Published online Sep 21, 2008. doi: 10.3748/wjg.14.5461
Revised: August 22, 2008
Accepted: August 29, 2008
Published online: September 21, 2008
AIM: To systematically investigate if cGMP/cGMP-dependent protein kinase G (PKG) signaling pathway may participate in dendroaspis natriuretic peptide (DNP)-induced relaxation of gastric circular smooth muscle.
METHODS: The content of cGMP in guinea pig gastric antral smooth muscle tissue and perfusion solution were measured using radioimmunoassay; spontaneous contraction of gastric antral circular muscles recorded using a 4-channel physiograph; and Ca2+-activated K+ currents (IK(Ca)) and spontaneous transient outward currents (STOCs) in isolated gastric antral myocytes were recorded using the whole-cell patch clamp technique.
RESULTS: DNP markedly enhanced cGMP levels in gastric antral smooth muscle tissue and in the perfusion medium. DNP induced relaxation in gastric antral circular smooth muscle, which was inhibited by KT5823, a cGMP-dependent PKG inhibitor. DNP increased IK(Ca). This effect was almost completely blocked by KT5823, and partially blocked by LY83583, an inhibitor of guanylate cyclase to change the production of cGMP. DNP also increased STOCs. The effect of DNP on STOCs was abolished in the presence of KT5823, but not affected by KT-5720, a PKA-specific inhibitor.
CONCLUSION: DNP activates IK(Ca) and relaxes guinea-pig gastric antral circular smooth muscle via the cGMP/PKG-dependent singling axis instead of cAMP/PKA pathway.
- Citation: Cai CY, Cai ZX, Gu XY, Shan LJ, Wang YX, Yin XZ, Qi QH, Guo HS. Dendroaspis natriuretic peptide relaxes gastric antral circular smooth muscle of guinea-pig through the cGMP/cGMP-dependent protein kinase pathway. World J Gastroenterol 2008; 14(35): 5461-5466
- URL: https://www.wjgnet.com/1007-9327/full/v14/i35/5461.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5461
Natriuretic peptides (NP) include atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP), dendroaspis natriuretic peptide (DNP) and urodilatin[1]. DNP is a recently isolated peptide that contains 38 amino residues and shares structural and functional properties with the other members of the natriuretic peptide family[2]. Studies about its physiologic functions mainly focus on cardiovascular[3-5], genital[6], and urinary systems[7]. Evolutionary studies[8] have suggested the presence of DNP-like immunoreactivity in rat colon, and the DNP-like molecule may control colonic motility as a local regulator. Interestingly, we found that DNP inhibits spontaneous contraction in gastric circular smooth muscle[9]. NPs are similar to nitric oxide (NO), which play important physiological functions by affecting the activity of cGMP and cAMP. Sabbatini et al reported[10] that CNP enhances amylase release by reducing cAMP in the exocrine pancreas. Borán et al[11] showed that ANP could play a beneficial role in the resolution of neuroinflammation by removing dead cells and decreasing levels of proinflammatory mediators in microglia via the cGMP-dependent protein kinase G (PKG) signaling pathway. ANP stimulates lipolysis in human adipocyte through a cGMP signaling pathway[12]. However, Wen et al[13] found that CNP activates the pGC-cGMP- phosphodiesterases 3 (PDE3)-cAMP signaling to play a role in hyperthyroid beating of rabbit atria. It is indicated that NP exerts its physiological function by a different pathway. In our previous study, we simply observed that cGMP participates in DNP-induced relaxing of circular smooth muscle, by using a pharmacologic approach. Thus, the aim of this study was to systematically investigate if the cGMP-PKG or cAMP signaling pathway may participate in DNP-induced relaxation using pharmacologic, radioimmunoassay and patch-clamp technique in gastric circular smooth muscle of guinea pigs.
