Published online Oct 7, 2008. doi: 10.3748/wjg.14.5701
Revised: July 15, 2008
Accepted: July 22, 2008
Published online: October 7, 2008
AIM: We studied the estrogen receptor (ER) and progesterone receptor (PR) isoforms expression in gastric antrum and corpus of female gerbils and their regulation by estradiol (E2) and progesterone (P4).
METHODS: Ovariectomized adult female gerbils were subcutaneously treated with E2, and E2 + P4. Uteri and stomachs were removed, the latter were cut along the greater curvature, and antrum and corpus were excised. Proteins were immunoblotted using antibodies that recognize ER-alpha, ER-beta, and PR-A and PR-B receptor isoforms. Tissues from rats treated in the same way were used as controls.
RESULTS: Specific bands were detected for ER-alpha (68 KDa), and PR isoforms (85 and 120 KDa for PR-A and PR-B isoforms, respectively) in uteri, gastric antrum and corpus. We could not detect ER-beta isoform. PR isoforms were not regulated by E2 or P4 in uterus and gastric tissues of gerbils. ER-alpha isoform content was significantly down-regulated by E2 in the corpus, but not affected by hormones in uterus and gastric antrum.
CONCLUSION: The presence of ER-alpha and PR isoforms in gerbils stomach suggests that E2 and P4 actions in this organ are in part mediated by their nuclear receptors.
- Citation: Saqui-Salces M, Neri-Gómez T, Gamboa-Dominguez A, Ruiz-Palacios G, Camacho-Arroyo I. Estrogen and progesterone receptor isoforms expression in the stomach of Mongolian gerbils. World J Gastroenterol 2008; 14(37): 5701-5706
- URL: https://www.wjgnet.com/1007-9327/full/v14/i37/5701.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5701
Mongolian gerbils (Meriones unguiculatus) have been used in scientific research for a long time, especially in neuronal protection studies and as a model of human H pylori infection. In spite of the numerous publications using Mongolian gerbils, and the characterization of their estrous cycle, which is 4 d to 6 d in duration[1,2], their sexual hormonal levels and receptors have not been characterized to date.
Estradiol (E2) and progesterone (P4) participate in numerous cellular functions, such as proliferation and differentiation in normal and cancer cells. Most of these functions are mediated by their nuclear receptors, ER and PR[3-5].
There are two well characterized isoforms or ER, alpha and beta, that are encoded by different genes[6]. The ER-alpha protein has 595 aminoacids, with a molecular weight of 66 KDa[7]. ER-beta is constituted by 485 amino acids, and has a molecular weight of 54 KDa. The ER-beta isoform is homologous to ER-alpha at the DNA binding domain (95%), and the heat shock protein binding domain (55%). Both isoforms bind estrogens with high affinity, but regulate different functions[8].
PR has two main isoforms, PR-A (72-94 kDa) and PR-B (108-120 kDa), encoded by the same gene, but regulated by distinct promoters. PR-A is a truncated form of PR-B, lacking 128-164 aminoacids, depending on the species, in the amino terminal end[9,10].
We have previously shown that E2 and P4 have clear and distinct effects on inflammatory response and gastric epithelial changes during early H pylori infection[11]. Recently, Ohtani et al have shown a protective role of E2 administration in H pylori-infected InGas mice[12]. Other studies have demonstrated that E2 and P4 have anti-ulcerative effects in murine gastric mucosa[13-16].
ER and PR have been reported in human, mouse and rat stomach. However, there is no information to date of their expression and regulation in the Mongolian gerbil stomach. Here we demonstrated the presence of ER-alpha, PR-A and PR-B isoforms in gerbil uterine and gastric tissues by Western blot, as well as a differential regulation of ER-alpha by E2 in gastric corpus.
