Published online Sep 14, 2008. doi: 10.3748/wjg.14.5331
Revised: July 27, 2008
Accepted: August 3, 2008
Published online: September 14, 2008
AIM: To identify the characteristics of morphology, location and collateral circulation involved in paraesophageal varices (para-EV) of portal hypertension patients with 64-row multidetector computed tomography (MDCT).
METHODS: Fifty-two of 501 patients with portal hypertensive cirrhosis accompanied with esophageal varices were selected for 64-row MDCT examination after the observation of para-EV. The CT protocol included unenhanced, arterial and portal phases with a slice thickness of 0.625 mm and a scanning field of 2 cm above the bifurcation to the lower edge of kidney. The CT portal venography (CTPV) was reformatted on AW4.3 workstation. The characteristics of origination, location, morphology and collateral circulation in para-EV were observed.
RESULTS: Among the 52 cases of para-EV, 50 showed the originations from the posterior branch of left gastric vein, while the others from the anterior branch. Fifty cases demonstrated their locations close to the esophageal-gastric junction, and the other two cases were extended to the inferior bifurcation of the trachea. The circuitous pattern was observed in 16 cases, while reticulated pattern was seen in 36 cases. Collateral circulation identified 4 cases of single periesophageal varices (peri-EV) communication, 3 cases of single hemiazygous vein, one case of single inferior vena cava, 41 cases of mixed type (collateral communications of at least 2 of above mentioned types) and 3 cases of undetermined communications. Among all the cases, 43 patients showed the communications between para-EV and peri-EV, while hemiazygous vein (43 cases) and inferior vena cava (5 cases) were also involved.
CONCLUSION: Sixty-four-row multidetector computed tomography portal venography could display the location, morphology, origin, and collateral types of para-EV, which provides important and referable information for clinical management and disease prognosis.
- Citation: Zhao LQ, He W, Chen G. Characteristics of paraesophageal varices: A study with 64-row multidetector computed tomography portal venography. World J Gastroenterol 2008; 14(34): 5331-5335
- URL: https://www.wjgnet.com/1007-9327/full/v14/i34/5331.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5331
| Drainage vein | Shape | Cases | Total | |
| Single | Peri-EV | Reti | 4 | 8 |
| Hemi | Cir | 3 | ||
| Sub | Cir | 1 | ||
| Peri-EV + Hemi | Cir | 8 | 41 | |
| Peri-EV + Hemi | Reti | 29 | ||
| Mixed | Sub + Peri-EV | Cir | 1 | |
| Sub + Hemi | Cir | 2 | ||
| Sub + Peri-EV + Hemi | Reti | 1 | ||
| Uncertain | Cir | 1 | 3 | |
| Reti | 2 | |||
| Total | 52 |
Esophageal varice is a common collateral circulation manifestation in portal hypertension, and may cause a severe complication if ruptured[1-3]. Endoscopic therapy is an effective approach that is being applied worldwide[4,5], although the recurrences have been reported in many cases[6]. Some authors reported that the existence of perforating veins, the communicating branches between periesophageal varices (peri-EV) and paraesophageal varices (para-EV), and their blood flow found by endoscopic color Doppler ultrasonography (ECDUS) affected the recurrences of hemorrhage caused by rupturing[7]. However, ECDUS is an invasive technique, requires specialized equipment and technology, and is incapable of demonstrating the drainage types and morphologic features of esophageal varices. Multidetector computed tomography portal venography (MDCTPV) has been used widely in collateral circulation studies of esophageal and gastric varices[8-11]. The drainage veins of esophageal varices can be clearly displayed on MDCTPV[10]. Our study focuses on the utilization of MDCTPV in observing para-EV drainage types to provide referable information for clinical management selection and prognosis evaluation.
A total of 501 portal hypertensive cirrhotic patients with esophageal varices were investigated from April 2007 to May 2008. Among them, 52 patients with presence of para-EV were selected for this study, including 35 male and 17 female, aged 21-71 years, averaging 45.2 years. There were 27 cases of hepatitis B, 3 hepatitis C, 16 alcoholic, 2 primary biliary and 4 cases of cryptogenic cirrhosis. Based on Child’s grading, 9 cases were Grade A, 28 cases Grade B and 15 cases of Grade C. Among the 52 patients, 50 were accompanied with gastric varices. Fifty patients were not treated with esophageal varice ligation (EVL), esophageal varice sclerotherapy (EVS) or any other endoscopic therapies, while 1 case received post-EVL therapy and 1 case received post-EVS therapy. Liver cirrhosis was confirmed in all patients through general consideration from clinical histories, laboratory findings, as well as CT, sonography or MRI examinations. Informed consent for CT scans and the use of contrast media were obtained from all patients before scanning procedures.