Guinea pigs of either sex, weighing 250-350 g, were purchased from the Experimental Animal Center, Dalian Medical University. The guinea pigs were housed in plastic cages containing corn-chip bedding with free access to food and water for 1 d before they were used for experiments. The care and use of the animals were followed strictly in accordance with the National Institutions of Health Guide for the Care and Use of Laboratory Animals. Guinea pigs were euthanized by a lethal intravenous dose of pentobarbital sodium (50 mg/kg). The abdomen of each guinea pig was opened along the midline, and the stomach was removed and placed in pre-oxygenated Tyrode’s solution at room temperature. After the mucous layer was removed, strips (approximately 2.0 mm × 15.0 mm) of gastric antral circular muscles were prepared. The muscle strips were placed in a bath chamber (2 mL volume). One end of the strip was fixed on the lid of the chamber through a glass claw, and the other end was attached to an isometric force transducer (TD-112S, Nihon Kohden-Kogyo Japan). The chamber was constantly perfused with pre-oxygenated Tyrode’s solution at 1 mL/min. The temperature was maintained at 37.0 ± 0.5°C with a water bath thermostat (WC/09-05, Chongqing, China). The muscle strips were allowed to incubate for at least 40 min before the experiments were started.
The longitudinal layer of muscle was dissected from the other muscle layers using fine scissors and then cut into small segments (1 mm × 4 mm). These segments were kept in modified Kraft-Bruhe (K-B) medium at 4°C for 15 min. They were then incubated at 36°C in 4 mL of digestion medium [Ca-free physiologic salt solution (Ca-free PSS)] containing 0.1% collagenase II, 0.1% dithioerythritol, 0.15% trypsin inhibitor, and 0.2% BSA for 25-35 min. The digested muscle segments were transferred into the modified K-B medium, and the single cells were dispersed by gentle disruption with a wide-bore, fire-polished glass pipette. The isolated gastric myocytes were kept in modified K-B medium at 4°C prior to use. Isolated cells were transferred to a 0.1 mL chamber on the stage of an inverted microscope (IX-70 Olympus, Tokyo, Japan) and allowed to settle for 10-15 min. The cells were continuously perfused with an isosmotic PSS at a rate of 0.9-1.0 mL/min. An 8-channel perfusion system (L/M-sps-8, List Electronics, Berlin, Germany) was used to exchange different solutions. The Ca2+-activated K+ currents (IK(ca)) were recorded using the conventional whole-cell patch-clamp technique. Patch-clamp pipettes were manufactured from borosilicate glass capillaries (GC 150T-7.5, Clark Electromedical Instruments, London, UK) using a 2-stage puller (PP-83, Narishige, Tokyo, Japan). The resistance of the patch pipette was 3-5 MΩ when filled with pipette solution. Liquid junction potentials were canceled prior to the seal formation. Whole-cell currents were recorded using an Axopatch 1-D patch-clamp amplifier (Axon Instruments, Foster City California, USA), and data were filtered at 1 KHz. Command pulses, data acquisition, and storage were applied using the IBM-compatible, 486-grade computer and pCLAMP 6.02 software (Axon Foster City, California, USA). Spontaneous transient outward currents (STOCs) were recorded simultaneously by an EPC-10-HEAKA amplifier (HEAKA Instruments, Berlin, Germany). All experiments were performed at room temperature (20-25°C).
Radioimmunoassay was performed as described elsewhere[14].
Tyrode solution contained (in mmol/L): NaCl 147, KCl 4, MgCl2.6H2O 1.05, CaCl2.2H2O 0.42, Na2PO4.2H2O 1.81, and 5.5 mmol/L glucose. Ca2+-free PSS was composed of (in mmol/L): NaCl 134.8, KCl 4.5, glucose 5, and N-(2-hydroxyethyl) piperazine-N-(2-ethanesulphonic acid) (HEPES; pH was adjusted to 7.4 with Tris (hydroxymethyl aminomethane). Modified K-B solution contained (in mmol/L): L-glutamate 50, KCl 50, taurine 20, KH2PO4 20, MgCl2.6H2O 3, glucose 10, HEPES 10, and egtazic acid 0.5 (pH 7.40 with KOH). PSS contained (in mmol/L): NaCl 134.8, KCl 4.5, MgCl2.6H2O 1, CaCl2.2H2O 2, glucose 5, HEPES 10, and sucrose 110 (pH 7.4 with Tris). In order to eliminate delayed rectifier K+ currents (IK(V)), external solution contained 4-aminopyridine (10 mmol/L), a selective inhibitor of IK(V). The pipette solution for recording IK(Ca) contained (in mmol/L): K+-aspartic acid 110, Mg-ATP 5, HEPES 5, MgCl2.6H2O 1.0, KCl 20, egtazic acid 0.1, di-tris-creatine phosphate 2.5, and disodium-creatine phosphate 2.5 (pH 7.3 with KOH). Tetraethylammonium (TEA), DNP, LY83583, zaprinast, KT5823 and KT5720 were made up as stock solutions. All chemicals in this experiment were purchased from Sigma (St Louis, MO, USA).