Mature (16-week-old) female Mongolian gerbils (Meriones unguiculatus) kept in micro isolators under a 12:12 h light-dark cycle, with food and water ad libitum were ovariectomized and after 10 d of recovery, subcutaneous injections of vehicle (corn oil/10% ethanol), 17-beta-estradiol (5 μg/100 μL) or E2 + P4 (1 mg/100 μL) where applied as previously reported[17]. Briefly, gerbils were injected with E2 in two consecutive days and then euthanized after 24 h, while E2 + P4 group received an additional P4 injection on the third day and euthanized 24 h later. Adult Sprague Dawley rats, ovariectomized and treated in the same manner, were used as positive controls.
Gerbils and rats were euthanized by exsanguination under anesthesia and uteri and stomach were removed. The stomachs were cut along the greater curvature, antrum and corpus mucosa were excised. All tissues were snap frozen in liquid nitrogen and kept at -80°C until protein extraction was performed. The experiments were carried out under the guidelines of the Committee for the use of Animals for Research of the Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán.
Uteri were homogenized in TDG lysis buffer with protease inhibitors (10 mmol/L Tris-HCl, 1 mmol/L dithiotreitol, 30% glycerol, 1% Triton X-100, 15 mmol/L sodium azide, 1 mmol/L EDTA, 4 μg/mL leupeptin, 22 μg/mL aprotinin, 1 mmol/L PMSF, 1 mmol/L sodium orthovanadate). Gastric samples were homogenized using TDG lysis buffer without EDTA. Supernatants were clarified by centrifugation at 15 000 r/min, 4°C for 15 min, and proteins quantified by the method of Bradford (Biorad, CA USA)[18]. Proteins (150 μg) were separated by electrophoresis on SDS-PAGE gels at 80 V. Prestained and ECL markers (Biorad, CA) were included for size determination. Gels were transferred to nitrocellulose membranes (Amersham, NJ, USA) at 20 V, room temperature, for 120 min, and were blocked at room temperature with 5% non-fat dry milk and 0.5% bovine serum albumin for 2 h. They were incubated at 4°C overnight with a mix of rabbit polyclonal anti-PR (Neo Markers, Fremont CA) and mouse monoclonal anti-PR (AB-52, sc-810, Santa Cruz, CA, USA) (1 μg/mL each), both antibodies recognize PR-A and PR-B isoforms with similar affinity[19]; 1 μg/mL rabbit polyclonal anti-RE-alpha (Santa Cruz sc-542), 1 μg/mL goat polyclonal anti ER-beta (Y-19, sc-6821, Santa Cruz) or goat polyclonal anti ER-beta (N-19, sc-6820, Santa Cruz) antibodies. Blots were then incubated with a 1:1500 dilution of goat anti-mouse IgG, donkey anti-rabbit IgG or donkey anti-goat IgG antibodies conjugated to horseradish peroxidase (Santa Cruz) for 1 hour at room temperature and detected by enhanced chemiluminescence (ECL, Amersham, NJ, USA).
To correct for differences in the amount of total protein loaded in each lane, blots were stripped with glycine (0.1 mol/L, pH 2.5, 0.5% SDS) overnight at 4°C, and 30 min at 37°C, and reprobed with 1 μg/mL of mouse anti-alpha-tubulin antibody (Santa Cruz) at room temperature for 2 h. Blots were incubated with a 1:1500 dilution goat anti-mouse IgG conjugated to horseradish peroxidase (Santa Cruz) for 1 h. Signals were detected by ECL. The intensity of ER and PR isoforms and alpha-tubulin signals was quantified by densitometry using the Scion Image software (Scion Corp., Maryland, USA).
Data were analyzed using one-way ANOVA and Student’s-t test for group comparisons, after homogeneity of variances was tested. A P < 0.05 value was considered as statistically significant. The analysis was performed using SPSS 12.0 for Windows.
