Published online Sep 7, 2008. doi: 10.3748/wjg.14.5186
Revised: August 13, 2008
Accepted: August 20, 2008
Published online: September 7, 2008
AIM: To investigate the role of phosphatidylinositol 3-kinase (PI 3-K)/Akt signaling pathway in the balance of HSC activation and apoptosis in rat hepatic stellate cells (HSC).
METHODS: An activated HSC cell line was used in this study. LY 294002, the PI 3-K/Akt signal pathway blocker was used to investigate the molecular events on apoptosis in HSC and to interpret the role of this pathway in HSC apoptosis. Immunocytochemistry, Western blot and reverse transcription polymerase chain reaction (RT-PCR) analysis were applied to detect the expression of PI 3-K, and simultaneously phosphorylated-Akt (p-Akt) and total-Akt were determined by Western blot. The HSC apoptosis was examined by annexin-V/propidium iodide double-labelled flow cytometry and transmission electron microscopy.
RESULTS: The apoptosis rates in LY 294002 (30.82% ± 2.90%) and LY 294002 + PDGF-BB (28.16% ± 2.58%) groups were significantly increased compared with those of control (9.02% ± 1.81%) and PDGF-BB (4.35% ± 1.18%). PDGF-BB augmented PI 3-K and p-Akt expression. LY 294002 significantly reduced the contents of PI 3-K and p-Akt. mRNA transcription evaluated by RT-PCR showed similar tendencies as protein expression.
CONCLUSION: Inhibition of PI 3-K/Akt signaling pathway induces apoptosis in HSC.
- Citation: Wang Y, Jiang XY, Liu L, Jiang HQ. Phosphatidylinositol 3-kinase/Akt pathway regulates hepatic stellate cell apoptosis. World J Gastroenterol 2008; 14(33): 5186-5191
- URL: https://www.wjgnet.com/1007-9327/full/v14/i33/5186.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5186
The activation and proliferation of hepatic stellate cells (HSC) is a key event in fibrogenesis. On the other hand, HSC apoptosis results in fibrolysis and fibrotic regression[1-3]. Therefore, by understanding pro- and anti-apoptogenic factors, new therapeutic targets will be identified for the treatment of liver fibrosis. However, intracellular signals that regulate HSC apoptosis are still obscure. Our previous study has demonstrated that a cross talk between platelet-derived growth factor (PDGF), the most potent proliferative cytokines for HSC, and focal adhesive kinase (FAK), a nonreceptor tyrosine kinase, is involved in an integrin signaling pathway[4-6]. This interaction is essential for PDGF to induce HSC proliferation[7].
Phosphatidylinositol 3-kinase (PI 3-K)/Akt signaling molecules, which are downstream of FAK, are also activated by PDGF. The activated PI 3-K/Akt participate in regulation of HSC migration, proliferation, collagen secretion and adhesion[5]. PI 3-K is involved in regulating a number of cellular responses, such as cell growth, survival and migration. Akt, a serine/threonine kinase, is downstream of PI 3-K and an important anti-apoptotic factor. The purpose of the present study was to determine the effects and the molecular mechanisms whereby PI 3K/Akt influence apoptosis in HSC.
RPMI 1640 culture medium was obtained from Gibco (Invitrogen Corporation Carlsbad, California USA), fetal bovine serum (FBS) from Sijiqing Company of Bio-products (Hangzhou, China) and LY 294002 from Sigma (Saint Louis, Missouri USA). Rat PDGF-BB was from Cytolab Biochemicals (Rehovot, Israel USA). Rabbit anti-phospho-Ser473 Akt polyclonal antibody and rabbit anti-PI 3-K p85α polyclonal antibody were from Santa Cruz Biotechnology (Santa Cruz, CA. USA). Rabbit anti-total Akt polyclonal antibody was purchased from Cell Signaling Biotechnology (Beverly, MA USA). RT-PCR amplification system was from Promega (Madison, WI USA). Annexin-V/Propidium iodide double-labelled flow cytometry kit was purchased from Baosai Company of Bio-products (Beijing, China). CO2 incubator was from SANYO Company (Chatsworth, CA Japan). Flow cytometer type FACS-420 was purchased from Becton Dickinson Company (Franklin Lakes, NJ USA).