All data was expressed as mean ± SD. Statistical significance was evaluated using Student t-test. Differences were considered to be significant when P < 0.05.
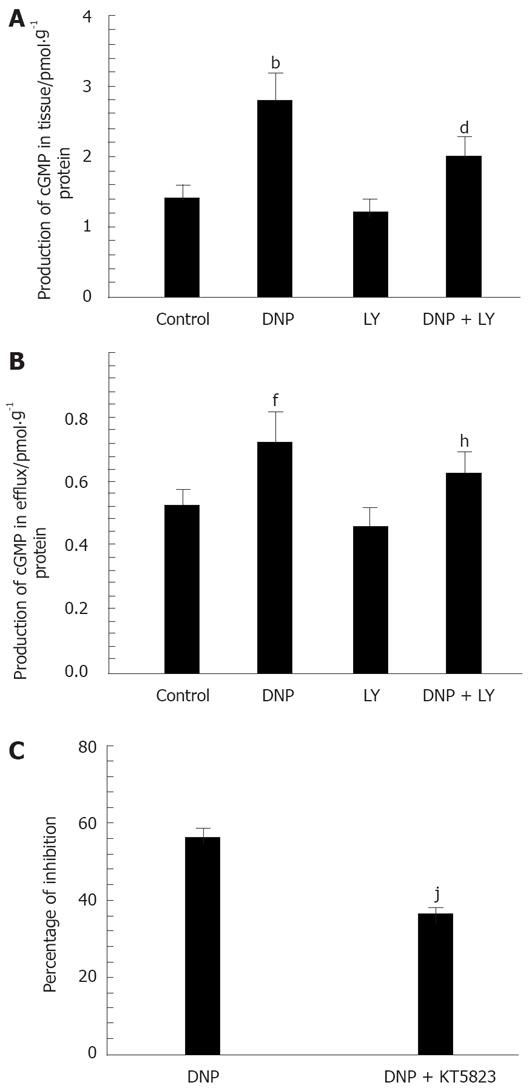
Our previous pharmacological study[15] suggests that DNP obviously inhibits spontaneous contraction in gastric antral circular smooth muscle through the cGMP-dependent signaling pathway. To directly confirm the involvement of cGMP on the effect of DNP, in the present study, we measured the content of cGMP in the smooth muscle tissue and in the perfusion solution using radioimmunoassay. The result indicated that cGMP in the smooth muscle tissue and perfusion solution was markedly increased after addition of 10 nmol/L DNP (Figure 1A and B). Pretreatment with 10 nmol/L LY83583 significantly diminished DNP-induced increase in the content of cGMP (Figure 1A and B).
Because cGMP activates PKG, we tested the effect of KT-5823 (1 μmol/L), a membrane-permeable PKG-specific inhibitor, on DNP-induced relaxation in the gastric antral circular smooth muscle to determine the potential involvement of PKG. The result indicated that KT-5823 could markedly diminish, although not completely abolish the inhibitory effect of DNP on spontaneous contraction (Figure 1C).
