Published online Oct 7, 2008. doi: 10.3748/wjg.14.5730
Revised: July 19, 2008
Accepted: July 26, 2008
Published online: October 7, 2008
AIM: To enrich hepatic progenitors using epithelial cell adhesion molecule (EpCAM) as a marker from human fetal liver and investigate the expression of human leukocyte antigen (HLA) and their markers associated with hepatic progenitor cells.
METHODS: EpCAM +ve cells were isolated using magnetic cell sorting (MACS) from human fetuses (n = 10) at 15-25 wk gestation. Expression of markers for hepatic progenitors such as albumin, alpha-fetoprotein (AFP), CD29 (integrin β1), CD49f (integrin α6) and CD90 (Thy 1) was studied by using flow cytometry, immunocytochemistry and RT-PCR; HLA class I (A, B, C) and class II (DR) expression was studied by flow cytometry only.
RESULTS: FACS analysis indicated that EpCAM +ve cells were positive for CD29, CD49f, CD90, CD34, HLA class I, albumin and AFP but negative for HLA class II (DR) and CD45. RT PCR showed that EpCAM +ve cells expressed liver epithelial markers (CK18), biliary specific marker (CK19) and hepatic markers (albumin, AFP). On immunocytochemical staining, EpCAM +ve cells were shown positive signals for CK18 and albumin.
CONCLUSION: Our study suggests that these EpCAM +ve cells can be used as hepatic progenitors for cell transplantation with a minimum risk of alloreactivity and these cells may serve as a potential source for enrichment of hepatic progenitor.
- Citation: Rao MS, Khan AA, Parveen N, Habeeb MA, Habibullah CM, Pande G. Characterization of hepatic progenitors from human fetal liver during second trimester. World J Gastroenterol 2008; 14(37): 5730-5737
- URL: https://www.wjgnet.com/1007-9327/full/v14/i37/5730.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5730
| Genes | Primer sequence | Product size (bp) |
| CK18 | Sense-TGGTACTCTCCTCAATCTGCTG | 148 |
| Anti-sense-CTCTGGATTGACTGTGGAAGT | ||
| CK19 | Sense-CCTGCGGGACAAGATTCTTG | 326 |
| Anti-sense-ACGGGCGTTGTCGATCTG | ||
| Albumin | Sense-GCTTTGCCGAGGAGGGTAA | 161 |
| Anti-sense-GTAGGCTGAGATGCTTTATGT | ||
| AFP | Sense-GCAAAGCTGAAAATGCAGTTGA | 216 |
| Anti-sense-GGAAAGTTCGGGTCCCAAAA |
| Antibody | Group 1 (n = 5), cells% | Group 2 (n = 5), cells% | P |
| CD29 | 3.9 ± 1.3 | 1.9 ± 0.7 | 0.005 |
| CD49f | 2.1 ± 0.6 | 1.5 ± 0.7 | 0.372 |
| CD90 | 3.5 ± 1.9 | 1.3 ± 0.3 | 0.044 |
| Albumin | 19.2 ± 3.1 | 21.3 ± 4.2 | 0.817 |
| AFP | 7.3 ± 0.6 | 7.2 ± 1.0 | 0.865 |
| HLA-A, B, C (HLA Class I) | 6.5 ± 2.4 | 8.2 ± 2.1 | 0.279 |
| HLA-DR (HLA class II ) | 0.6 ± 0.4 | 0.35 ± 0.1 | 0.612 |
Hepatocyte transplantation has been reported as a useful bridge/alternative therapy for the treatment of various types of liver diseases. The potential of hepatocyte transplantation to provide metabolic support for acutely injured liver tissues has been suggested by earlier workers[1,2]. However, due to the scarcity of HLA matched adult human liver donor cells and the inability of hepatocytes to proliferate in vitro the usage of hepatocyte-based cell therapy in liver diseases has been limited. The existence of bi-potential progenitor cells in the liver of rodents using different phenotypic markers was demonstrated many years ago[3]. More recently the same markers have been demonstrated in human fetal liver and it has been argued that these progenitor cells are derived from a common cell compartment and they can be referred to as the hepatoblasts[4]. Similar cells obtained from the rat fetus could reconstitute bile ducts and structures resembling hepatocytes after being transplanted in retrosine damaged liver of syngeneic rats[5].