The activated HSC phenotype, from CCl4 induced cirrhotic liver, was provided by Professor Greenwel (USA). This cell line is similar to that of primary cultured HSC except for absent expression of collagen type IV[8]. HSC was cultured in RPMI 1640 media supplemented with 10% FBS, penicillin (100 IU/mL)/streptomycin (100 μg/mL), and glutamine (4 mmol/L) in a 95% air and 5% CO2 humidified atmosphere. Cell viability was determined by trypan blue exclusion staining. When exponential growing HSC populations were found to be nearly 100% confluence, HSC (2 × 105/mL) was incubated in serum free media for 24 h and then split into four groups: control, PDGF-BB (PDGF 20 ng/mL), PDGF-BB + LY 294002 group (LY 294002 50 μmol/L, PDGF-BB 20 ng/mL) and LY 294002 (LY 294002 50 μmol/L). Activated HSC was serum-starved for 24 h, and then treated as described above. One set of the cells was harvested for 4 h later for mRNA transcription and protein expression assay. The same set of cells was harvested 20 h later for electron microscopic examination, flow cytometric analysis and immunostaining.
The cultured HSC of the four groups were collected, digested, washed with phosphate buffered solution (PBS), fixed with 4% glutaraldehyde for 2 h, and then fixed with osmium tetroxide for 1 h, stained with uranium acetate, embedded into 6.8# epoxide resin, after sectioning into ultra-thin slices, the cells were stained with lead citrate and examined under transmission electron microscopy.
Four groups of HSC were collected, washed with pre-cooled PBS twice, fixed with cold 70% ethanol, digested with 50 μg/mL RNase at 37°C, and stained with 65 μg/mL propidium iodide at 4°C for 1 h, and then the flow cytometric analysis was conducted. 104 cells were used in apoptotic analyses. Avian red blood cells were used as control. The variation coefficient (CV) of the assay was less than three percent. The data collected were analyzed in a computer (type HP-300 Consort 30), and analyzed with Histogram Statistics software.
Total RNA from cultured HSC was extracted by the Trizol method. After RT (41°C, 45 min) and pre-denaturation (94°C, 2 min), PCR amplification was carried out for 35 cycles. The primers, as designed according to GenBank, were as follows: PI 3-K p85: sense 5’- CCCAGGAGCGGTACAGCAAAGAA-3’, antisense 5’-TGGGGCAAATCCTCATCATCTTC-3’; β-actin: sense 5’-ACAGAGTACTTGCGCTCAGGAG-3’, antisense 5’-GTCACCCACACTGTGCCCATCT-3’. The initial reaction mixture contained 1 μmol/L primers, AMV reverse transcriptase, RNA Tfl DNA polymerase and 25 mmol/L MgSO4. Total volume was 50 μL. The amplicon lengths of the PCR products were: PI 3-K p85 355 bp and β-actin 585 bp. Denaturation (94°C for 40 s), annealing (52°C for 1 min), and extension (72°C for 1.5 min). PCR products were analyzed by electrophoresis in a 1.5% agarose gel. β-actin amplification was used as internal control.
Cultured cells were seeded onto glass coverslips and allowed to adhere for 20 h under routine culture conditions. After incubation, cells were washed twice with cold PBS, fixed and permeabilized by cold methanol for 8 min, and coverslips were then air-dried at room temperature. Immunostaining was performed using the SP kit according to the instructions of the manufacturer. Briefly, HSC was washed with washing buffer (0.2% Tween PBS), incubated with peroxidase-blocking reagent and normal goat serum. After rinsing, coverslips were incubated with primary antibodies (rabbit anti-phospho-Ser473, Akt polyclonal antibody (1:100) and rabbit anti-PI 3-K p85α polyclonal antibody (1:50) at room temperature for 12 h. After rinsing primary antibody with washing buffer, horseradish peroxidase-conjugated goat anti-rabbit IgG antibody was added, and incubated at 37°C for 30 min. Following rinsing with washing buffer, 3,3-diamino benzidine/enhancer (DAB) solution was added and incubated at room temperature for 5 min. After washing, the coverslips were counterstained with hematoxylin for 1 min, rinsed with water and dried for 10 min. The slides were dehydrated with 100% ethanol for 20 s twice, and finally with xylene for 20 s. The coverslips were mounted onto glass slices and viewed under microscopy.