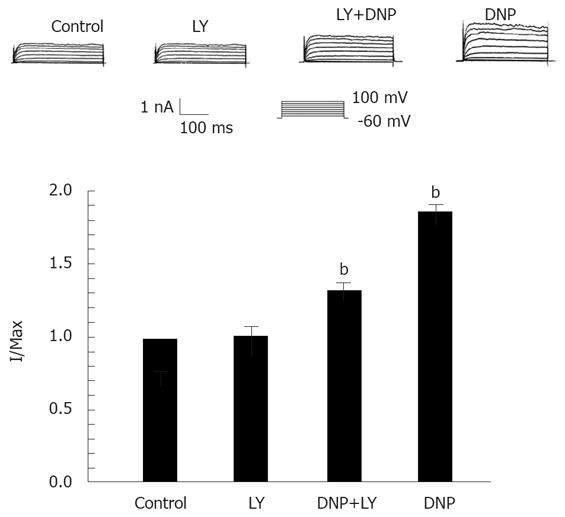
Considering our previous finding that DNP relaxed smooth muscle by increasing IK(ca)[15], here we further investigated the relationship between cGMP and DNP-induced increase of IK(ca), and found that the effect of DNP on IK(ca) was observed in the presence of LY83583, an inhibitor of guanylate cyclase to change the production of cGMP. LY83583 (10 nmol/L) significantly blocked DNP-induced increase of IK(ca). The percentage of DNP-induced increase was diminished from 63.24% ± 4.32% to 28.53% ± 3.31% at 60 mV (Figure 2).
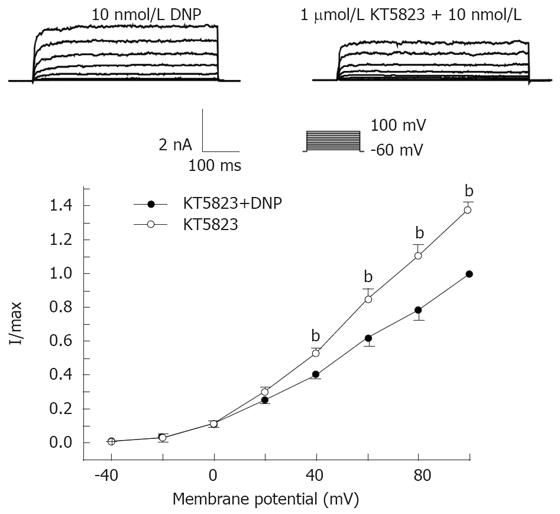
To extend our understanding of the role of DNP in the regulation of IK(ca) through the cGMP/PKG pathway, the effect of KT-5823 (a membrane-permeable PKG-specific inhibitor) on channel activity was tested. As the results show in Figure 3, the addition of KT-5823 (1 μmol/L) completely inhibited DNP-induced increase of IK(ca). This data suggests the involvement of PKG-mediated phosphorylation in DNP-mediated regulation of IK(ca).
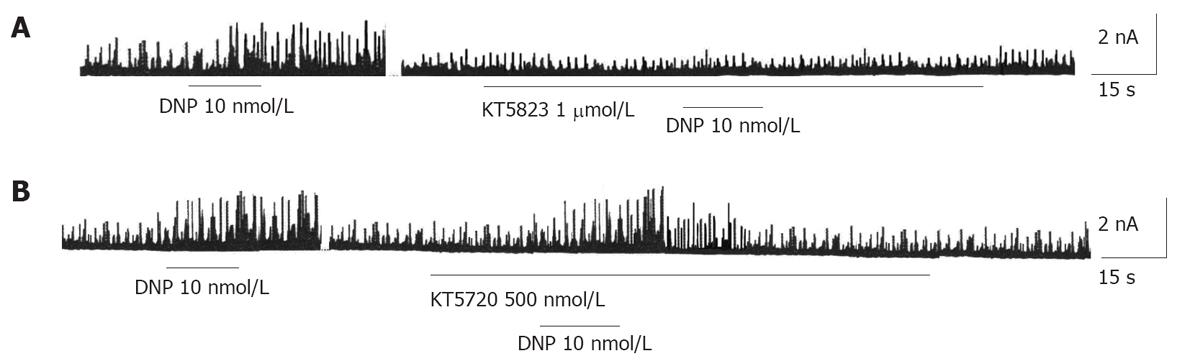
STOCs, which can be activated by extracellular Ca2+ influx and intracellular Ca2+ release, were recorded at -20 mV. The currents were sensitive to TEA (a nonselective K+ channel blocker) and CHTX (a selective Ca2+-activated K+ channel blocker). As described in our previous study, DNP increased STOCs in gastric circular myocytes. To further investigate the relationship between cGMP/PKG pathway and DNP-induced increase in STOCs, we examined the effect of KT5823 on DNP-induced increase in STOCs. The result indicated that KT5823 almost completely abolished DNP-induced increase of STOCs (Figure 4A). However, KT-5720 (500 nmol/L), a PKA-specific inhibitor, could not suppress DNP-induced increase in STOCs (Figure 4B).