A GE VCT 64-row MDCT scanner was applied to perform unenhanced, arterial and portal vein phase enhanced scans on all patients. The scanning range covers from 2 cm above the tracheal bifurcation to the lower edge of the kidney. One hundred mL of non-ionic contrast medium (Omnipaque 350) was introduced with an infusion rate of 4.0 mL/s. The arterial phase scanning time delay was determined with Smart technology, and portal phase scanning was initiated at 25 s after the beginning of arterial phase. Slice thickness was set at 5.0 mm, and reconstitution thickness was 0.625 mm.
Post-processing methods: vertebrae, costal bones and other structures with bony densities were removed using GE AW4.3 workstation, and then maximum intensity projection (MIP), multi-planner reformation (MPR) technique were applied to reveal the origin, location, morphology and communicating veins of para-EV. The 3D structures of esophageal varices were displayed with volume rendering (VR) technique.
Origin of para-EV: The relationship between para-EV and anterior or posterior branches of the left gastric vein were examined and determined.
Morphology and location of para-EV: The morpho-logical patterns of para-EV were categorized into circuitous pattern and reticulated pattern, in which circuitous pattern was denoted as para-EV linear, near-parallel pathways, while reticulated pattern was denoted as prominent para-EV winding and distortion, forming meshwork or loops. In case of the presence of both circuitous and reticulated patterns, the categorization was determined according to their diameter ratios and then was defined as “mainly circuitous pattern” or “mainly reticulated pattern”. Large-diameter vessels with a ratio of over 1 were defined as dominant type. The para-EV was divided into 3 sections according to the extent, including the upper section located at a level superior to the tracheal bifurcation; the middle section located at or near the tracheal bifurcation level, and the lower section located at the abdominal as well as the lower thoracic segment of the esophageal periphery.
Communicating veins of para-EV: The communica-tions between para-EV and peri-EV, hemiazygous vein, subphrenic vein, inferior vena cava and other vessels were investigated. The criteria set for CTPV judgment on the communication of para-EV and its collaterals were as follows: (1) Para-EV was directly conjoint with the above-mentioned vessels; (2) Para-EV and the vessels mentioned above were conjoint with their peripheral circuitous and reticulated blood vessels, respectively.
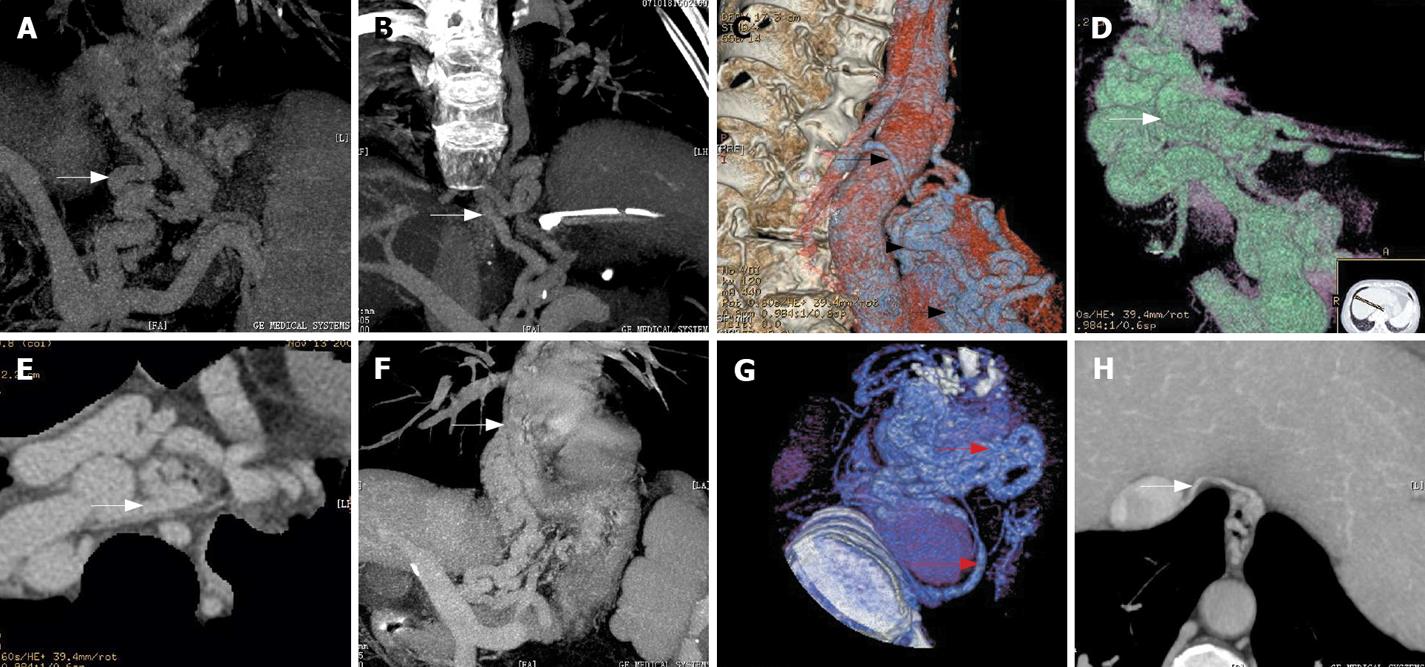