Cultured HSC was washed with PBS and lysed with protein sample buffer (50 mmol/L Tris-HCl pH 7.5, 150 mmol/L NaCl, 0.5% sodium deoxycholate, 1% NP-40, 0.1% SDS, 1 mmol/L EDTA, 1 mmol/L phenylmethyl sulfonylfluoride, and 2 mg/L leupeptin). The protein concentrations were measured using Coomassie brilliant blue chromatometry. Equal amounts (100 μg) of the denatured proteins per lane were loaded and separated on sodium dodecyl sulfate-10% polyacrylamide gels for p-Akt (Ser473), Akt and β-actin, and 8% SDS-polyacrylamide gels for PI 3-K. After electrophoresis, proteins were transferred to nitrocellulose. The membranes were blocked for 12 h with 5% powdered skim milk in Tris-HCl-buffered saline containing 0.05% Tween 20 (TBS-T). After washed with TBS-T for three times, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (1:3000). After washing 5 times, the blots were detected with the enhanced chemiluminescence method (ECL) assay.
Results were expressed as mean ± SD. Statistical analysis was performed by one-way ANOVA, and, when the F value was significant, by Student-Newman-Keuls test. P value less than 0.05 was considered statistically significant.
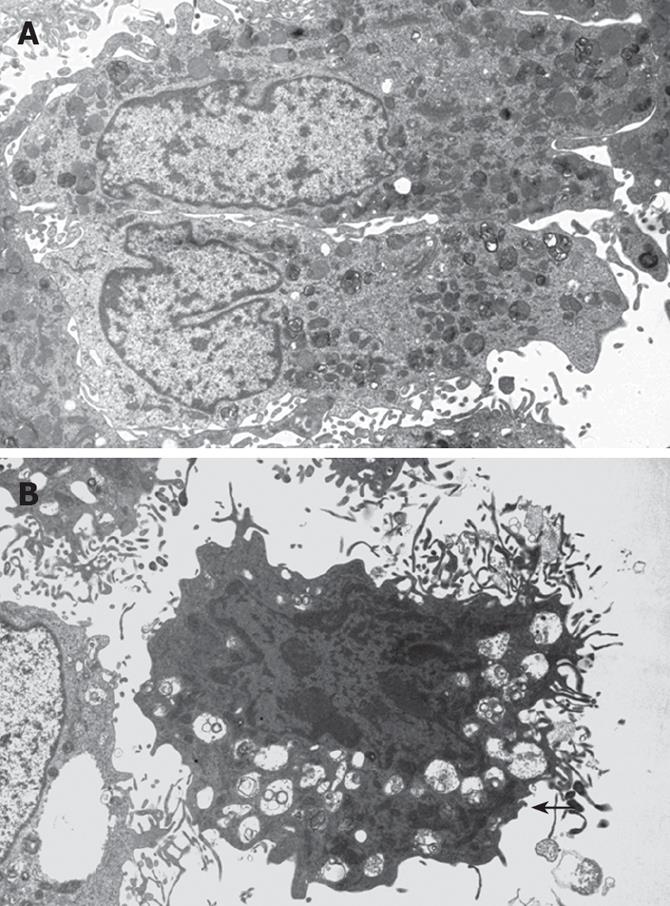
Under the transmission electron microscopy (Figure 1), the cells in LY 294002 and LY 294002 + PDGF-BB groups showed condensed chromatin, shrunk and aggregated along inside the nuclear membrane. The morphology of the cells showed spherical, petal or crescent shape, apoptotic bodies were found in some cells, while in control and PDGF-BB groups, HSC revealed normal silhouettes.
