P value |
| HBF (mL/min per 100 mL) | 294.594 ± 162.104 | 203.941 ± 147.717 | 0.096 | 133.277 ± 111.124 | 205.580 ± 217.101 | 0.054 |
| HBV (mL/100 mL) | 37.569 ± 19.818 | 19.252 ± 11.847 | 0.001 | 14.097 ± 4.276 | 21.558 ± 21.085 | 0.220 |
| MTT (s) | 10.385 ± 4.747 | 10.687 ± 2.132 | 0.637 | 14.097 ± 4.276 | 12.235 ± 4.276 | 0.421 |
| PS (mL/min per 100 mL) | 20.066 ± 19.398 | 19.997 ± 22.516 | 0.638 | 27.357 ± 21.960 | 22.504 ± 18.439 | 0.310 |
| HAF (%) | 63.671 ± 20.218 | 38.154 ± 22.357 | 0.030 | 42.343 ± 14.025 | 33.793 ± 18.575 | 0.248 |
| HAP (mL/min/100 mL) | 201.857 ± 145.608 | 57.266 ± 33.537 | 0.001 | 62.621 ± 65.274 | 78.230 ± 76.442 | 0.172 |
| PVP (mL/min/100 mL) | 92.590 ± 69.771 | 146.681 ± 144.414 | 0.330 | 70.664 ± 50.880 | 127.349 ± 167.413 | 0.054 |
| PD (n = 10) | Pre-treatment | Post-treatment | P value |
| HBF (mL/min per 100 mL) | 178.508 ± 63.650 | 263.828 ± 91.731 | 0.005 |
| HBV (mL/100 mL) | 28.101 ± 26.862 | 29.978 ± 9.261 | 0.385 |
| MTT (S) | 9.336 ± 2.517 | 10.001 ± 2.457 | 0.798 |
| PS (mL/min/100 mL) | 33.103 ± 3.784 | 26.384 ± 10.544 | 0.092 |
| HAF (%) | 45.856 ± 41.332 | 69.628 ± 30.126 | 0.012 |
| HAP (mL/min per 100 mL) | 32.686 ± 7.145 | 94.200 ± 55.928 | 0.005 |
| PVP (mL/min per 100 mL) | 19.820 ± 5.546 | 33.480 ± 8.765 | 0.035 |
Transarterial chemoembolization (TACE) has been widely accepted as a choice of treatment for advanced hepatocellular carcinoma (HCC) and shows promising results[1-3]. The therapeutic efficacy of TACE has been evaluated by various imaging modalities. Digital subtraction angiography (DSA) is probably the most sensitive and effective imaging modality[4,5]. However, angiography is an invasive procedure and its routine use in evaluation of therapeutic effectiveness needs justification[5]. CT examination after TACE has been widely used to assess the efficacy of TACE[6], but its limitation is that a quantitative evaluation cannot be provided and an incomplete lipiodol accumulation may disturb the assessment of viable tumors on contrast-enhanced CT imaging[7].
Alternatively, CT perfusion imaging is a non-invasive technique for assessment of tissue perfusion in locally advanced HCC[8-10]. The response to TACE may be evaluated by comparing the difference in perfusion parameters pre- and post-treatment[11,12].
To our knowledge, there are few reports on the application of this technique in evaluating the efficacy of TACE based on the quantitative analysis of perfusion parameters. In this study, we prospectively assessed the changes in perfusion parameters of viable tumors after TACE for different treatment response groups, and correlated the changes in CT perfusion parameters to different responses of tumors to TACE.
Thirty-nine consecutive patients (28 men and 11 women; age range, 36-82 years; mean, 60.1 years) with histopathologically proven HCC (fine needle aspiration) referred to our center for TACE from September 2007 to June 2008 were included in the study. The average size of HCC was 6.1 ± 3.3 cm, ranging 2.5-16.5 cm. All patients underwent CT perfusion examinations pre-(1 d before TACE) and post-treatment (4 wk after TACE).
CT perfusion was performed with a 64-row multi-detector CT scanner (VCT 64 slices; GE Medical Systems). For initial localization of the tumor, a CT scan of the abdomen was performed without contrast medium during a breath hold at the end of expiration. After tumor localization, a 4-cm tumor region was selected independently for the dynamic study of the tumor maximal diameter. A dynamic study of the selected area was performed in a single breath hold at a static supine position. A total of 40-50 mL of non-ion iodinated contrast medium (Iohexol300, 300 mg of iodine per milliliter) was injected at a rate of 4 mL/s through an 18-gauge intravenous cannula. The following CT parameters were used to acquire dynamic data: 1-second gantry rotation time, 120 kV, 80 mA, acquisition in 264 transverse mode (64 sections per gantry rotation), and 5-mm reconstructed section thickness. The examination was repeated in all patients using the same technique before and 4 wk after TACE.