Progenitors isolated from adult or fetal liver can generate hepatocytes in vitro and mature liver cells in vivo. Fetal liver CD117+/CD34+/Lin-progenitors and their progeny proliferated in vitro and also functionally differentiated into mature hepatic cells in an acute liver injury model[6]. Epithelial progenitor cells (EPC) from human fetal liver possessed highly proliferative ability and sub-passaged for more than 25 passages. Two months after EPC transplantation, the grafted cells differentiate into hepatocyte like cells[7]. Epithelial cell adhesion molecule positive (EpCAM +) cells are 80% hepatoblasts and 0.1%-0.7% is hepatic stem cells in human fetal liver[8]. Transplantation of these EpCAM + cells or hepatic stem cells expanded in culture into NOD/SCID mice results in mature liver tissue expressing human specific proteins[9]. It has been reported that cells in embryonic day (ED) 13.5 fetal mouse liver cells, which co-express CD49f and CD29 (α6, β1 integrin subunits) but do not express c-kit, CD45 or TER 119 are the best candidate for hepatic stem /progenitor cells[10].
Human fetal liver cells also offer an important source for isolating hepatic and hematopoietic progenitors for clinical application. After 5 wk of gestation hematopoiesis starts to shift from the yolk sac to the liver[11], and during the first trimester fetal liver contains both hepatic and hematopoietic progenitors[12-14]. During this period, fetal liver cells express hepatoblasts and biliary cell specific markers, such as albumin, alpha-fetoprotein (AFP), β-1 microglobulin, glycogen, glucose-6-phosphatase (G-6-P), gamma glutamyl transpeptidase (GGT), dipeptidyl peptidase IV (DPPIV) and cytokeratin (CK) 19; they also express hematopoietic markers such as CD34[15,16]. During the second trimester markers for hepatoblasts continue to be expressed but the expression of hematopoietic precursors is reduced. This offers an opportunity to isolate and study large numbers of hepatic progenitor cells without hematopoietic potential during the second trimester of gestation. Several years ago we, for the first time, had shown the use of human fetal hepatocytes isolated from the second trimester, for the treatment of fulminant hepatic failure patients[17]. Subsequently other investigators have also isolated hepatic progenitor cells from human fetal liver in the second trimester and demonstrated the higher ability to proliferate without changing their karyotypes[4]. It is therefore important to characterize the cells that could be responsible for the regenerative potential of the fetal hepatocytes in the second trimester of gestation.
Recently we have reported the expression of CD34 antigen (a hematopoietic marker) in human fetal liver cells during the second trimester, and the co-expression of hepatic markers such as AFP and albumin in these cells[18]. In this study we have described the expression of hepatic progenitor and HLA markers in the EpCAM +ve cells isolated from the second trimester fetal liver. Our results show that EpCAM +ve fetal liver cells at this stage express nil levels of HLA-DR marker but they show expression of progenitor and liver specific markers suggesting that these EpCAM +ve cells are hepatic progenitors. Our results also indicate that the EpCAM +ve cells include most of the CD34 +ve cells that are seen in the fetal liver during the second trimester.
Human fetal liver tissues were obtained from aborted fetuses at 15-25 wk gestation in accordance with the Institute ethical guidelines. The fetuses were collected under sterile condition within 2 h of the termination of pregnancy. The liver tissue from the fetus was initially perfused twice with cold PBS for 5 min to eliminate circulating cell contaminants, followed by digestion with 0.025% collagenase prepared in PBS for 5 min. Then the liver was disintegrated into a single cell suspension by passing through 70 μm cell strainer (BD Biosciences). Viable cell count was determined by the trypan blue dye exclusion test. All donors of the fetus used in the study had been serologically screened for syphilis, toxoplasmosis, rubella, hepatitis B and C, human immunodeficiency virus 1, cytomegalovirus, parvovirus, and herpes simplex types 1 and 2. All cell isolation procedures were carried out under sterile condition in class 100-biosafety cabinet.
EpCAM positive cells were sorted by using the magnetic cell sorter Auto MACS according to the manufacturer’s instructions (Miltenyi Biotec, Germany, http//http://www.miltenyibiotec.com). Labeling of EpCAM positive cells was done by incubating with 5 × 107 total fetal liver cells in 500 μL of buffer containing FCR blocking reagent and EpCAM micro beads at 4°C for 30 min. Cells were filtered using 70 μm cell strainer to remove clumps and loaded on magnetic column. EpCAM +ve cells were collected in the second fraction form the column.
The enriched EpCAM +ve cells were collected and suspended in RPMI 1640 medium (Sigma) supplemented with 10% fetal bovine serum (FBS, Sigma). Cells were plated at 1.5 × 106 per well in a six well plates and maintained at 37°C in a humidified environment containing 5% carbon dioxide. After 72 h, the nonadherent cells were removed and the medium replaced. Colonies that formed during the culture period were picked after 10 d and 20 d and kept on ice for further study of CK18 and albumin expression by immunofluorescence.