As listed in Table 1, the apoptotic rates in LY 294002 and LY 294002 + PDGF-BB groups were significantly increased compared with that of control group (30.82% ± 2.90%, 28.16% ± 2.58% and 9.02% ± 1.81%, respectively; P < 0.01). There was no significant difference between LY 294002 and LY 294002 + PDGF-BB groups (P = 0.12).
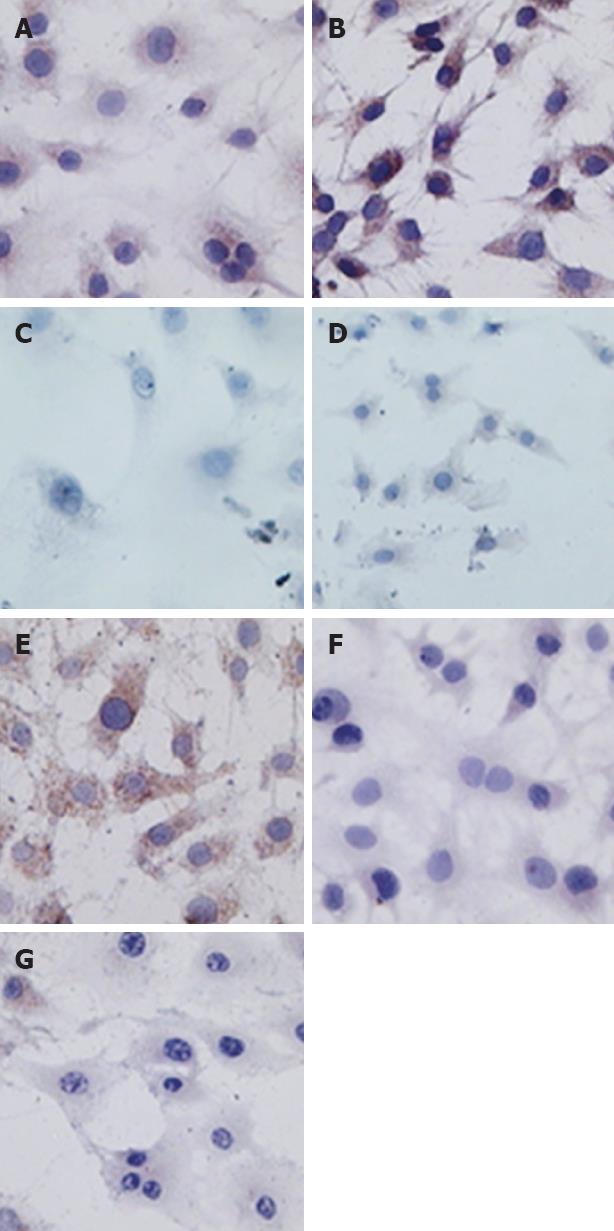
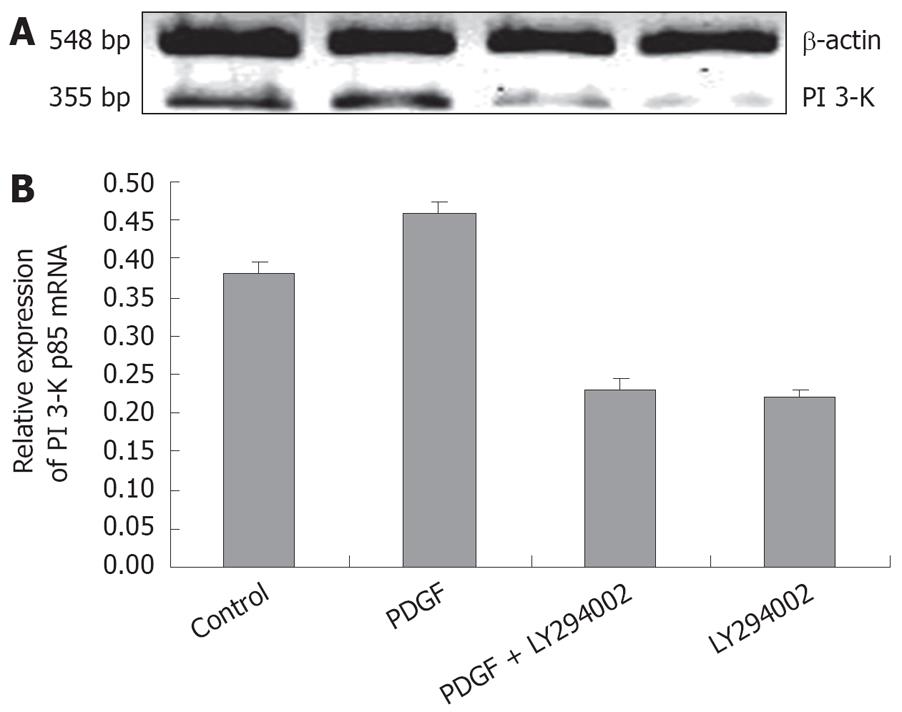
PI 3-K assay: PDGF-BB could significantly increase PI 3-K expression in rat HSC. LY 294002 not only decreased the PI 3-K positive cells in control group, but also reduced PI 3-K content in PDGF-BB activated cells (Figure 2 and Table 2). These immunocytochemical results were supported by Western blots: the band density in PDGF-BB group was the strongest. LY 294002 not only decreased the PI 3-K expression in control cells, but also decreased the protein content in PDGF-BB activated cells (Figure 3). The effects of LY 294002 were reflected not only by the protein expression levels, but also by mRNA transcription (Figure 4).
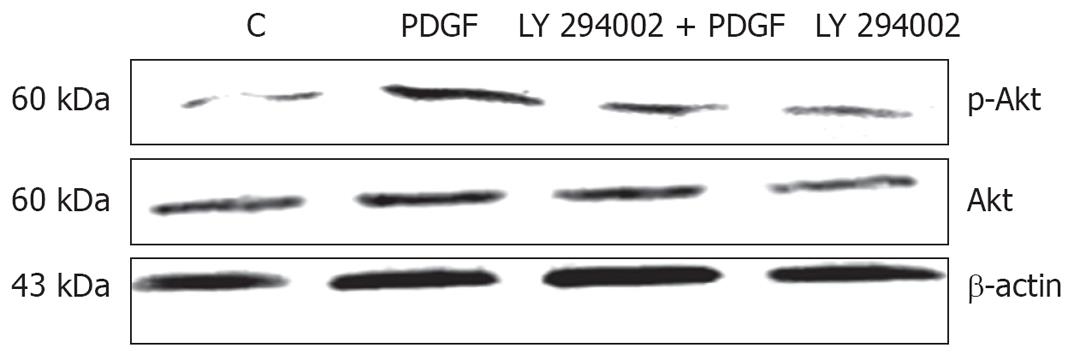
Akt assay: Immunocytochemistry showed that p-Akt expression was parallel with PI 3-K: PDGF-BB stimulated p-Akt protein expression, while LY 294002 not only inhibited p-Akt concentration in control group, but also abolished PDGF stimulated p-Akt expression (Figure 2 and Table 2). Western blots showed the same pattern as immunocytochemistry for p-Akt. Noticeably, the total Akt levels assessed by Western blotting revealed no change in all four groups (Figure 5).
All the results above showed that PDGF activated the whole PI 3-K/Akt/p-Akt, pathway, while LY 294002 decreased the entire pathway activity not only in control cells, but also in PDGF stimulated circumstances.
It is activation of HSC that initiates liver fibrosis, and regardless of the etiology, is the final pathway is to activate HSC. The activated HSC is proliferative, fibrogenic, and contractile myofibroblasts[5,9]. The proliferation and apoptosis of HSC keep balance in physiological situation. Once this equilibrium is broken under chronic injury, continuing HSC proliferation and collagen secretion will cause fibrosis[10]. Therefore, inhibiting the proliferation of HSC and inducing its apoptosis are two ways to delay or stop fibrogenesis. PI 3-K and Akt are two molecules that stimulate HSC proliferation[1,2].