After image acquisition, the data were transferred to an image processing workstation (Advantage Windows 4.3; GE Medical Systems) and analyzed using CT Perfusion 3.0 (GE Medical Systems). For derivation of the functional maps of perfusion parameters, a reference arterial input curve was obtained by placing a region of interest (ROI) in the aorta (range, 10-15 mm2) manually, and a reference input curve of portal vein was obtained by placing a ROI in the portal vein (range, 8-12 mm2) manually. A ROI for tumor was hand drawn in a selected section in which the lesion demonstrated the maximal diameter.
The parameters generated by the software included hepatic blood flow (HBF), hepatic blood volume (HBV), mean transit time (MTT), and permeability-surface area product (PS), hepatic arterial fracture (HAF), hepatic artery perfusion (HAP), and portal vein perfusion (PVP).
All TACE procedures were performed by one experienced interventional radiologist. Diagnostic arteriogram of the common, right or left hepatic artery was obtained in all patients prior to TACE. For solitary lesions in liver, TACE with superselective catheterization of the feeding hepatic artery branch was performed. Chemoembolization was performed at the common hepatic artery for multiple lesions involving both lobes of liver. After a combined chemotherapy with an average dose of epirubicin (60 mg), hydroxycamptothecin (20 mg), and fluorouracil (750 mg), an average dose of lipiodol (6 mL) with epirubicin (10 mg) mixture and/or gelfoam particles were used in our study. The total volume of emulsion was judged by the tumor size and achievement of stagnant arterial flow. Follow-up hepatic angiograms were performed in the latter sessions, 4-5 wk and 8-9 wk following the first TACE. Additional chemoembolization would be given at the same session setting if there was evidence of residual hypervascularity in hepatic arteriograms.
DSA has been recognized as the gold standard for detection of viable hepatic tumors[5,7]. Therefore, in this study, DSA was used as the assessment method for measuring target lesions responsive to TACE. The first angiogram and follow-up angiograms in the latter sessions 4-5 wk and 8-9 wk following the first TACE were compared. The following response evaluation criteria [complete response (CR): disappearance of all target lesions; partial response (PR): at least a 30% decrease in the sum of the longest diameters (LD) of target lesions; progressive disease (PD): at least a 20% increase in the sum of the LD of target lesions; stable disease (SD): neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD][13] for solid tumors (RECIST) were referred to when it was necessary to assess the therapeutic effect after TACE.
All data were expressed as mean ± SD, and statistical analysis was performed with SPSS 11.0. Wilcoxon-signed ranks test (nonparametric test) of variance was used to compare the differences in CT perfusion parameters pre- and post-TACE for different treatment response groups. P < 0.05 was considered statistically significant.
In all patients of our study cohort, only one patient who underwent a period of TACE and CT perfusion examinations pre- and post-treatment had treatment response to CR. His CT perfusion imaging of post-treatment lesion displayed complete absence of blood perfusion on CT perfusion maps. Therefore, in the CR group, the perfusion parameters of post-treatment tumor could not be generated.
In remaining 38 HCC patients, response to treatment was observed in the PR group (n = 14), SD group (n = 14), and PD group (n = 10).