There were 50 patients with their varices originating from the posterior branch of the left gastric vein (50/52, 96.15%) (Figure 1A) while the varices of the other 2 patients originated from the anterior branch of the left gastric vein (2/52, 3.85%), in which the posterior branch of the left gastric vein was absent and the anterior branch of left gastric vein routes up and enters the fundus through the gastroesophageal junction to form gastric fundal varices. The latter routes up to form peri-EV, then penetrate through esophageal wall to form para-EV.
Sixteen cases of circuitous morphological pattern (16/52, 36.54%) (Figure 1B) and 36 cases of reticulated morphological pattern (36/52, 63.46%) (Figure 1C) were observed. The locations of para-EV were identified as follows: 50 cases in lower section (50/52, 96.15%) (Figure 1C), 2 cases in middle section (2/52, 3.85%), and none in upper section.
Among the 52 cases, 3 showed undetermined communicating vessels (3/52, 5.77%) with 1 of circuitous morphological pattern, while the others were of reticulated morphological pattern. In the other 49 cases, 43 cases involved the peri-EV (43/49, 87.76%) (Figure 1D-G), and a similar number of patients also demonstrated the hemiazygous vein (Figure 1B, C and G). Inferior vena cava was included in 5 cases (5/49, 10.20%) (Figure 1H).
The specific communicating patterns found in this study were listed as follows (Table 1): 8 cases with single communication, 41 with mixed communications and, in 3 cases, the drainage veins of the para-EV remained uncertain. Among the 8 cases of single communication, the para-EV connecting to peri-EV (Figure 1E and F) with reticulated shape was found in 4 cases (4/49, 8.16%), connecting to Hemiazygos vein of circuitous appearance in 3 cases (3/49, 5.77%), and to the subphrenic vein draining circuitously in 1 case (1/49, 1.92%).
In the 41 cases of mixed type, the para-EV was found connecting to peri-EV and hemiazygous vein in 37 cases (37/49, 71.15%) (Figure 1G), with circuitous shape in 8 cases and reticulated in 29 cases. Three cases presented para-EV communicating to subphrenic vein: 1 to peri-EV (1/49, 1.92%) and 2 to Hemiazygos vein circuitously (2/49, 3.84%). In 1 case (1/49, 1.92%), all the drainage mentioned above was observed with reticulated appearance. The para-EV was circuitous in 1 case and reticular in 2 cases of uncertain drainage.
The prevalence rate of esophageal varices is high in patients with liver cirrhosis, and the mortality rate is very high imposed by massive hemorrhage due to rupture[2,12]. It is reported that more than 70% of portal hypertension patients with a variceal bleeding history may suffer from recurrent bleeding[13]. The esophageal varice was divided into three groups: peri-EV, para-EV and perforating veins[14]. The factors associated with the rupture of esophageal varices included the extent and collateral circulation of peri-EV and para-EV[15,16]. Therefore, the acquisition of reliable images for the above mentioned factors will provide valuable information for further treatment implementation and prognosis of the patients.