EpCAM +ve cells were stained with Phycoerythrin (PE) labeled anti-CD29 (Integrin β1, 1:100, BD Biosciences, USA), CD90 (Thy 1) or fluorescein isothiocynate (FITC) labeled anti CD49f (integrin α6) antibodies (1:100, BD Biosciences, USA). Staining for HLA-A, HLA-B, HLA-C and HLA-DR was done incubating with the respective primary antibodies (Chemicon, USA) at 4°C for overnight. Subsequently the cells were incubated with FITC labeled rabbit anti-mouse IgG FITC (1:100, Sigma) at room temperature for 40 min. Unstained EpCAM positive cells were used as negative control to subtract auto fluorescence.
For albumin and AFP staining in the cytoplasm, cells were fixed with 4% paraformaldehyde in PBS and then permeated with 0.5% Triton X-100 for 10 min. After two washes cells were blocked with 0.5% bovine serum albumin (BSA, Sigma), at room temperature for 1 h. Cells were incubated with primary antibodies at room temperature for 2 h and subsequently incubated with secondary antibodies, FITC labeled rabbit anti-goat IgG (1:200, Bangalore Genei, India) at room temperature for 40 min. Stained cells were analyzed on FACS Calibur flow cytometry (BD Biosciences, USA).
In addition to phenotyping by flow cytometry, EpCAM +ve cells were stained for albumin and CK18 by immunocytochemistry. For albumin and CK18 staining in the cytoplasm, Cultured EpCAM +ve cells were fixed with 4% paraformaldehyde in PBS and then permeated with 0.5% Triton X-100 for 10 min. After two washes cells were blocked with 0.5% BSA at room temperature for 1 h. Cells were incubated with primary antibodies include albumin (1:100, MP biomedical), CK18 (1:200, Sigma) at room temperature for 2 h and subsequently incubated with secondary antibodies, FITC labeled rabbit anti-goat IgG (1:200, Bangalore Genei, India) at room temperature for 40 min. The signal was detected using a fluorescence microscope (Axio Plan upright; Carl Zeiss Vision Co., Ltd., Hallbergmoos, Germany).
RT PCR analysis of cultured EpCAM +ve cells was done for 3 samples (n = 3). Total RNA from cultured EpCAM +ve cells was isolated using RNAeasy Mini Kit (Qiagen). One µg of RNA was reversed transcribed using Moloney murine leukemia virus Reverse transcriptase and Oligo dT (Promega). PCR reaction was performed using cDNA amount representing 100 ng of total RNA and 1 unit of Taq polymerase (Invitrogen, reaction mixture containing 10 mmol/L Tris-Hcl, 200 mmol/L dNTP and 20 pmol of gene specific primer). PCR was performed in a thermal cycler (Programmable Thermal Controller) following an initial denaturation step of 5 min at 95°C. Thermal program was consisting of 35 cycles of 94°C for 1 min, 56°C for 1 min and 72°C for 2 min. This was followed by a final extension of 10 min at 72°C. PCR products were separated by electrophoresis in 1.5% agarose gel. The primer sequences (Invitrogen) used in the study are listed in Table 1.
The data were categorized as group 1, cells obtained from abortuses of gestational age 15 to 20 wk, and group 2, gestational age 21 wk to 25 wk. Student t-test was used to determine the likelihood of a significant difference (P < 0.05) between these two groups in the percentage of EpCAM +ve cells that express phenotypes with hepatic and progenitor markers and in the percentage of cells that express HLA classes I and II.