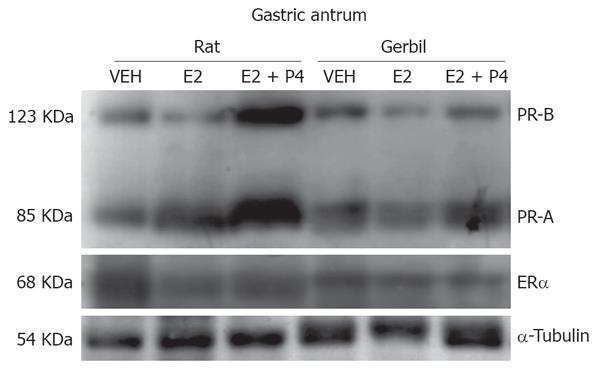
We were able to distinguish two bands of 120 and 85 KDa corresponding to PR-B and PR-A isoforms, respectively, and a 66 KDa band corresponding to ER-alpha isoform in the uteri and stomach of the female gerbil (Figure 1), even when ER and PR have not been sequenced or identified before in gerbils tissues and there are no specific antibodies against these receptors in this species. Unfortunately, we were unable to detect the ER-beta isoform with the antibodies tested in this study in any gerbil tissue.
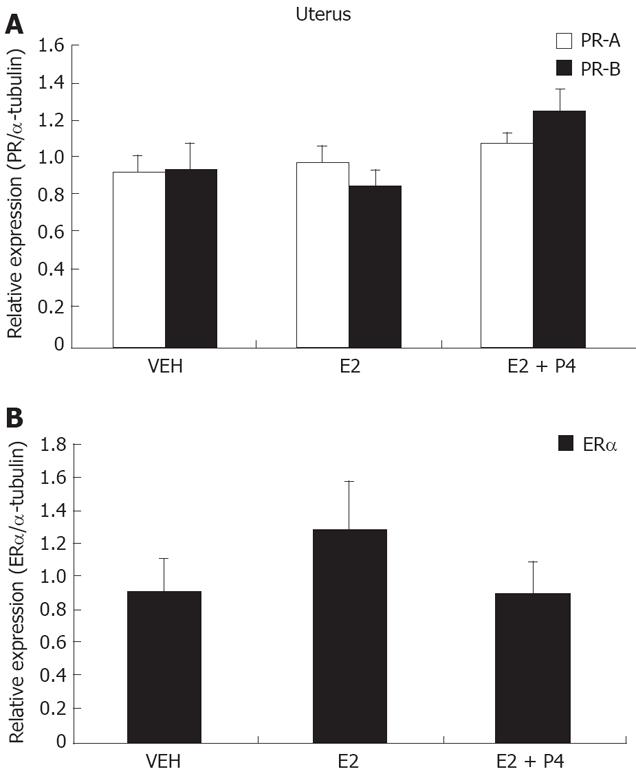
Gerbil uterine samples presented PR-A and PR-B in similar amounts. Interestingly, their expression was not regulated by E2 or P4 (Figure 2A). ER-alpha isoform in uteri (Figure 2B) was detected and, although there is a tendency to an increase after E2 treatment, this was not statistically significant.
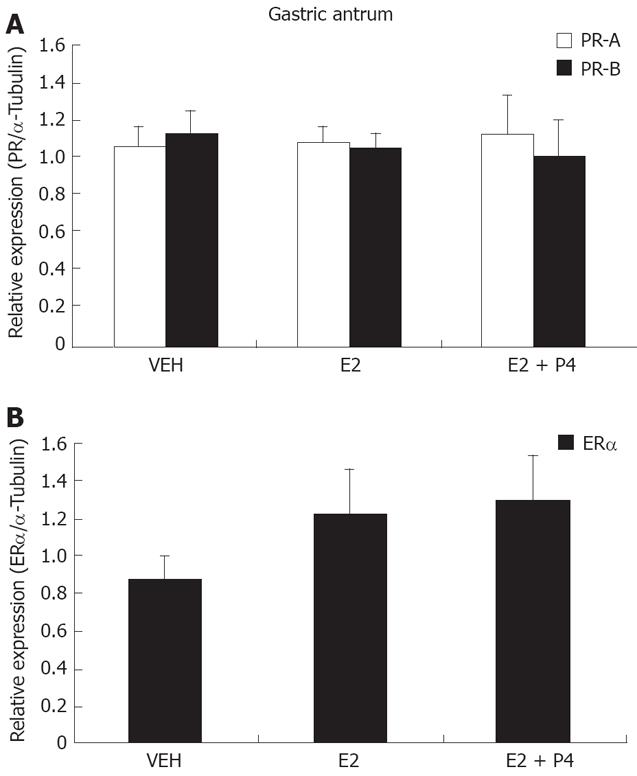
In gastric antrum, PR isoforms were present in similar amounts and were not regulated by hormonal treatments (Figure 3A). ER-alpha isoform was also present in antrum and showed a tendency to be increased under E2 and E2 + P4 treatments, however, this was not significant (Figure 3B).