In the present study, the patch clamp technique, radioim-munoassay and specific pharmacological inhibitors were used to determine involvement of the pGC-cGMP-PKG pathway in DNP-mediated relaxation in guinea-pig gastric antral circular smooth muscle.
In this study, we found that cGMP in the smooth muscle tissue and perfusion solution both were markedly increased after the addition of DNP. The effect of DNP was diminished after treatment with LY83583, an inhibitor of guanylate cyclase to change the production of cGMP. These data indicate that DNP may inhibit spontaneous contraction by increasing cGMP levels. Consistent with this view, KT5823, a PKG inhibitor, markedly diminished the inhibitory effect of DNP on spontaneous contraction. In an attempt to understand how DNP relaxes smooth muscle by affecting the cGMP-dependant pathway, patch clamp experiments were carried out. We observed that KT5823 inhibited DNP-induced increase of IK(ca), and almost completely abolished the DNP-induced increase of STOCs. However, KT-5720 (500 nmol/L), a PKA-specific inhibitor, had no effect on DNP-induced increase in STOCs.
NPs, similar to NO, can increase the generation of cGMP and cAMP, and play important physiological functions in a variety of cell types. In smooth muscle cells, NPs exhibited an inhibitory effect on motility via the cGMP pathway. For example, ANP increases intracellular cGMP levels and mediates the role of endothelium- and cardiac-derived NO in regulating sympathetic control functions of the heart and the microvasculature in conscious rats by affecting cGMP-dependent release of catecholamines[16]. Additionally, Kedia et al[17] observed that CNP is involved in the cGMP-dependent control of the normal function of human prostatic smooth muscle. Our previous study also found that CNP inhibited spontaneous contraction by increasing cGMP in gastric antral circular smooth muscle[18]. All these previous reports are consistent with our current findings that DNP-induced relaxation is related to cGMP in gastric circular smooth muscle.
It has been reported that intracellular cGMP may not only result in activation of PKG, but also inhibits activity of PDE3[19]. The latter action would lead to an increase in cAMP, and hence may stimulate another cyclic nucleotide-dependent protein kinase, PKA. NPs exert some physiological functions by affecting PKA. Birukova et al[20] have found that Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. However, our present study indicates that DNP-induced increase of IK(ca) and STOCs were significantly blocked by LY83583 and KT-5823. DNP stimulated STOCs even in the presence of a PKA-specific inhibitor (KT-5720), suggesting that the DNP-induced increase of STOCs was due to stimulation of PKG, rather than PKA. The direct effect of cGMP on the activity of ion channels has been reported previously. Yao et al[21] showed that a cGMP-gated K+ channel is expressed in the kidney. Nakamura et al[22] revealed that protein kinase G activates inwardly rectifying K+ channels in cultured human proximal tubule cells. Hirsch et al[23] demonstrated the existence of cGMP-regulated K+ channels that were inhibited by cGMP without PKG-mediated phosphorylation. In our current study, however, DNP-induced relaxation in gastric antral myocytes was inhibited by KT5823. This indicates that PKG-mediated phosphorylation participates in DNP-induced relaxation. Consistent with our data, a previous report has shown that CNP can inhibit L-type Ca2+ channel currents, and the inhibitory effect is mediated by pGC-cGMP-PKG-dependent signal pathway in gastric antral myocytes of guinea pigs[24]. However, it should be pointed out that the results of our current study can not determine whether a direct or indirect activation of Ca2+-activated K+ channels by PKG participates in DNP-induced relaxation of in gastric antral smooth muscle cells. As such, further experiments are necessary to decode this intriguing question.