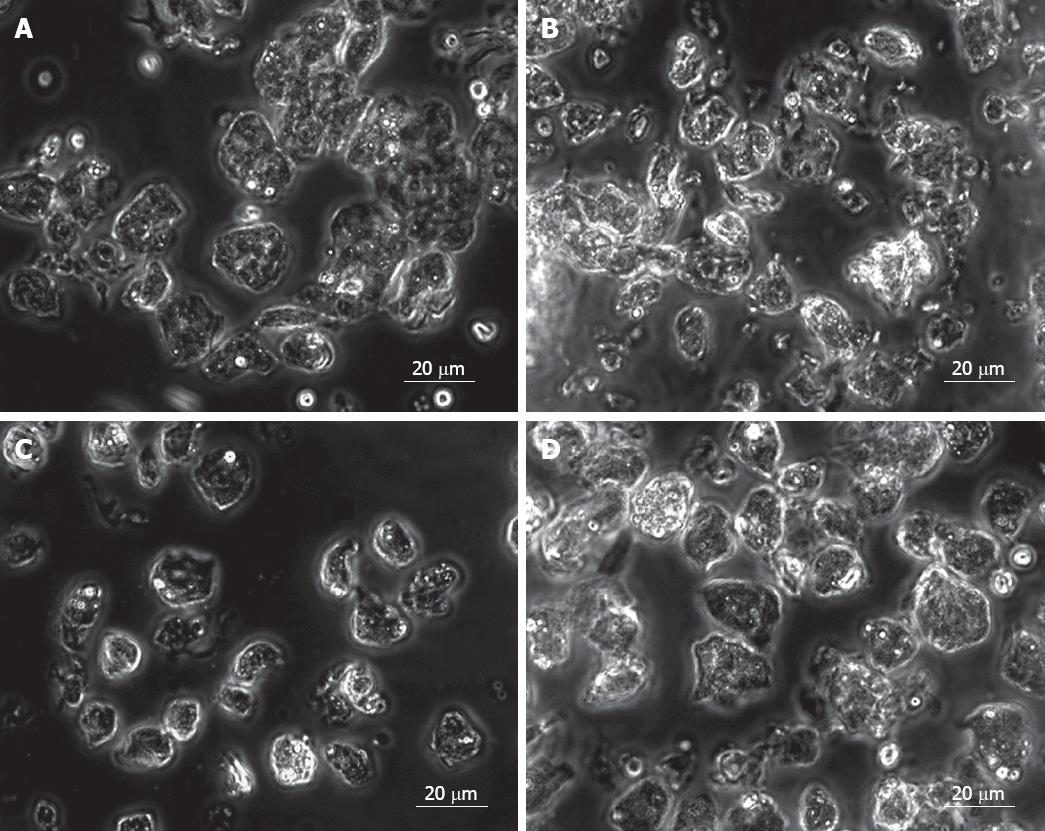
Fetal liver cells in the study have been divided into two age groups, 15-20 wk (group 1) and 21-25 wk (group 2). The total number of cells obtained from each group was (140.0 ± 30.8) × 106 and (193.4 ± 96.8) × 106, respectively, and the cell viability in both groups was > 80%. This indicated that the total number of cells in fetal liver during the late second trimester is higher in the early second trimester. Microscopic examination of the cells before EpCAM +ve cell enrichment showed a heterogeneous population in both age groups (Figure 1B). Purification of EpCAM +ve cells was done by using magnetically tagged EpCAM antibodies and MACS as described. The number of EpCAM +ve cells obtained in both fetal age groups were (22 ± 4.8) × 106 to (34.4 ± 11.4) × 106, thus indicating a decrease in the yield of EpCAM +ve cells during the last part of the second trimester from about 16% to 15% of the total fetal liver cells, during the later second trimester. Morphologically most EpCAM +ve cells were homogenous (Figure 1C). On the contrary EpCAM -ve cells were a mixed population of cells (Figure 1D).
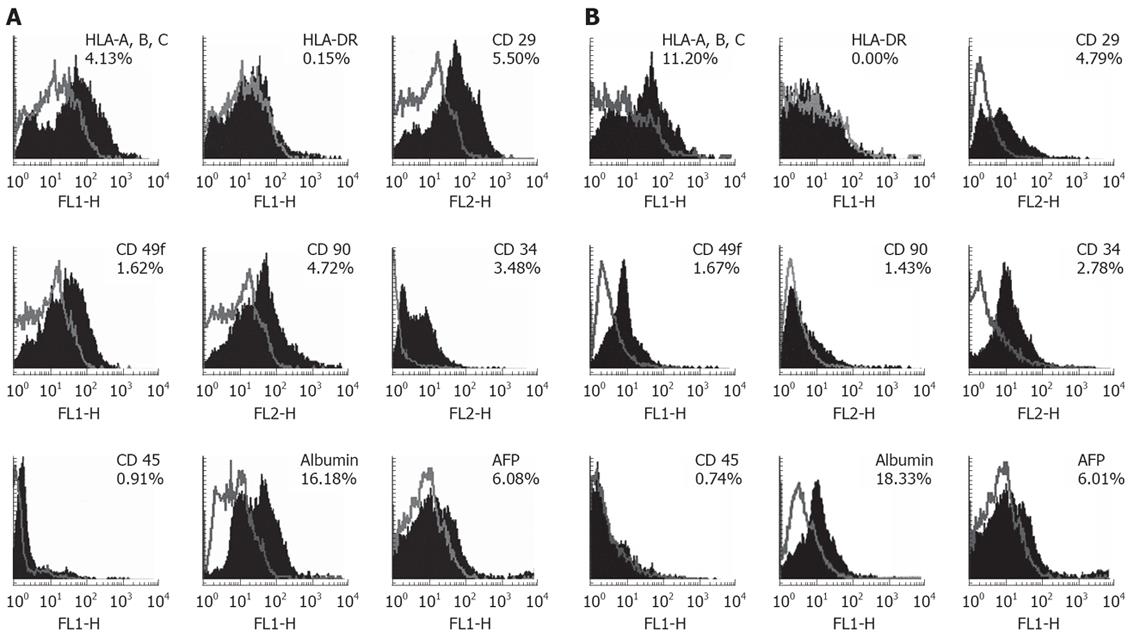
We have analyzed the expression of HLA class I and II and hepatic progenitor markers such as CD29, CD49f, CD90 and hepatic specific markers such as albumin and AFP in EpCAM +ve cells by flow cytometry (Figure 2, Table 2). The results are depicted in Figure 2, gives representative histograms of the FACS analysis for HLA and hepatic progenitor markers in EpCAM +ve cells in group 1 (Figure 2A) and group 2 (Figure 2B). Numbers indicated in Table 2, gives mean percentage expression of HLA and hepatic progenitor markers in EpCAM +ve cells in group 1 and group 2.