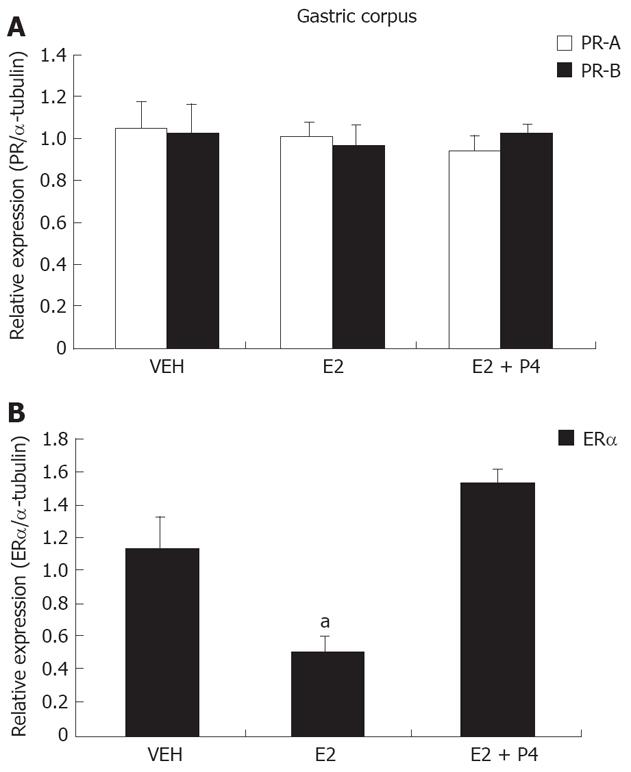
Similar results were obtained for PR isoforms in gastric corpus (Figure 4A). Interestingly, ER-alpha isoform in this tissue was significantly diminished after E2 treatment, and this effect was blocked by P4 (Figure 4B).
The antibodies used for Western blot analysis were tested for immunohistochemical staining in both uterine and gastric gerbil tissues, but we were not able to detect ER and PR using this approach (data not shown).
Mongolian gerbils (Meriones unguiculatus) have become a regular laboratory species, mainly employed in neuronal damage models. However, since 1998, when the H pylori infection model was described[12,20], showing persistent H pylori infection, and similar gastric damage to that observed in human stomach, their use in research has been constantly increasing.
To our knowledge, this is the first study in evaluating the presence of ER and PR in gerbils. We demonstrated the presence of both PR (PR-A and PR-B) isoforms and ER-alpha in gerbils uteri and stomach. These receptors are similar enough to rat receptors since they can be recognized by commercially available antibodies, rendering same size bands than those observed for rat ER-alpha and PR isoforms.
ER-alpha and ER-beta isoforms have been identified in different tissues and their expression is tissue and species-specific. In rat uteri, ER-alpha expression is down-regulated by E2[21]. P4 also down-regulates ER-alpha expression in rat uteri, but increases ER-beta expression in the decidual tissue[10,21,22].
Both PR-A and PR-B are present in rodents, primates and birds[10,23,24], except for rabbits, where only PR-B isoform has been detected[25]. In humans and rats, both PR isoforms are encoded by the same gene, but transcribed from two different promoter sequences[26,27]. ER and PR isoforms in Mongolian gerbils have not been sequenced to date.
In the gerbil uterus, we observed a similar content of both PR isoforms, but they were not regulated by E2 and P4. This lack of regulation contrasts with the up-and down-regulation of PR expression by E2 and P4, respectively, reported in rat uterus[17].
