Published online Aug 28, 2008. doi: 10.3748/wjg.14.5025
Revised: August 8, 2008
Accepted: August 15, 2008
Published online: August 28, 2008
AIM: To investigate whether hepatocytes isolated from macroscopically normal liver during hepatic resection for neoplasia could provide a novel source of healthy hepatocytes, including the development of reliable protocols for malignant cells removal from the hepatocyte preparation.
METHODS: Hepatocytes were procured from resected liver of 18 patients with liver tumors using optimised digestion and cell-enrichment protocols. Suspensions of various known quantities of the HT-29 tumor cell line and patient hepatocytes were treated or not with Ep-CAM-antibody-coated immunomagnetic beads in order to investigate the efficacy of tumor-purging by immunomagnetic depletion, using a semi-quantitative RT-PCR method developed to detect tumor cells. Immunomagnetic bead-treated or bead-untreated tumor cell-hepatocyte suspensions were transplanted intra-peritoneally in Balb/C nude mice to assess the rates of tumor development.
RESULTS: Mean viable hepatocyte yield was 9.3 x 106 cells per gram of digested liver with mean viability of 70.5%. Immunomagnetic depletion removed tumor cells to below the RT-PCR detection-threshold of 1 tumor cell in 106 hepatocytes, representing a maximum tumor purging efficacy of greater than 400 000-fold. Transplanted, immunomagnetic bead-purged tumor cell-hepatocyte suspensions did not form peritoneal tumors in Balb/C nude mice. Co-transplantation of hepatocytes with tumor cells did not increase tumorigenesis of the tumor cells.
CONCLUSION: Immunomagnetic depletion appears to be an effective method of purging contaminating tumor cells to below threshold for likely tumorigenesis. Along with improved techniques for isolation of large numbers of viable hepatocytes, normal liver resected for neoplasia has potential as another clinically useful source of hepatocytes for transplantation.
- Citation: Gunasegaram A, Akhter J, Yao P, Johnson LA, Riodan SM, Morris DL. Hepatocytes isolated from neoplastic liver-immunomagnetic purging as a new source for transplantation. World J Gastroenterol 2008; 14(32): 5025-5031
- URL: https://www.wjgnet.com/1007-9327/full/v14/i32/5025.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5025
| Parameters | n |
| Age (yr; mean ± SD, range) | 59 ± 11 (33-78) |
| Male | 10 |
| Female | 8 |
| Primary tumor | |
| Colorectal cancer | 12 |
| Benign liver lesions | 2 |
| Renal carcinomas | 1 |
| Prostate carcinoma | 1 |
| Cholangiocarcinoma | 1 |
| Pseudopapillary pancreas tumor | 1 |
| Operation | |
| Right hemi-hepatectomy | 7 |
| Left hemi-hepatectomy | 4 |
| Extended right hemi-hepatectomy | 2 |
| Right lateral sectorectomy | 2 |
| Extended left hemi-hepatectomy | 1 |
| Left lateral sectorectomy | 1 |
| Non-anatomic resection | 1 |
| Treatment groups | Mouse group | IP injection | No. of mice |
| Negative control (Hepatocyte only) | 1 | 5 x 106 hepatocytes | 3 |
| Positive control 1 (HT-29 only) | 2 | 5000 HT-29 cells | 3 |
| 3 | 100 000 HT-29 cells | 3 | |
| 4 | 500 000 HT-29 cells | 3 | |
| 5 | 2 million HT-29 cells | 3 | |
| Positive control 2 (Hepatocytes + HT-29) | 6 | 5 million hepatocytes + 5000 HT-29 | 3 |
| 7 | 5 million hepatocytes + 100 000 HT-29 | 3 | |
| 8 | 5 million hepatocytes + 500 000 HT-29 | 3 | |
| 9 | 5 million hepatocytes + 2 million HT-29 | 3 | |
| Bead (Hepatocyte + HT-29) | 10 | 5 million hepatocytes + 5000 HT-29 | 3 |
| 11 | 5 million hepatocytes + 100 000 HT-29 | 3 | |
| 12 | 5 million hepatocytes + 500 000 HT-29 | 3 | |
| 13 | 5 million hepatocytes + 2 million HT-29 | 3 |
| Treatment groups | IP injection | Mouse with tumour | Percentage expression (%) |
| Negative control (Hepatocyte only) | 5 x 106 hepatocytes | 0/3 | 0 |
| Positive control 1 (HT-29 only) | 5000 HT-29 cells | 0/3 | 0 |
| 100 000 HT-29 cells | 1/31 | 01 | |
| 500 000 HT-29 cells | 2/3 | 67 | |
| 2 million HT-29 cells | 3/3 | 100 | |