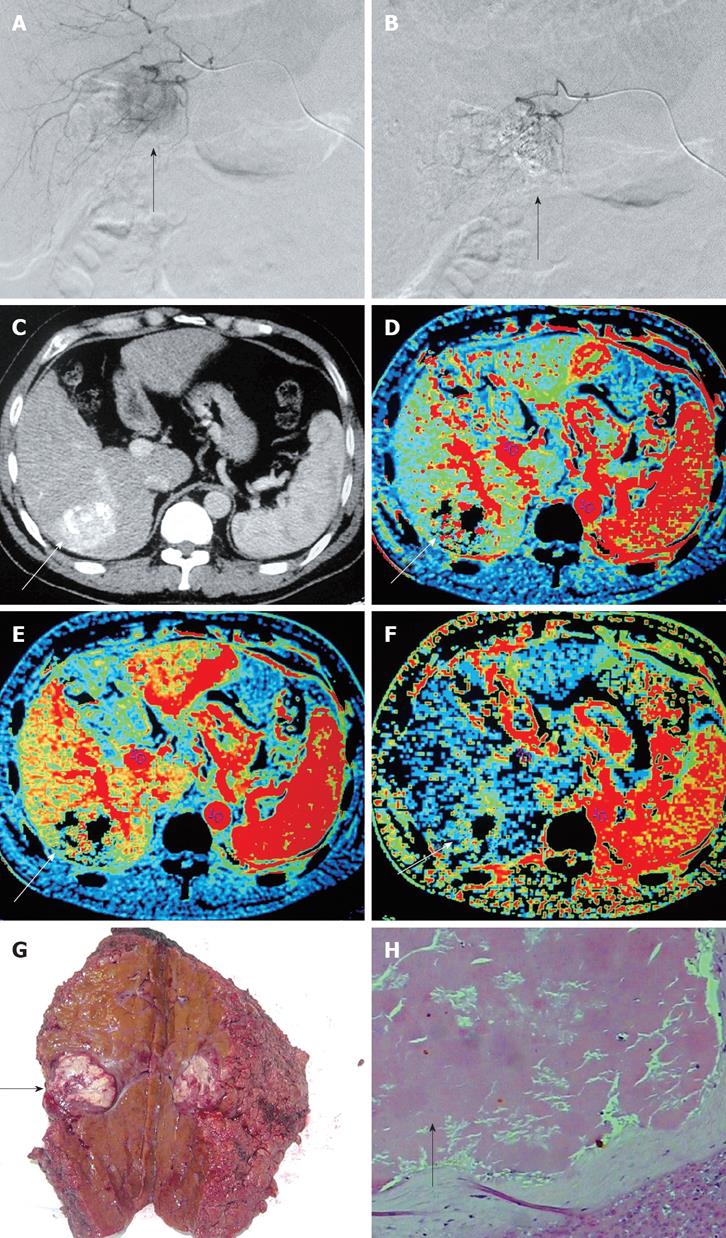
In the PR treatment response group, some perfusion parameters (HAP, HAF, and HBV) of HCC post-TACE were significantly reduced compared with pre-TACE (Figure 1) and significantly different pre- and post-TACE (P = 0.001, 0.030 and 0.001, respectively), while no significant difference was observed in other perfusion parameters (HBF,MTT,PS, and PVP) pre- and post- TACE (Table 1).
In the SD treatment response group, no significant difference was found in all CT perfusion parameters pre- and post-TACE (Table 1).
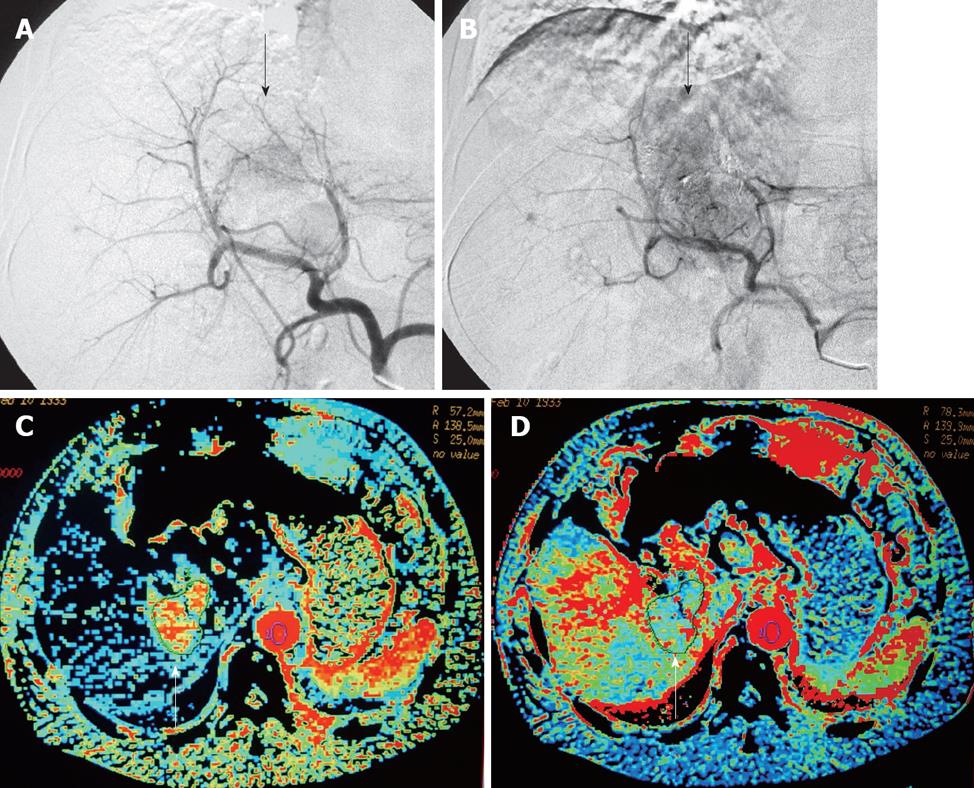
In the PD treatment response group, some CT perfusion parameters (HAP, HAF, PVP, and HBF) of HCC post-TACE were significantly increased compared to pre-TACE (Figure 2) and their difference showed a statistical significance (P = 0.005, 0.012, 0.035 and 0.005, respectively), while no significant difference was found in other perfusion parameters (HBV, MTT, and PS) pre- and post-TACE (Table 2).