There was a tendency of E2 treatment to increase ER-alpha content, but it was not significantly different. Since sexual hormone levels in gerbils have not been characterized, it is not known yet whether other hormone doses in gerbils are necessary to regulate ER and PR content.
To date, there are no reports on the regulation of the ER and PR isoforms in either the human or murine stomach; however, it is well known that this regulation is tissue-specific. In our previous study, we demonstrated an important role for E2 and P4 in the Mongolian gerbil gastric mucosa in response to early H pylori infection influencing the inflammatory response, proliferation, apoptosis and gastrin producing cell number in the gastric antrum[11]. Although the mechanisms involved in those effects are still unclear, the presence of ER-alpha and both PR isoforms in gastric tissue suggest that their effects are mediated by these receptors.
We have detected ER-alpha and both PR isoforms in gerbil gastric antrum and corpus. In the antrum, none of the receptors was regulated by E2 and P4, but in gastric corpus we observed a down-regulation by E2. Thus, our results show that ER-alpha regulation also depends on the type of cells present in the mucosa. The mechanisms involved in the lack of regulation of PR in gerbil stomach, as well as the differential regulation of ER-alpha by E2, deserve further research.
Parietal cells, the acid producing cells characteristic of gastric corpus, have steroidogenic activity. These cells synthesize E2, P4, testosterone and their metabolites and, therefore, an important role of sexual hormones in hepatic-gastric axis regulation has been suggested[28]. It has been shown that E2 participates in gastric mucous production, epithelial cell exchange, and induces G-cells to release gastrin. E2 produced in stomach is probably processed in the liver[28-30]. Parietal cells are localized only in gastric corpus, thus, it is possible for cells in the corpus to be more sensitive or prone to E2 regulation on ER as compared with antral cells. It is necessary to explore the specific cell types expressing these receptors in both corpus and antrum.
PR and ER expression has been reported in normal human stomach, in human gastric cancer samples[31,32] and in gastric cell lines[33-35]. Also, the differential expression of ER isoforms alpha and beta has been reported in intact rat antrum and corpus, with a higher expression of ER-beta both in human[31,32] and rat[36,37] stomach. In our study, we were unable to detect ER-beta probably due to a lack of cross reactivity of the tested antibodies with this gerbil receptor.
In conclusion, PR isoforms and ER-alpha are expressed in gastric tissue of female gerbils and these receptors are probably involved in E2 and P4 actions in the stomach.
Sex steroid hormones, estradiol and progesterone in particular, have multiple effects that have been widely described. These hormones act either via their nuclear receptors or activating second-messengers pathways. We, and others, have previously reported effects of estradiol and progesterone in the stomach. In this work, we studied the presence of estrogens receptor alpha isoform and progesterone receptors isoforms A and B, and their regulation by their respective ligands in Mongolian gerbils.
Estradiol and progesterone have shown to have effects on the stomach response to damage and Helicobacter pylori (H pylori) infection; Mongolian gerbils are a very useful model for studying the latter. However, the general information available on Mongolian gerbils genome and proteome is scarce. This study will provide basis for further studies on the role of sex steroids on H pylori infection and gastric cancer.
In this study, we reported the presence of estrogen receptor alpha isoform, and progesterone receptor A and B isoforms in Mongolian gerbil antrum and corpus, as well as their regulation. The results showed that these receptors are detectable in whole tissue protein with commercially available antibodies, and that the regulation of sex steroid receptors in the stomach is tissue specific.
This study provides support to the possible genomic mechanism of action of sex steroid hormones in the stomach. Also, it describes an accessible method to detect the presence of these receptors in gastric tissue.
In this manuscript, the authors examined the expression of receptors for estrogen (ERs) and progesterone (PRs) in ovarectomized female gerbils. PR isoforms and ER-alpha are expressed in gastric tissue of female gerbils. In humans, male are more prone than female to H pylori-related diseases such as peptic ulcer diseases and gastric cancers. These gender-related differences appear diminished after menopausal age. This paper is of special value in considering some interesting and unknown aspects of gastric physiology.