HLA class I expression was seen in approximately 6.52% cells in group 1. 8.2% in group 2. Expression of HLA class II expression in the same period was similar 0.6% and 0.4% cells respectively (Table 2). HLA class I and II expression levels were not significantly different with respect to their gestational age groups.
CD29 (β-1 integrin) and CD49f (α-6 integrin) are cell-adhesion molecules that are important for cell-matrix interactions, these markers are also used to study early hepatocyte differentiation. The expression of β1 integrin was observed in about 3.9% cells in-group 1 and in 2.0% cells in group 2. During the corresponding periods CD49f expression was seen in 2.2% and 1.6% cells. These data indicate the β-1 integrin cells in the EpCAM +ve population reduce by half in group 2 but there is no significant change in α-6 integrin +ve cells (Table 2).
CD90 is a cell surface protein is also one of the hepatic progenitor markers. The expression of CD90 was observed in 3.5% cells in group 1 and in 1.3% cells in group 2 and significant difference of expression of CD90 marker was observed with respect to gestational ages (Table 2).
Albumin is used as a classic hepatic indicative markers differentiation for hepatic stem cells. We observed strong levels of albumin expression in EpCAM +ve but it did not differ significantly in gestational age groups (Table 2). FACS data revealed that albumin expression was 19.2% cells in group 1 and 21.3% cells in group 2.
AFP used as a marker for hepatic stem cells was observed strong levels in EpCAM +ve cells (Figure 2A and B), but it did not differ significantly in gestational age groups (Table 2). FACS data revealed that AFP expression was 7.3% in group 1 and 7.2% in group 2.
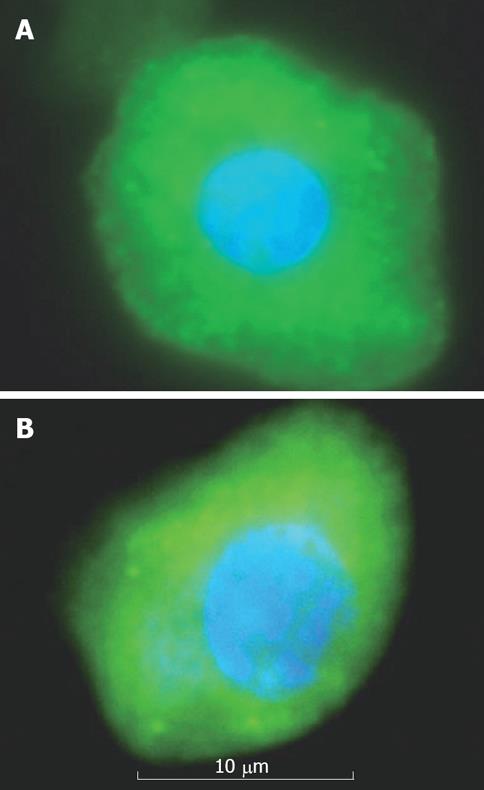
On Immunocytochemical staining, EpCAM +ve cells were more strongly stained for CK18 and albumin. Albumin positive stained cells possessed large nuclei and more cytoplasm (Figure 3A). CK18 positive stained cells possessed large nuclei and highly granulated cytoplasm (Figure 3B).