PI 3-K is comprised of an 85-kDa regulatory subunit, and a catalytic 110-kDa subunit. Activated by growth factors such as PDGF and insulin-like growth factor (IGF), PI 3-K catalyzes the phosphorylation of phosphatidylinositol (PI) at the 3-OH position to generate phosphatidylinositol triphosphate (PIP3). Activated growth factor receptor migrates to the cellular membrane, and this results in PI 3-K activation, that later combines with its substrates. Akt, a serine/threonine kinase, is also known as protein kinase B (PKB). Three isoforms have been identified thus far in the mammalian cells: Akt1/PKBα, Akt2/PKBβ, and Akt3/PKBγ. The three isoforms have similar phosphorylation sites. One is in the N-terminal Thr308. And the other in the COOH-terminal hydrophobic motif Ser473. The phosphorylation of both sites is necessary for full activity. Activated PI 3-K/Akt signaling inhibits apoptosis, and stimulates cell proliferation, migration, survival as well as other biological behaviours[11].
Among the three PDGF isoforms, PDGF-AA, PDGF-BB and PDGF-AB, PDGF-BB has been identified as the most potent mitogen for HSC[7,12]. Liver fibrosis is associated with an increase in PDGF protein expression and increased PDGF receptor expression. PDGF receptors have intrinsic tyrosine kinase activity and upon binding to its ligand, PDGF receptors can phosphorylate itself at tyrosine residues. In cultured human HSC, PDGF can activate PI 3-K which is necessary for both mitogenesis and chemotaxis[13-15]. This pathway is up-regulated during liver injury in vivo. Wortmannin and LY 294002, two specific PI 3-K inhibitors, can both dose-dependently block PI 3-K activity induced by PDGF and inhibit DNA synthesis[16].
Our immunocytochemistry in the present study found that PI 3-K and p-Akt were mainly in HSC cytoplasm. Immunocytochemistry, Western blot and RT-PCR demonstrated that PDGF-BB can significantly stimulate PI 3-K and p-Akt productions in both mRNA transcription (PI 3-K) and protein expression levels, LY 294002 not only reduced PI 3-K and p-Akt contents in control group, but also reduced PDGF stimulated PI 3-K expression and Akt phosphorylation. There was no significant difference between the groups of LY 294002 only and LY 294002 + PDGF. This means that LY 294002 can abolish the simulatory effect of PDGF. Our results also showed that there was no significant difference of total-Akt levels among four groups, indicating that instead of total Akt syntheses, LY 294002 mainly inhibits Akt phosphorylation. This in vitro study is consistent with the well-documented data which confirmed Akt phosphorylation being inhibited by LY 294002 in bile duct-ligated (BDL) animals. In addition, PDGF can induce Akt phosphorylation and LY 294002 blocks this phosphorylation.
The Akt family represents pivotal factors to promote cell survival, proliferation and inhibit apoptosis. Akt plays an important role in inhibiting apoptosis in variety of cells such as uterine leiomyoma cell lines, hematopoietic progenitor/stem cell lines, pancreatic beta cells and islet beta cells etc[17-20]. Our results showed that Akt also inhibited apoptosis in HSC. Following PI 3-K/Akt pathway inhibition, HSC revealed typical apoptotic morphous under transmission electron microscopy. Microvilli on the cell surface decreased,became short, and even disappeared. Cells shrank, cytoplasm condensed, ribosome and mitochondria aggregated. The chromatin condensed and shrank and aggregated along inside of the nuclear membrane to form of balls, petals and crescents. Sometimes,apoptotic bodies formed. The apoptosis rates of both LY 294002 treated groups were significantly increased compared with that of the control group and the PDGF group and thus, we conclude that PI 3-K/Akt inhibition enhances HSC apoptosis.
In summary, the data obtained have shown that inhibition of PI 3-K/Akt signaling pathway can induce apoptosis in rat HSC, even under strong mitogen stimulator (PDGF). The activation of HSC is essential for fibrogenesis, and PI 3-K/Akt signaling is indispensable to support HSC activation. Therefore, blocking the PI 3-K/Akt signaling pathway may provide a potential therapeutic benefit for liver fibrosis.