| Positive control 2 (Hepatocytes + HT-29) | 5 million hepatocytes + 5000 HT-29 | 0/3 | 0 |
| 5 million hepatocytes + 100 000 HT-29 | 0/3 | 0 | |
| 5 million hepatocytes + 500 000 HT-29 | 3/3 | 100 | |
| 5 million hepatocytes + 2 million HT-29 | 2/3 | 67 | |
| Bead (Hepatocyte + HT-29) | 5 million hepatocytes + 5000 HT-29 | 0/3 | 0 |
| 5 million hepatocytes + 100 000 HT-29 | 0/3 | 0 | |
| 5 million hepatocytes + 500 000 HT-29 | 0/3 | 0 | |
| 5 million hepatocytes + 2 million HT-29 | 0/3 | 0 |
While advances in supportive medical care have improved survival, liver failure in its most severe form continues to carry a high mortality rate unless orthotopic liver transplantation (OLT) is performed. Nonetheless, there is a limit to the number of patients that can be treated in this way. The rapidity with which the clinical syndrome often progresses and a worldwide shortage of donor organs are significant limiting factors and many patients listed for OLT die or develop contraindi-cations to transplantation before a donor liver becomes available. Further, the presence of co-morbidities in a substantial number of patients often precludes listing for OLT altogether. Consequently, there is considerable ongoing interest in the provision of other means of liver support, including extracorporeal artificial or bioartificial devices and hepatocyte transplantation (HT).
The feasibility of HT as a therapeutic tool has been demonstrated in studies performed in animals with liver-based metabolic defects such as analbuminaemic and Gunn rats, hypercholesterolaemic rabbits and dogs with impaired purine metabolism[1-3]. Experience with HT in experimental animals with liver failure due to chemically-induced hepatic necrosis, surgical models of hepatic ischaemia or resection has also been favourable, with evidence of improved survival even when small numbers of cells (0.5% to 3% of normal hepatocyte mass) are used[4-7].
Transplantation of human hepatocytes has, to date, been performed in only a small number of paediatric and adult patients with liver failure[8-11]. Instances of mostly short-term improvement in encephalopathy and some metabolic parameters have been recorded both in OLT and non-OLT candidates. Potential advantages of HT over OLT include its minimal invasiveness, ease of treatment, substantially lower cost and the possibility of on-demand use following the establishment of a cell bank. Limited availability of primary human hepatocytes for transplantation however, represents a major limitation. Currently, the standard approach is to isolate hepatocytes from livers rejected for liver transplantation due to excessive steatosis, cirrhosis and prolonged ischemia. However, availability of hepatocytes from this source is in increasingly short supply and concerns with the functional capability of such cells have been raised[12].
Our group recently reported the feasibility of harvesting tumor-free hepatocytes from macroscopically normal liver unavoidably removed during hepatic resection for malignancy[13]. Here we report further improvements in our hepatocyte isolation and tumor-purging techniques, resulting in the harvest of sufficiently large numbers of viable hepatocytes from resected liver specimens to offer the prospect of effective clinical support and a high degree of tumor-purging efficacy. Furthermore, we demonstrate the safety of transplantation of hepatocytes harvested in this way, in terms of lack of complicating tumor development in an athymic mouse model.
The human isolation study was approved by the South East Sydney Area Health Service Ethics Committee (approval No. 01/123). Hepatocytes were harvested from liver resection specimens of consenting patients undergoing partial hepatectomy for neoplasia. Patients with hepatocellular carcinoma were excluded. Patient demographic and disease details are outlined (Table 1). The bulk of specimens (61%) arose from livers with colorectal metastases.