Angiography is one of the earliest investigations applied in hemodynamic evaluation of portal hypertension-induced esophageal varices by displaying esophageal varices and their drainage vessels through arterial portography[17]. However, the invasive procedure and the incapability to differentiate peri-EV and para-EV prevent it to be applied in a general clinical setting.
Color Doppler ultrasound has been widely used to investigate the relationship between EV and hemodynamics associated with portal hypertension and liver cirrhosis. But it could not show the para-EV and peri-EV clearly[18].
Endoscopy is a more popular approach for the observation of morphological patterns, extension and severity of esophageal submucotic varices with the limitations to intramural and para-EV[19].
ECDUS is gradually becoming a useful tool for esophageal varicose inspection, which is able to display peri-EV, para-EV, perforating veins and the blood flow directions of perforating veins[7,14,20]. However, it is unable to clearly display the pathway and collaterals of para-EV. On the other hand, endoscopic sonography is costly, and also an invasive procedure that requires a skillful operator, which limited its application in clinical settings.
MDCTPV has already been a matured investigation technique used to examine esophageal and gastric varices. It is competent in displaying the types of morphological pattern, origin and collateral circulations of esophageal and gastric varices[10,11]. Sixty-four-row MDCT consists of thin slice of 0.625 mm, and a high spatial resolution, which makes it capable of displaying the communicating patterns of para-EV and peri-EV. Among the 52 cases in our study, this pattern of collateral circulation was shown in 49 (94.23%) cases. Generally, it is a non-invasive technique, and hence, it can be used to evaluate para-EV conveniently. However, displaying of this sort of collaterals is relatively rough and unsatisfactory in detail if compared with EUS. In addition, it is incapable to assess the blood flow directions of paraesophageal-periesophageal collateral circulation due to the characters of CTPV.
According to our study, vast majority of para-EV originate from the posterior branch of left gastric vein, and only 2 cases originated from the anterior branch of left gastric vein. The latter is highly associated with gastric fundal varices. A previous report suggested that[21], in cases of coexisting gastric fundal varices and esophageal varices in lower esophageal segment, the extent of esophageal varices could be aggravated after imposing obliteration therapy for gastric fundal varices. In our study, there was one patient who experienced alleviation of gastric varices with aggravated para-EV and peri-EV after receiving gastroesophageal varices treatment. The origination of para-EV may be related directly to its anatomical formation.
In our study, para-EV was found more abundant near the superior part of the gastroesophageal junction, which is also the lower esophageal segment (96.15%) than in the middle segment (3.85%). There was no para-EV involved in the upper segment. This suggests that para-EV usually occurs in the middle and lower esophageal segment and drains into the vena cava system through collateral vessels. The involved drainage vessels that we have noted were peri-EV, hemiazygous veins and subphrenic veins.
The communicating branches of para-EV and hemiazygous vein manifest as drainage “detour” veins bypassing along the anterior descending aorta to the hemiazygous vein, and subsequently drain into the superior vena cava. Ibukuro et al[22] described the above anatomic changes as preaortic vein using CT and CTAP application. This “detour” is a commonly seen drainage route, and has an occurrence rate of 87.76%.
The hemodynamics of para-EV communicating peri-EV is relatively complicated. The communicating vessels of para-EV and peri-EV are mostly situated close to the top of the cardia level. Sato et al[7] observed the presence of perforating veins between peri-EV and para-EV with Levovist ECDUS, and meanwhile, he found the bidirectional blood flow of peri-EV and para-EV, which included the directions from para-EV to peri-EV and from peri-EV to para-EV, as well as mixed type that presented both blood flow directions. This may explain why previous para-EV studies about the recurrence risk of post-esophageal varices treatment remain controversial[20,23,24].
Para-EV can still drain into the inferior vena cava through the subphrenic vein, or directly communicate with the inferior vena cava. The subphrenic veins are the bilateral vessels which end up at inferior vena cava at the diaphragm level[25]. The communication between para-EV and subphrenic veins shown on CTPV was consistent to the precaval route and morphological pattern as reported by Ibukuro et al[25] from their CTAP and autopsy findings.