Taken together, it can be concluded that DNP relaxes gastric circular smooth muscle by activating Ca2+-activated K+ channels, mediated by a pGC-cGMP-PKG-dependent signal pathway. cAMP did not participate in the process.
Dendroaspis natriuretic peptide (DNP) is a recently isolated peptide that contains 38 amino residues and shares structural and functional properties with the other members of the natriuretic peptide (NP) family. Studies about its physiologic functions mainly focus on cardiovascular, nervous, and urinary systems. In a previous study, these authors found that DNP inhibited spontaneous contraction in gastric circular smooth muscle. NP plays important physiological functions by affecting the activity of cGMP and cAMP. However, it is unclear whether cGMP or cAMP participates in regulating DNP-induced inhibition of gastric motility.
Studies about the physiologic functions of DNP mainly focus on cardiovascular, nervous, and urinary systems. There are few reports about the relationship between DNP and gastrointestinal functions. Studies have demonstrated that the DNP system is present in the rat colon and regulates colonic motility as a local regulator. The relationship between DNP and gastrointestinal function has become a focus of study. A previous study has indicated that DNP inhibits gastric motility, which is the first report about DNP regulating gastric motility. However, the mechanism on how DNP regulates gastric motility is still unclear and is the focus of the author’s study.
The author’s of this paper have shown that, for the fist time, DNP activates IK(Ca) and relaxes guinea-pig gastric antral circular smooth muscle via the cGMP/PKG-dependent signaling axis, instead of the cAMP/PKA pathway. The combined use of pharmacologic, radioimmunoassay and patch-clamp techniques can sufficiently demonstrate the mechanism involved in DNP regulation of gastric motility.
This work enhanced the understanding of the mechanism on how DNP regulates gastric motility.
This is a very interesting study. The authors demonstrated that DNP activates IK(Ca) and relaxes guinea-pig gastric antral circular smooth muscle via the cGMP/PKG-dependent signaling axis instead of the cAMP/PKA pathway. This study is well designed, and the analysis is reasonable.
Peer reviewers: Leonard R Johnson, Professor, Department of Physiology, University Tennessee College of Medicine, 894 Union Ave, Memphis, TN 38163, United States; Per M Hellström, MD, Professor, Gastrocentre Medicine, Karolinska University Hospital Solna, Stockholm SE-17176, Sweden
S- Editor Li DL L- Editor Rippe RA E- Editor Yin DH
| 2. | Lisy O, Jougasaki M, Heublein DM, Schirger JA, Chen HH, Wennberg PW, Burnett JC. Renal actions of synthetic dendroaspis natriuretic peptide. Kidney Int. 1999;56:502-508. |
| 3. | Singh G, Maguire JJ, Kuc RE, Skepper JN, Fidock M, Davenport AP. Characterization of the snake venom ligand [125I]-DNP binding to natriuretic peptide receptor-A in human artery and potent DNP mediated vasodilatation. Br J Pharmacol. 2006;149:838-844. |
| 4. | Singh G, Kuc RE, Maguire JJ, Fidock M, Davenport AP. Novel snake venom ligand dendroaspis natriuretic peptide is selective for natriuretic peptide receptor-A in human heart: downregulation of natriuretic peptide receptor-A in heart failure. Circ Res. 2006;99:183-190. |
| 5. | Ha KC, Piao CS, Chae HJ, Kim HR, Chae SW. Dendroaspis natriuretic peptide protects the post-ischemic myocardial injury. Regul Pept. 2006;133:13-19. |
| 6. | Piao FL, Park SH, Han JH, Cao C, Kim SZ, Kim SH. Dendroaspis natriuretic peptide and its functions in pig ovarian granulosa cells. Regul Pept. 2004;118:193-198. |