Hepatic fibrosis represents a reversible and dynamic process in response to a variety of chronic stimuli. Activation of the hepatic stellate cell (HSC), a perisinusoidal cell that resides in the liver in a quiescent state, is responsible for the increased synthesis and deposition of extracellular matrix (ECM) in the liver, and plays a critical role in fibrogenesis. The paradigms of HSC activation and apoptosis remain valuable frameworks for understanding pathways of hepatic fibrogenesis and fibrosis regression. HSC apoptosis results in fibrolysis and fibrotic regression. Therefore, by understanding the pro- and anti-apoptogenic factors, new therapeutic targets will be identified for the treatment of liver fibrosis. Phosphatidylinositol 3-kinase (PI 3-K)/Akt are components of the major intracellular signaling pathways elicited by platelet-derived growth factor (PDGF) in HSC. Intracellular signals that regulate HSC apoptosis are still obscure. Our previous study has demonstrated that a cross talk between PDGF, the most potent proliferative cytokines for the HSC, and focal adhesion kinase (FAK), a nonreceptor tyrosine kinase, is involved in integrin signaling pathway. This interaction is essential for PDGF to induce HSC proliferation.
The activated HSC is primarily responsible for excessive deposition of ECM proteins and collagen deposition during liver fibrosis. Substantial insight is being gained into the molecular mechanisms responsible for apoptosis in the HSC. The activated HSC becomes responsive to both proliferative and fibrogenic cytokines. These cytokines activate both mitogen-activated protein kinase (MAPK) signaling, involving p38, and FAK-PI 3-K-Akt-p70 (S6K) signaling cascades. PI 3-K/Akt constitutes an important pathway regulating the signaling of multiple biological processes such as apoptosis, metabolism, cell proliferation and cell growth. Activating mutations, which have been reported for PI 3-K and Akt in tumors, are able to confer tumorigenic properties in several cellular systems. These regulate the proliferative response, activating cell cycle progression as well as collagen gene expression. It is anticipated that by understanding the molecular mechanisms responsible for HSC proliferation, apoptosis, and excess ECM production new therapeutic targets will be identified for the treatment of liver fibrosis.
HSC apoptosis results in fibrolysis and further on, fibrotic regression. Intracellular signals that regulate HSC apoptosis are still obscure. Our previous study has demonstrated that a cross talk between PDGF and FAK is involved in integrin signaling pathway. PI 3-K/Akt signaling molecules, which are downstream of FAK, are also activated by PDGF. Up to now, few studies have addressed the function of PI 3-K/Akt signal transduction pathways on the apoptosis of HSC. The present study determined the effects and the molecular mechanisms whereby PI 3K/Akt influences apoptosis in HSC, and showed that PDGF activated the whole PI 3-K/Akt/p-Akt, pathway, while LY 294002 decreased the entire pathway activity not only in control cells, but also in PDGF stimulated circumstances. PI 3K/Akt signal molecules will be new therapeutic targets for the treatment of liver fibrosis.
The present study has shown that inhibition of PI 3-K/Akt signaling pathway can induce apoptosis in rat HSC, even under strong mitogen stimulators (PDGF). The activation of HSC is essential for fibrogenesis, and PI 3-K/Akt signaling is indispensable to support HSC activation. Therefore, blocking the PI 3-K/Akt signaling pathway may provide a potential therapeutic benefit for liver fibrosis.
The paper by Wang et al presents an interesting study of the role of PI 3-K/Akt in controlling apoptosis of hepatic stellate cells. The purpose of the present work was to determine whether inhibition of PI 3-K affected apoptosis in HSC. The methods used to measure expression of PI 3-K and phosphorylation of Akt as well as total Akt were acceptable, and the assessment of apoptosis in HSC also reliable. The results very clearly show that the apoptotic rate was markedly increased in presence of LY294002.