The degree of varices will be more severe if the morphological patterns of para-EV are reticulated. Under such condition, para-EV and peri-EV are individually communicated, or may be accompanied with hemiazygous vein drainage. They will mostly drain into the hemiazygous veins or subphrenic veins if the collaterals of para-EV are circuitous. Explanations about this correlation and its pathological significance require further studies.
Even though CTPV is incapable of displaying the blood flow directions of para-EV, it has a certain guidance value for EUS application. If para-EV is detected on CTPV, EUS should be further implemented to confirm the presence of specific communicating branches between peri-EV and para-EV as well as the location and hemodynamic characteristics of their communicating branches so as to provide relevant referable information for treatment selections.
The existence of para-EV and perforating veins and the direction of their blood flow can affect the recurrence of hemorrhage caused by rupturing.
The morphology, location and collateral circulation characteristics of para-EV in portal hypertensive patients were studied with 64-row multidetector computer tomography portal venography (MDCTPV), which is a non-invasive method.
In majority cases of this study, the collateral circulation pattern of para-EV and the morphological characteristics of para-EV were revealed thanks to the high spatial resolution images of the advanced MDCT and the appropriate image post-processing.
MDCTPV is a noninvasive method to display the morphological characteristics of para-EV, which is useful in clinical management and disease prognosis.
Paraesophageal varices are the varices that exist outside the esophagus. Peri-EV is the varices that exist in the esophageal wall or the submucotic varices. Perforating vein is the communicating branch between peri-EV and para-EV. The subphrenic vein is the bilateral vessel that ends up at inferior vena cava at the diaphragm level. CTPV is the abbreviation of CT portal venography.
This is a good review with excellent images. MDCTPV is a convenient method to display the morphological and collateral circulations of the para-EV. It is of important value in the selection of clinical therapy and evaluation of prognosis.
Peer reviewers: Juan G Abraldes, MD, Hepatic Hemodynamic Laboratory, Liver Unit, Hospital Clinic, University of Barcelona, Villarroel 170, Barcelona 08036, Spain; Aydin Karabacakoglu, PhD, Assistant Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Li DL L- Editor Ma JY E- Editor Yin DH
| 1. | Bhasin DK, Malhi NJ. Variceal bleeding and portal hypertension: much to learn, much to explore. Endoscopy. 2002;34:119-128. |
| 3. | Brandenburger LA, Regenstein FG. Variceal Hemorrhage. Curr Treat Options Gastroenterol. 2002;5:73-80. |
| 4. | Gotoh Y, Iwakiri R, Sakata Y, Koyama T, Noda T, Matsunaga C, Ogata SI, Ishibashi S, Sakata H, Tsunada S. Evaluation of endoscopic variceal ligation in prophylactic therapy for bleeding of oesophageal varices: a prospective, controlled trial compared with endoscopic injection sclerotherapy. J Gastroenterol Hepatol. 1999;14:241-244. |
| 5. | Mizumoto H, Matsutani S, Fukuzawa T, Ishii H, Sato G, Maruyama H, Saisho H. Hemodynamics in the left gastric vein after endoscopic ligation of esophageal varices combined with sclerotherapy. J Gastroenterol Hepatol. 2001;16:495-500. |
| 6. | Grace ND, Groszmann RJ, Garcia-Tsao G, Burroughs AK, Pagliaro L, Makuch RW, Bosch J, Stiegmann GV, Henderson JM, de Franchis R. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology. 1998;28:868-880. |
| 7. | Sato T, Yamazaki K, Toyota J, Karino Y, Ohmura T, Akaike J, Kuwata Y, Suga T. Perforating veins in recurrent esophageal varices evaluated by endoscopic color Doppler ultrasonography with a galactose-based contrast agent. J Gastroenterol. 2004;39:422-428. |