CT perfusion is a feasible, reproducible technique for assessing tissue perfusion in locally advanced HCC[14]. Measures of tumor perfusion have been correlated with angiogenesis and microvessel density within the tumor[14,15]. It is logical to speculate that high perfusion values indirectly suggest a high rate of angiogenesis and microvessel density within the tumor.
However, to our knowledge, there are few studies on the role of CT perfusion in evaluating the therapeutic efficacy of TACE, although Tsushima et al[11] presented their data on CT perfusion and demonstrated decreased values of HAP and HAF in viable tumors post-treatment compared with pre- treatment, which are consistent with our findings.
In our study, the number of cases collected allowed us to divide them into different treatment response groups for observation. In the CR group, CT perfusion imaging of post-TACE lesion displayed complete absence of signal on the CT perfusion maps. We observed significant differences in some CT perfusion parameters of viable tumors per- and post-TACE in the treatment response PR and PD groups. In the PR group of HCC, after TACE treatment, the change in perfusion parameters was consistent with previous findings[11,16]. The decreased HBV and HBF within liver tumors indicate the reduction of vascular capacity and microvessel density, which are due to the lipiodol embolism (Figure 1G and H). Kan et al[16] reported that post-embolization MTT is elongated in intrahepatic lesions of a rat model, while post-embolization PS is significantly decreased. However, we did not find any significant difference in values of MTT and PS pre- and post-treatment, which may be due to the difference in observational time windows, in which the microvascular function within tumors dynamically changes. In the SD group, the perfusion parameters remained unchanged pre- and post-TACE treatment, suggesting a perfusion recovery within the tumor after treatment. In the PD group, HBF, HAF, and HAP were increased after treatment, reflecting the increased angiogenesis and microvessel density within the liver tumor. The value of PVP was further increased in HCC of the PD group after treatment, suggesting involvement of hepatic portal vein during tumor advancement. In our study, the value of HBV was not further increased during tumor advancement, suggesting that the vascular capacity within the tumor mass has reached its limit and thus, cannot further expand. Further histological research is needed to prove this speculation.
In addition, many CT perfusion analyses were performed using a single arterial input[8,9,15], but the liver has a dual arterial-portal blood supply, and the tumor and portal vein could not be consistently included. In our study, 64-rows multi-detector CT was introduced to offer a greater coverage (up to 4 cm), thus overcoming this limitation by including both the tumor and portal vein in dual-input analysis. The introduction of multi-detector CT has stimulated further interest in perfusion CT techniques and their future implementation in clinical practice[14].
We believe that comparison of the results from different CT perfusion studies has to be made cautiously, as the values measured are dependent on mathematic model and pharmacokinetics of the contrast medium used. Thus, application of different models to the same data may well yield different perfusion values. It should be emphasized that our results were specific to the method of analysis and the software employed in this study.
The limitations of our study are as follows. The grouping of patients did not involve factors such as differentiation of HCC, cirrhosis, invasion of portal vein, dosage of chemotherapeutant and lipiodol, and use of gelfoam particles, all of which could influence the therapeutic response of HCC to TACE and change the CT perfusion parameters after TACE. Because the determinants for therapeutic response of HCC to TACE are complicated and numerous, this study did not take into account these determinants of therapeutic response to TACE, but rather it only correlated the changes in CT perfusion parameters with different treatment responses to TACE.