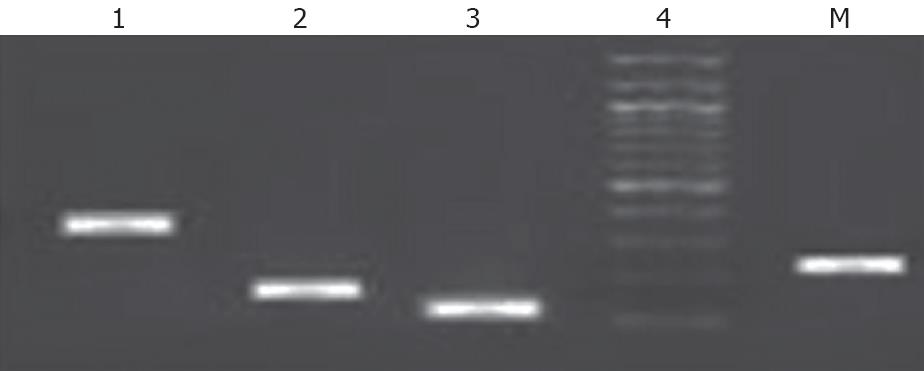
To confirm that the sorted cells were of hepatic lineage, cultured Epcam +ve sorted cells were further analyzed for the expression of CK18, CK19, albumin, and AFP. These results suggest that EPCAM +ve cells strongly express genotypic CK18, CK19, Albumin, AFP (Figure 4) and these cells were capable of differentiating into hepatic and biliary lineages.
A progenitor or precursor for hepatocytes seen in fetal or adult tissues give rise to differentiated cells in a specialized way. Researchers often distinguish progenitor precursor cells from adult stem cells in the following way: when a stem cell divides, one of the two new cells is often a stem cell capable of replicating itself again where as the other cell is commited to differentiation. In contrast, when a progenitor/precursor cell divides, it can either form more progenitor/precursor cells or it can form two differentiated cells directly, neither of which is capable of replicating itself. During normal homeostasis of tissues progenitor/precursor cells are used to replace cells that are damaged or dead, thus maintaining the integrity and functions of a tissue like liver.
This idea of progenitor cell dependent liver regeneration started with oval cells. Although neither cell replacement during normal tissue turnover nor after injury by partial hepatectomy requires stem cells for organ regeneration, this is not true for all types of liver injury. In some types of liver damage, for example primary biliary cirrhosis Alcoholic liver disease NASH, small cells (oval cells) with a high nuclear/cytoplasmic ratio emerge in the portal zone, proliferative extensively, and migrate into the lobule[19]. But the engraftment of oval cells may be less efficient than for hepatoctyes because of their smaller size and less efficient trapping in the liver[20].
Earlier studies have shown hepatic progenitors that express markers such as CD117/CD34[21], CD90[22], CD34/AFP[18]. Hepatoblasts are common progenitors for hepatocytes and biliary epithelial cells[23]. EpCAM has first been identified as a tumor-specific antigen on several carcinomas of different origin.
In our study we have observed EpCAM +ve cell enrichment of up to 15% after MACS sorting. These cells did not clump during purification and there was no change in the cell morphology or viability before and after sorting. In an earlier study have reported 12% EpCAM + enrichment from human fetal liver[8].
In our recent study we have shown an average of 5% CD34 +ve cells with hepatic progenitor properties on human total fetal liver cells[18]. Interestingly in the present study we observed CD34 expression (an average of 3%) on EpCAM +ve cells also. However these cells showed low or nil (0.91%) expression of CD45, the marker that distinguishes hematopoietic cells from non-hematopoietic cells. Therefore we can say that EpCAM +ve and CD34 +ve cells are not committed to hematopoietic lineage and they may differentiate into hepatic cells in the second trimester of fetal liver. Recently CD90 expressing cells were recognized as hepatic progenitors [22]. In our study progenitor markers such as CD90+, CD34+ expressions were observed on EpCAM +ve cells and these cells were negative for hematopoietic lineage (CD45). Where as CD90 and CD34 double positive expression ranged 0.44%-2.04% in total fetal liver cells and these cells were positive for both hepatic lineage (CK19) and hematopoietic lineage (CD45) markers in the second trimester[22].
In our study we found that positive cells of integrin CD29 (β-1 integrin) and CD49f (α-6) on EpCAM +ve cells. Earlier investigators have reported that CD29 as a specific hepatic stem cell marker, was expressed on an average of 0.08%-2% of the human total fetal liver cells[21]. It has been shown that CD49f +ve cells (an average of 48.51%) are primitive hepatic endodermal cells with the capacity to differentiate into hepatocytes in the mouse fetal liver[24]. Taken together presence of stem cell marker (β-1 integrin) and primitive hepatic endodermal (α-6 integrin) indicate that it is possible to designate our cells as possessing progenitor phenotype.