Following resection, the specimen was transferred to a sterile table where the diseased portion of the liver was dissected with a 1cm margin, leaving the remaining macroscopically normal liver for hepatocyte harvest. The tissue was placed immediately into a sterile cooling solution (< 4°C) and 2-4 of the largest vessels were cannulated. Warm ischemic time, defined as clamping time of hepatic inflow and/or outflow during resection plus specimen processing time before being cooled, was recorded. Cannulae ranging from 0.95 mm to 2 mm diameter were suture-ligated around the respective vessels and capped with one-way valved bungs to prevent backflow. Histoacryl® (Braun, Germany) was used to seal most of the remaining liver surface, in addition to the cannula entry points. This was done to prevent leakage of perfusate and to maximize perfusion of the microcirculation during isolation, having been previously shown to increase hepatocyte yield and viability[14,15]. The specimen was then flushed with 500-1000 mL of heparinized (5000 units per litre) Custodiol® histidine-tryptophan-ketoglutarate (HTK) solution (Kohler Chemie, Germany)[16] to prevent obstruction of the hepatic microcirculation by thrombus and facilitate organ preservation. The specimen was then transported under sterile conditions to the laboratory where it was re-warmed and digested by a modified version of Seglen’s original 2-step technique[17]. Cold ischemic time, defined as time that the specimen had been on ice prior to re-warming, was recorded. Pre-warmed (37°C) buffers were perfused in the following order: (1) Wash buffer-HBSS without Ca2+/Mg2 (Gibco, Auckland, New Zealand) + 208.1 mg/L EDTA (Sigma, St Louis, MO, USA) + ascorbic acid 50 mg/L (Sigma, St Louis, MO, USA) + bovine serum albumin (BSA) (Sigma, St Louis, MO, USA) 0.5% w/v-10 min perfusion and then discarded. (2) EDTA washout-HBSS + BSA 0.5% w/v; 5 min perfusion and then discarded. (3) Digestion buffer-HBSS + 0.05% w/v Collagenase P (Roche, Germany) + 0.5% w/v BSA-re-circulated for 20-30 min.
Perfusion was carried out manually due to the varying size of the specimens (range, 55-690 g) at 20-40 mL/min per cannula depending on the weight of the specimen and cannula size. Digestion buffer was perfused until the specimen was soft and friable. A cell suspension was obtained by gentle mechanical dissociation of the digested specimen in 500 mL of ice-cold suspension buffer DMEM (Gibco, Auckland, New Zealand) + 10% (v/v) FCS (Gibco, Auckland, New Zealand) + 1% antibiotic-antimycotic Penicillin 10 000 units/mL + streptomycin 25μg/mL + amphotericin B as Fungizone® (Gibco, Auckland, New Zealand) and sequentially filtered through three sterile stainless steel filters of decreasing pore size (425 μm, 150 μm and 75 μm) (Endecotts, UK). The raw fraction was pelleted by centrifugation at 50 g for 3 min at 4°C and washed three times in the suspension buffer. Cells were counted in quadruplicate and viability assessed by Trypan blue exclusion, using a hemocytometer (Neubauer, Germany). Cells were cryopreserved in suspension medium containing 10% DMSO in 5-mL tubes by freezing to -80°C in 1°C decrements per minute and then transferred to liquid nitrogen after 24 h.
In vitro and in vivo studies were performed using suspensions of isolated human hepatocytes spiked with various numbers of HT-29 human colorectal cell-line tumor cells and then treated or not with immunomagnetic beads (CELLection® Epithelial Enrich; Dynal AS, Oslo, Norway), in the method described by Kielhorn et al[18] and Flatmark et al[19]. Specifically, we used 4.5 μm magnetic beads coated with mouse IgG1 Ber-EP4 antibody against Ep-CAM, an antigen highly expressed on colorectal cancer (CRC) cells (including HT-29 tumor cells), but not on mature hepatocytes[20].