| 7. | Lee S, Park SK, Kang KP, Kang SK, Kim SZ, Kim W. Relationship of plasma Dendroaspis natriuretic peptide-like immunoreactivity and echocardiographic parameters in chronic haemodialysis patients. Nephrology (Carlton). 2004;9:171-175. |
| 8. | Kim JH, Yang SH, Yu MY, Lee HK, Kim SY, Kim SH. Dendroaspis natriuretic peptide system and its paracrine function in rat colon. Regul Pept. 2004;120:93-98. |
| 9. | Guo HS, Cai ZX, Wu TH, Xu J, Qiu Y, Xu WX. Inhibitory effect of dendroaspis natriuretic peptide on spontaneous contraction in gastric antral circular smooth muscles of guinea pigs. Acta Pharmacol Sin. 2007;28:1797-1802. |
| 10. | Sabbatini ME, Rodríguez M, di Carlo MB, Davio CA, Vatta MS, Bianciotti LG. C-type natriuretic peptide enhances amylase release through NPR-C receptors in the exocrine pancreas. Am J Physiol Gastrointest Liver Physiol. 2007;293:G987-G994. |
| 11. | Borán MS, Baltrons MA, García A. The ANP-cGMP-protein kinase G pathway induces a phagocytic phenotype but decreases inflammatory gene expression in microglial cells. Glia. 2008;56:394-411. |
| 12. | Moro C, Klimcakova E, Lafontan M, Berlan M, Galitzky J. Phosphodiesterase-5A and neutral endopeptidase activities in human adipocytes do not control atrial natriuretic peptide-mediated lipolysis. Br J Pharmacol. 2007;152:1102-1110. |
| 13. | Wen JF, Quan HX, Zhou GH, Cho KW. Altered role of C-type natriuretic peptide-activated pGC-cGMP-PDE3-cAMP signaling in hyperthyroid beating rabbit atria. Regul Pept. 2007;142:123-130. |
| 14. | Cui X, Lee SJ, Kim SZ, Kim SH, Cho KW. Effects of pituitary adenylate cyclase activating polypeptide27 on cyclic AMP efflux and atrial dynamics in perfused beating atria. Eur J Pharmacol. 2000;402:129-137. |
| 15. | Guo HS, Yang YZ, Zou Y, Xu J, Cai ZX, Qi QH. Effects of dendroaspis natriuretic peptide on calcium-activated potassium current and its mechanism. J Physiol Sci. 2008;58:1-6. |
| 16. | Whalen EJ, Saurer TB, Johnson AK, Lewis SJ. Intracellular cGMP may promote Ca2+-dependent and Ca2+-independent release of catecholamines from sympathetic nerve terminals. Vascul Pharmacol. 2006;45:102-111. |
| 17. | Kedia G, Uckert S, Scheller F, Chigogidze T, Managadze L, Jonas U, Truss MC. In vitro functional responses of isolated normal human prostatic tissue to compounds interacting with the cyclic guanosine monophosphate pathway. Urology. 2006;67:1292-1297. |
| 18. | Guo HS, Cui X, Cui YG, Kim SZ, Cho KW, Li ZL, Xu WX. Inhibitory effect of C-type natriuretic peptide on spontaneous contraction in gastric antral circular smooth muscle of rat. Acta Pharmacol Sin. 2003;24:1021-1026. |
| 19. | Degerman E, Belfrage P, Manganiello VC. Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J Biol Chem. 1997;272:6823-6826. |
| 20. | Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008;215:715-724. |
| 21. | Yao X, Segal AS, Welling P, Zhang X, McNicholas CM, Engel D, Boulpaep EL, Desir GV. Primary structure and functional expression of a cGMP-gated potassium channel. Proc Natl Acad Sci USA. 1995;92:11711-11715. |
| 22. | Nakamura K, Hirano J, Itazawa S, Kubokawa M. Protein kinase G activates inwardly rectifying K(+) channel in cultured human proximal tubule cells. Am J Physiol Renal Physiol. 2002;283:F784-F791. |
| 23. | Hirsch JR, Weber G, Kleta I, Schlatter E. A novel cGMP-regulated K+ channel in immortalized human kidney epitheliall cells (IHKE-1). J Physiol. 1999;519 Pt 3:645-655. |
| 24. | Sun JB, Huang X, Xu HY, Li XL, Gao L, Kim YC, Xu WX. Inhibitory effect of C-type natriuretic peptide on L-type calcium channel currents in gastric antral myocytes of guinea pigs. Gen Physiol Biophys. 2006;25:365-377. |