It has been speculated that progenitor cells are less immunogenic than mature cells; however, immunological rejection would still be an issue. We believe that transplantation of cells having negligible would certainly reduce GvHd development. Earlier investigators reported that high percentage of cells expressing the histocompatibility markers HLA class I and HLA class II were found in the 5th and 8th wk of gestation in total fetal liver cells after which the expression levels were decreased with advanced gestation age 21 wk This higher expression of HLA molecules in these cells may be because of hematopoietic cell contamination in the fetal liver in early gestation. Therefore in our study, we have enriched the EpCAM +ve cells without hematopoietic cell contamination and investigated the pattern of HLA molecules expressed by EpCAM +ve cells from human fetal liver by using flow cytometry. We found that EpCAM +ve cells expressed intermediate levels HLA class I but no HLA class II. Although controversial, it seems that, to varying degree, the human fetus can respond to allogenic cells from the beginning of the second trimester[25]. The allogenic response is usually HLA mediated and it is expected that transplantation with cells that do not express HLA class II antigen may minimize the risk of alloreactivity. It has been stated that embryonic hepatocytes were still immature cells bearing incomplete MHC II surface antigen, thus possessing lower immunogenicity[26].
Earlier investigators reported that EpCAM +ve cells were not immunogenic as they failed to stimulate a significant amount of T cell proliferation. Besides, these cells were not immunosuppressive as they failed to suppress the mixed lymphocyte reaction (MLR) significantly. Therefore EpCAM +ve cells might be an ideal candidate donor cells for hepatic cell therapy in liver disorders.
RT-PCR analysis on the EpCAM +ve cells showed that expression of albumin, AFP, CK18 and CK19 genes was detectable significantly with gestational ages. CK18 expression further confirms that the cells are epithelial lineage. It has been shown that EpCAM sorted hepatic progenitor cells have the high gene expression levels of AFP and albumin on hepatoblasts[8]. This suggests that EpCAM is a hepatic progenitor cell marker.
Fetal liver cells have been shown to be a rich source of progenitor/stem cells. However enrichment of hepatic progenitors from the developing human liver, with more viability and with minimum rejection in allogenic transplantation remains a significant challenge for the advancement of therapeutic approaches to liver disorders.
To summarize, the current study demonstrates that EpCAM is a novel and useful marker for enrichment of hepatic progenitors with moderate expression of HLA-class I and negative for HLA- class II. Thus this finding supports the use of EpCAM positive fetal liver cells for allogenic transplantation since these cells negative for class II HLA antigen on their surface which otherwise can trigger a rejection reaction in allogenic transplantation. In our present study we determined the characteristic of cell type and purity. Further more, MLR and animal model studies are warranted to assess immunogenicity and in vivo engraftment respectively, of enriched EpCAM positive cells of fetal liver prior to pre-clinical transplantation.
Liver transplantation is the primary treatment for various end-stage hepatic diseases but is hindered by the lack of donor organs and by complications associated with rejection and immunosuppression. There is increasing evidence to suggest that the aborted human fetal liver is a transplantable source of hepatic progenitors. The aim of this study was to critically analyze the various phenotypic markers to enrich hepatic progenitor cells from human fetal liver. We have previously reported a marker (CD34) to enrich hepatic progenitor from human fetal liver.
The hepatic progenitors cells offer a potential source for cell therapy and doing bridge transplantation for treatment of liver diseases.
This article helps to enrich hepatic progenitor cells using epithelial cell adhesion molecule (EpCAM) as a marker. We have found that EpCAM +ve cells expressed intermediate levels human leukocyte antigen (HLA) class I but no HLA class II. Our results provide evidence why enriched EpCAM + cells can be used for the treatment of liver diseases.
The sorting of stem cells using EpCAM can be used as hepatic as an alternative for hepatic cell therapy in liver cell disorders.
This article tries to explore the enrichment of hepatic progenitor using EpCAM as a marker for the treatment of liver diseases. The result revealed that the EpCAM +ve cells can be used as hepatic progenitors for transplantation in patient with liver diseases.