Peer reviewer: Trond Berg, Professor, Department of Molecular Biosciences, University of Oslo, PO Box 1041 Blindern, Oslo 0316, Norway
S- Editor Zhong XY L- Editor Ma JY E- Editor Ma WH
| 1. | Murphy FR, Issa R, Zhou X, Ratnarajah S, Nagase H, Arthur MJ, Benyon C, Iredale JP. Inhibition of apoptosis of activated hepatic stellate cells by tissue inhibitor of metalloproteinase-1 is mediated via effects on matrix metalloproteinase inhibition: implications for reversibility of liver fibrosis. J Biol Chem. 2002;277:11069-11076. |
| 2. | Saile B, Knittel T, Matthes N, Schott P, Ramadori G. CD95/CD95L-mediated apoptosis of the hepatic stellate cell. A mechanism terminating uncontrolled hepatic stellate cell proliferation during hepatic tissue repair. Am J Pathol. 1997;151:1265-1272. |
| 3. | Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427-436. |
| 4. | Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409-1416. |
| 5. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. |
| 6. | Jiang HQ, Zhang XL, Liu L, Yang CC. Relationship between focal adhesion kinase and hepatic stellate cell proliferation during rat hepatic fibrogenesis. World J Gastroenterol. 2004;10:3001-3005. |
| 7. | Carloni V, Defranco RM, Caligiuri A, Gentilini A, Sciammetta SC, Baldi E, Lottini B, Gentilini P, Pinzani M. Cell adhesion regulates platelet-derived growth factor-induced MAP kinase and PI-3 kinase activation in stellate cells. Hepatology. 2002;36:582-591. |
| 8. | Greenwel P, Schwartz M, Rosas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Differences in the production of interleukin-6. Lab Invest. 1991;65:644-653. |
| 9. | Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125-172. |
| 10. | Guo J, Friedman SL. Hepatic fibrogenesis. Semin Liver Dis. 2007;27:413-426. |
| 11. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. |
| 12. | Rovida E, Navari N, Caligiuri A, Dello Sbarba P, Marra F. ERK5 differentially regulates PDGF-induced proliferation and migration of hepatic stellate cells. J Hepatol. 2008;48:107-115. |
| 13. | Robino G, Parola M, Marra F, Caligiuri A, De Franco RM, Zamara E, Bellomo G, Gentilini P, Pinzani M, Dianzani MU. Interaction between 4-hydroxy-2,3-alkenals and the platelet-derived growth factor-beta receptor. Reduced tyrosine phosphorylation and downstream signaling in hepatic stellate cells. J Biol Chem. 2000;275:40561-40567. |
| 14. | Bell CA, Tynan JA, Hart KC, Meyer AN, Robertson SC, Donoghue DJ. Rotational coupling of the transmembrane and kinase domains of the Neu receptor tyrosine kinase. Mol Biol Cell. 2000;11:3589-3599. |
| 15. | Gutierrez-Ruiz MC, Gomez-Quiroz LE. Liver fibrosis: searching for cell model answers. Liver Int. 2007;27:434-439. |
| 16. | Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor’s actions on hepatic stellate cells. Gastroenterology. 1997;112:1297-1306. |
| 17. | Yin XJ, Wang G, Khan-Dawood FS. Requirements of phosphatidylinositol-3 kinase and mammalian target of rapamycin for estrogen-induced proliferation in uterine leiomyoma- and myometrium-derived cell lines. Am J Obstet Gynecol. 2007;196:176.e1-e5. |
| 18. | Wandzioch E, Edling CE, Palmer RH, Carlsson L, Hallberg B. Activation of the MAP kinase pathway by c-Kit is PI-3 kinase dependent in hematopoietic progenitor/stem cell lines. Blood. 2004;104:51-57. |
| 19. | Maeda H, Rajesh KG, Maeda H, Suzuki R, Sasaguri S. Epidermal growth factor and insulin inhibit cell death in pancreatic beta cells by activation of PI3-kinase/AKT signaling pathway under oxidative stress. Transplant Proc. 2004;36:1163-1165. |
| 20. | Scassa ME, Guberman AS, Varone CL, Canepa ET. Phosphatidylinositol 3-kinase and Ras/mitogen-activated protein kinase signaling pathways are required for the regulation of 5-aminolevulinate synthase gene expression by insulin. Exp Cell Res. 2001;271:201-213. |