In conclusion, CT perfusion imaging can be used in the assessment of perfusion changes resulting from TACE therapy. Post-treatment changes in perfusion parameters are correlated with different therapeutic efficacies for HCC. Thus, CT perfusion imaging is a feasible and non-invasive technique for monitoring treatment response to TACE.
Computed tomography (CT) perfusion imaging is a non-invasive and non-invasive technique for assessing tissue perfusion in locally advanced HCC.
CT perfusion scan for patients with HCC allows assessment of perfusion changes due to transarterial chemoembolization (TACE). There are few reports on the application of this technique in evaluating the efficacy of TACE based on the quantitative analysis of perfusion parameters.
In this study, changes in parameters of CT perfusion pre- and post-TACEwere observed in different treatment response groups.
CT perfusion imaging, a feasible and non-invasive technique, can be used for monitoring treatment response to TACE.
This study reported the changes of CT perfusion parameters in different treatment response groups and provides a useful imaging modality for monitoring treatment response to HCC.
Peer reviewer: Dr. Serdar Karakose, Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Zhong XY L- Editor Wang XL E- Editor Ma WH
| 1. | Rossi P, Salvatori FM, D’Erme M, Maradei A, Rossi M, Santoro P, Mastantuono M, Gualdi G. [Therapy of hepatic carcinoma by the intra-arterial injection of lipiodol, antineoplastic agents and gelfoam]. Radiol Med (Torino). 1989;77:37-43. |
| 2. | Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170:783-786. |
| 3. | Ngan H, Lai CL, Fan ST, Lai EC, Yuen WK, Tso WK. Treatment of inoperable hepatocellular carcinoma by transcatheter arterial chemoembolization using an emulsion of cisplatin in iodized oil and gelfoam. Clin Radiol. 1993;47:315-320. |
| 4. | Yoshioka H, Nakagawa K, Shindou H, Ono Y, Kawakami A, Mabuchi N, Arita S, Fujii K, Hamada T, Ishida O. MR imaging of the liver before and after transcatheter hepatic chemo-embolization for hepatocellular carcinoma. Acta Radiol. 1990;31:63-67. |
| 5. | Castrucci M, Sironi S, De Cobelli F, Salvioni M, Del Maschio A. Plain and gadolinium-DTPA-enhanced MR imaging of hepatocellular carcinoma treated with transarterial chemoembolization. Abdom Imaging. 1996;21:488-494. |
| 6. | Okusaka T, Okada S, Ueno H, Ikeda M, Yoshimori M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, Iwata R. Evaluation of the therapeutic effect of transcatheter arterial embolization for hepatocellular carcinoma. Oncology. 2000;58:293-299. |
| 7. | Tsui EY, Chan JH, Cheung YK, Cheung CC, Tsui WC, Szeto ML, Lau KW, Yuen MK, Luk SH. Evaluation of therapeutic effectiveness of transarterial chemoembolization for hepatocellular carcinoma: correlation of dynamic susceptibility contrast-enhanced echoplanar imaging and hepatic angiography. Clin Imaging. 2000;24:210-216. |
| 8. | Miles KA, Hayball MP, Dixon AK. Functional images of hepatic perfusion obtained with dynamic CT. Radiology. 1993;188:405-411. |
| 9. | Fournier LS, Cuenod CA, de Bazelaire C, Siauve N, Rosty C, Tran PL, Frija G, Clement O. Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol. 2004;14:2125-2133. |
| 10. | Tsushima Y, Funabasama S, Aoki J, Sanada S, Endo K. Quantitative perfusion map of malignant liver tumors, created from dynamic computed tomography data. Acad Radiol. 2004;11:215-223. |
| 11. | Tsushima Y, Unno Y, Koizumi J, Kusano S. Hepatic perfusion changes after transcatheter arterial embolization (TAE) of hepatocellular carcinoma: measurement by dynamic computed tomography (CT). Dig Dis Sci. 1998;43:317-322. |
| 12. | Lin WY, Wang SJ, Yeh SH. Hepatic perfusion index in evaluating treatment effect of transcatheter hepatic artery embolization in patients with hepatocellular carcinoma. Neoplasma. 1995;42:89-92. |
| 13. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. |
| 14. | Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol. 2003;76 Spec No 1:S36-S42. |