Peer reviewers: Shingo Tsuji, Professor, Department of Internal Medicine and Therapeutics, Osaka University Graduate School of Medicine(A8), 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan; Julio H Carri, Professor, Internal Medicine – Gastroenterology, Universidad Nacional de Córdoba, Av.Estrada 160-P 5-Department D, Córdoba 5000, Argentina
S- Editor Zhong XY L- Editor Rippe RA E- Editor Lin YP
| 1. | Barfield MA, Beeman EA. The oestrous cycle in the Mongolian gerbil, Meriones unguiculatus. J Reprod Fertil. 1968;17:247-251. |
| 2. | Nishino N, Totsukawa K. Study on the estrous cycle in the Mongolian gerbil (Meriones unguiculatus). Exp Anim. 1996;45:283-288. |
| 3. | Cunha GR, Cooke PS, Kurita T. Role of stromal-epithelial interactions in hormonal responses. Arch Histol Cytol. 2004;67:417-434. |
| 4. | Peluso JJ. Multiplicity of progesterone's actions and receptors in the mammalian ovary. Biol Reprod. 2006;75:2-8. |
| 5. | Yamashita S. Localization and functions of steroid hormone receptors. Histol Histopathol. 1998;13:255-270. |
| 7. | Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134-139. |
| 8. | Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925-5930. |
| 9. | Anzaldua SR, Camacho-Arroyo I, Reyna-Neyra A, Perez-Martinez M, Cerbon M. Regional differences in expression of progesterone receptor in oviduct and uterus of rabbit during early pregnancy. Comp Biochem Physiol A Mol Integr Physiol. 2007;147:685-690. |
| 10. | Camacho-Arroyo I, Gonzalez-Arenas A, Gonzalez-Moran G. Ontogenic variations in the content and distribution of progesterone receptor isoforms in the reproductive tract and brain of chicks. Comp Biochem Physiol A Mol Integr Physiol. 2007;146:644-652. |
| 11. | Saqui-Salces M, Rocha-Gutierrez BL, Barrios-Payan JA, Ruiz-Palacios G, Camacho-Arroyo I, Gamboa-Dominguez A. Effects of estradiol and progesterone on gastric mucosal response to early Helicobacter pylori infection in female gerbils. Helicobacter. 2006;11:123-130. |
| 12. | Ohtani M, Garcia A, Rogers AB, Ge Z, Taylor NS, Xu S, Watanabe K, Marini RP, Whary MT, Wang TC. Protective role of 17 beta -estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis. 2007;28:2597-2604. |
| 13. | Campbell-Thompson M, Lauwers GY, Reyher KK, Cromwell J, Shiverick KT. 17Beta-estradiol modulates gastroduodenal preneoplastic alterations in rats exposed to the carcinogen N-methyl-N'-nitro-nitrosoguanidine. Endocrinology. 1999;140:4886-4894. |
| 14. | Aguwa CN. Effects of exogenous administration of female sex hormones on gastric secretion and ulcer formation in the rat. Eur J Pharmacol. 1984;104:79-84. |
| 15. | Machowska A, Szlachcic A, Pawlik M, Brzozowski T, Konturek SJ, Pawlik WW. The role of female and male sex hormones in the healing process of preexisting lingual and gastric ulcerations. J Physiol Pharmacol. 2004;55 Suppl 2:91-104. |
| 16. | Montoneri C, Drago F. Effects of pregnancy in rats on cysteamine-induced peptic ulcers: role of progesterone. Dig Dis Sci. 1997;42:2572-2575. |
| 17. | Gonzalez-Arenas A, Villamar-Cruz O, Guerra-Araiza C, Camacho-Arroyo I. Regulation of progesterone receptor isoforms expression by sex steroids in the rat lung. J Steroid Biochem Mol Biol. 2003;85:25-31. |
| 18. | Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. |
| 19. | Estes PA, Suba EJ, Lawler-Heavner J, Elashry-Stowers D, Wei LL, Toft DO, Sullivan WP, Horwitz KB, Edwards DP. Immunologic analysis of human breast cancer progesterone receptors. 1. Immunoaffinity purification of transformed receptors and production of monoclonal antibodies. Biochemistry. 1987;26:6250-6262. |