| 8. | Kang HK, Jeong YY, Choi JH, Choi S, Chung TW, Seo JJ, Kim JK, Yoon W, Park JG. Three-dimensional multi-detector row CT portal venography in the evaluation of portosystemic collateral vessels in liver cirrhosis. Radiographics. 2002;22:1053-1061. |
| 9. | Agarwal A, Jain M. Multidetector CT portal venography in evaluation of portosystemic collateral vessels. J Med Imaging Radiat Oncol. 2008;52:4-9. |
| 10. | Zhang LQ, He W. CT portal venography of collateral veins in esophageal varices. Zhongguo Yixue Yingxiang Jishu. 2007;23:242-245. |
| 11. | Zhao LQ, He W, Zhao H, Yu YZ. The value of CT portalvenography in the diagnosis of collateral veins in patients with gastric varices. Zhonghua Fangshexue Zazhi. 2006;40:1175-1178. |
| 12. | Seewald S, Seitz U, Yang AM, Soehendra N. Variceal bleeding and portal hypertension: still a therapeutic challenge? Endoscopy. 2001;33:126-139. |
| 13. | Grace ND, Groszmann RJ, Garcia-Tsao G, Burroughs AK, Pagliaro L, Makuch RW, Bosch J, Stiegmann GV, Henderson JM, de Franchis R. Portal hypertension and variceal bleeding: an AASLD single topic symposium. Hepatology. 1998;28:868-880. |
| 14. | Irisawa A, Shibukawa G, Obara K, Saito A, Takagi T, Shishido H, Odajima H, Abe M, Sugino T, Suzuki T. Collateral vessels around the esophageal wall in patients with portal hypertension: comparison of EUS imaging and microscopic findings at autopsy. Gastrointest Endosc. 2002;56:249-253. |
| 15. | Irisawa A, Obara K, Sato Y, Saito A, Takiguchi F, Shishido H, Sakamoto H, Kasukawa R. EUS analysis of collateral veins inside and outside the esophageal wall in portal hypertension. Gastrointest Endosc. 1999;50:374-380. |
| 16. | Irisawa A, Saito A, Obara K, Shibukawa G, Takagi T, Yamamoto G, Sakamoto H, Takiguchi F, Shishido H, Hikichi T. Usefulness of endoscopic ultrasonographic analysis of variceal hemodynamics for the treatment of esophageal varices. Fukushima J Med Sci. 2001;47:39-50. |
| 17. | Watanabe K, Kimura K, Matsutani S, Ohto M, Okuda K. Portal hemodynamics in patients with gastric varices. A study in 230 patients with esophageal and/or gastric varices using portal vein catheterization. Gastroenterology. 1988;95:434-440. |
| 18. | Li FH, Hao J, Xia JG, Li HL, Fang H. Hemodynamic analysis of esophageal varices in patients with liver cirrhosis using color Doppler ultrasound. World J Gastroenterol. 2005;11:4560-4565. |
| 19. | Chikamori F, Kuniyoshi N, Shibuya S, Takase Y. Correlation between endoscopic and angiographic findings in patients with esophageal and isolated gastric varices. Dig Surg. 2001;18:176-181. |
| 20. | Choudhuri G, Dhiman RK, Agarwal DK. Endosonographic evaluation of the venous anatomy around the gastro-esophageal junction in patients with portal hypertension. Hepatogastroenterology. 1996;43:1250-1255. |
| 21. | Cho SK, Shin SW, Lee IH, Do YS, Choo SW, Park KB, Yoo BC. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol. 2007;189:W365-W372. |
| 22. | Ibukuro K, Tsukiyama T, Mori K, Inoue Y. Preaortic esophageal veins: CT appearance. AJR Am J Roentgenol. 1998;170:1535-1538. |
| 23. | Dhiman RK, Choudhuri G, Saraswat VA, Agarwal DK, Naik SR. Role of paraoesophageal collaterals and perforating veins on outcome of endoscopic sclerotherapy for oesophageal varices: an endosonographic study. Gut. 1996;38:759-764. |
| 24. | Hino S, Kakutani H, Ikeda K, Uchiyama Y, Sumiyama K, Kuramochi A, Kitamura Y, Matsuda K, Arakawa H, Kawamura M. Hemodynamic assessment of the left gastric vein in patients with esophageal varices with color Doppler EUS: factors affecting development of esophageal varices. Gastrointest Endosc. 2002;55:512-517. |
| 25. | Ibukuro K, Tsukiyama T, Mori K, Inoue Y. Precaval draining vein from paraesophageal varices: radiologic-anatomic correlation. AJR Am J Roentgenol. 1999;172:651-654. |