Peer reviewer: Paul E Sijens, PhD, Associate Professor, Department of Radiology, UMCG, Groningen 9713GZ, the Netherlands
S- Editor Zhong XY L- Editor Alpini GD E- Editor Lin YP
| 1. | Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery. 1977;82:124-132. |
| 2. | Sommer BG, Sutherland DE, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation for treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc. 1979;11:578-584. |
| 3. | Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142-148. |
| 4. | Malhi H, Irani AN, Gagandeep S, Gupta S. Isolation of human progenitor liver epithelial cells with extensive replication capacity and differentiation into mature hepatocytes. J Cell Sci. 2002;115:2679-2688. |
| 5. | Sigal SH, Brill S, Reid LM, Zvibel I, Gupta S, Hixson D, Faris R, Holst PA. Characterization and enrichment of fetal rat hepatoblasts by immunoadsorption ("panning") and fluorescence-activated cell sorting. Hepatology. 1994;19:999-1006. |
| 6. | Nowak G, Ericzon BG, Nava S, Jaksch M, Westgren M, Sumitran-Holgersson S. Identification of expandable human hepatic progenitors which differentiate into mature hepatic cells in vivo. Gut. 2005;54:972-979. |
| 7. | Liu YN, Zhang J, He QH, Dai X, Shen L. Isolation and characterization of epithelial progenitor cells from human fetal liver. Hepatol Res. 2008;38:103-113. |
| 8. | Schmelzer E, Wauthier E, Reid LM. The phenotypes of pluripotent human hepatic progenitors. Stem Cells. 2006;24:1852-1858. |
| 9. | Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao HL, Moss N, Melhem A, McClelland R, Turner W. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973-1987. |
| 10. | Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol. 2002;156:173-184. |
| 11. | Migliaccio G, Migliaccio AR, Petti S, Mavilio F, Russo G, Lazzaro D, Testa U, Marinucci M, Peschle C. Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac----liver transition. J Clin Invest. 1986;78:51-60. |
| 12. | Rowley PT, Ohlsson-Wilhelm BM, Farley BA. Erythroid colony formation from human fetal liver. Proc Natl Acad Sci USA. 1978;75:984-988. |
| 13. | Hann IM, Bodger MP, Hoffbrand AV. Development of pluripotent hematopoietic progenitor cells in the human fetus. Blood. 1983;62:118-123. |
| 14. | Roy V, Miller JS, Verfaillie CM. Phenotypic and functional characterization of committed and primitive myeloid and lymphoid hematopoietic precursors in human fetal liver. Exp Hematol. 1997;25:387-394. |
| 15. | Haruna Y, Saito K, Spaulding S, Nalesnik MA, Gerber MA. Identification of bipotential progenitor cells in human liver development. Hepatology. 1996;23:476-481. |
| 16. | Badve S, Logdberg L, Sokhi R, Sigal SH, Botros N, Chae S, Das KM, Gupta S. An antigen reacting with das-1 monoclonal antibody is ontogenically regulated in diverse organs including liver and indicates sharing of developmental mechanisms among cell lineages. Pathobiology. 2000;68:76-86. |
| 17. | Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951-952. |
| 18. | Nyamath P, Alvi A, Habeeb A, Khosla S, Khan AA, Habibullah CM. Characterization of hepatic progenitors from human fetal liver using CD34 as a hepatic progenitor marker. World J Gastroenterol. 2007;13:2319-2323. |
| 19. | Shinozuka H, Lombardi B, Sell S, Iammarino RM. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res. 1978;38:1092-1098. |
| 20. | Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11881-11888. |
| 21. | Nava S, Westgren M, Jaksch M, Tibell A, Broome U, Ericzon BG, Sumitran-Holgersson S. Characterization of cells in the developing human liver. Differentiation. 2005;73:249-260. |
| 22. | Masson NM, Currie IS, Terrace JD, Garden OJ, Parks RW, Ross JA. Hepatic progenitor cells in human fetal liver express the oval cell marker Thy-1. Am J Physiol Gastrointest Liver Physiol. 2006;291:G45-G54. |
| 23. | Tanimizu N, Nishikawa M, Saito H, Tsujimura T, Miyajima A. Isolation of hepatoblasts based on the expression of Dlk/Pref-1. J Cell Sci. 2003;116:1775-1786. |
| 24. | Hoppo T, Fujii H, Hirose T, Yasuchika K, Azuma H, Baba S, Naito M, Machimoto T, Ikai I. Thy1-positive mesenchymal cells promote the maturation of CD49f-positive hepatic progenitor cells in the mouse fetal liver. Hepatology. 2004;39:1362-1370. |
| 25. | Lindton B, Markling L, Ringden O, Kjaeldgaard A, Gustafson O, Westgren M. Mixed lymphocyte culture of human fetal liver cells. Fetal Diagn Ther. 2000;15:71-78. |
| 26. | Shi Z, Liang XL, Lu BX, Pan SY, Chen X, Tang QQ, Wang Y, Huang F. Diminution of toxic copper accumulation in toxic milk mice modeling Wilson disease by embryonic hepatocyte intrasplenic transplantation. World J Gastroenterol. 2005;11:3691-3695. |