| 20. | Ikeno T, Ota H, Sugiyama A, Ishida K, Katsuyama T, Genta RM, Kawasaki S. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am J Pathol. 1999;154:951-960. |
| 21. | Varayoud J, Ramos JG, Monje L, Bosquiazzo V, Munoz-de-Toro M, Luque EH. The estrogen receptor alpha sigma3 mRNA splicing variant is differentially regulated by estrogen and progesterone in the rat uterus. J Endocrinol. 2005;186:51-60. |
| 22. | Tessier C, Deb S, Prigent-Tessier A, Ferguson-Gottschall S, Gibori GB, Shiu RP, Gibori G. Estrogen receptors alpha and beta in rat decidua cells: cell-specific expression and differential regulation by steroid hormones and prolactin. Endocrinology. 2000;141:3842-3851. |
| 23. | Guerra-Araiza C, Villamar-Cruz O, Gonzalez-Arenas A, Chavira R, Camacho-Arroyo I. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol. 2003;15:984-990. |
| 24. | Guerra-Araiza C, Cerbon MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci. 2000;66:1743-1752. |
| 25. | Loosfelt H, Atger M, Misrahi M, Guiochon-Mantel A, Meriel C, Logeat F, Benarous R, Milgrom E. Cloning and sequence analysis of rabbit progesterone-receptor complementary DNA. Proc Natl Acad Sci USA. 1986;83:9045-9049. |
| 26. | Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603-1614. |
| 27. | Kraus WL, Katzenellenbogen BS. Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists. Endocrinology. 1993;132:2371-2379. |
| 28. | Ueyama T, Shirasawa N, Numazawa M, Yamada K, Shelangouski M, Ito T, Tsuruo Y. Gastric parietal cells: potent endocrine role in secreting estrogen as a possible regulator of gastro-hepatic axis. Endocrinology. 2002;143:3162-3170. |
| 29. | Le Goascogne C, Sananes N, Eychenne B, Gouezou M, Baulieu EE, Robel P. Androgen biosynthesis in the stomach: expression of cytochrome P450 17 alpha-hydroxylase/17,20-lyase messenger ribonucleic acid and protein, and metabolism of pregnenolone and progesterone by parietal cells of the rat gastric mucosa. Endocrinology. 1995;136:1744-1752. |
| 30. | Ueyama T, Shirasawa N, Ito T, Tsuruo Y. Estrogen-producing steroidogenic pathways in parietal cells of the rat gastric mucosa. Life Sci. 2004;74:2327-2337. |
| 31. | Singh S, Poulsom R, Wright NA, Sheppard MC, Langman MJ. Differential expression of oestrogen receptor and oestrogen inducible genes in gastric mucosa and cancer. Gut. 1997;40:516-520. |
| 32. | Matsuyama S, Ohkura Y, Eguchi H, Kobayashi Y, Akagi K, Uchida K, Nakachi K, Gustafsson JA, Hayashi S. Estrogen receptor beta is expressed in human stomach adenocarcinoma. J Cancer Res Clin Oncol. 2002;128:319-324. |
| 33. | Karat D, Brotherick I, Shenton BK, Scott D, Raimes SA, Griffin SM. Expression of oestrogen and progesterone receptors in gastric cancer: a flow cytometric study. Br J Cancer. 1999;80:1271-1274. |
| 34. | Wu CW, Chang YF, Yeh TH, Chang TJ, Lui WY, P'Eng FK, Chi CW. Steroid hormone receptors in three human gastric cancer cell lines. Dig Dis Sci. 1994;39:2689-2694. |
| 35. | Wu CW, Chi CW, Chang TJ, Lui WY, P'eng FK. Sex hormone receptors in gastric cancer. Cancer. 1990;65:1396-1400. |
| 36. | Campbell-Thompson ML. Estrogen receptor alpha and beta expression in upper gastrointestinal tract with regulation of trefoil factor family 2 mRNA levels in ovariectomized rats. Biochem Biophys Res Commun. 1997;240:478-483. |