Cryopreserved human hepatocytes were thawed in a 37°C water bath, diluted in suspension medium, pelleted by centrifuging at 50 g for 3 min at 4°C and re-suspended in suspension medium. Density gradient centrifugation was performed to purify the thawed hepatocytes. The suspension was then mixed with a Percoll® (Amersham Biosciences, Uppsala, Sweden) density gradient (25% final concentration) medium and centrifuged at 75 g for 5 min at 4°C to separate viable from dead hepatocytes. Pellets were re-suspended in PBS with 0.1% BSA and placed on ice. Cells were counted in quadruplicate, using a hemocytometer (Neubauer, Germany), and their viability assessed by Trypan blue exclusion. HT-29 cells grown in 75 cm2 culture flasks (Greiner Bio-One, Germany) in 95% O2 and 5% CO2 at 37°C were trypsinised, washed in PBS (Invitrogen, Auckland, New Zealand), pelleted by centrifugation at 75 g for 5 min, re-suspended in PBS and counted as above.
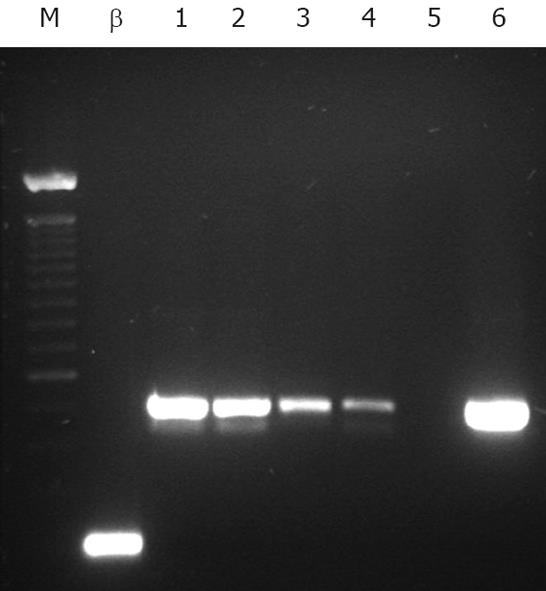
One, 10, 50, 100, 1000, 5000 or 10 000 HT-29 cells were added to suspensions of 1 million human hepatocytes to establish the lower limit of detection of tumor cells by RT-PCR. Suspensions of 1 million HT-29 cells alone and 1 million hepatocytes alone served as positive and negative controls respectively.
Total RNA was extracted using TRIzol® reagent (15596026, Invitrogen) as per the manufacturer’s instructions. Following DNase I treatment (18068-015, Invitrogen), the total RNA concentration and quantity was assessed by spectrophotometry at 260 nm and the RNA stored at -80°C.
cDNA was synthesized from total RNA using One-Step SuperScript III® system (12574-026, Invitrogen) with target specific primers as per the manufacturer’s instructions. EpCAM primers were as published by Sakaguchi et al[21] with actin housekeeping primers as follows; antisense 5'-GGAGCAATGATCTTGATCTT -3'; sense 5'-CTTCCTGGGCATGGAGTCCT-3'. The RT-PCR program was as follows; cycle one, 56°C, 30 min, 94°C for 3 min, followed by 40 cycles of 30 s at 94°C , 30 s at 60°C, 30 s at 72°C, with a final extension for 10 min at 72°C. The cDNA products were visualized on a 1% agarose gel, and sequenced to confirm product identity.
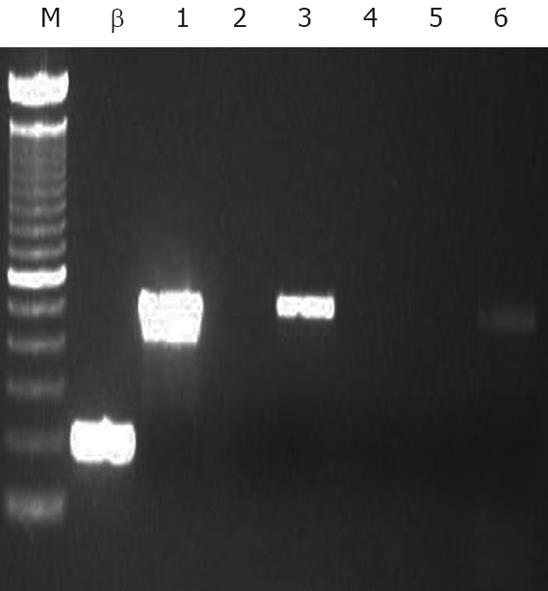
Five mL suspensions of 1 × 106 hepatocytes spiked with 1000, 10 000 and 50 000 HT-29 cells per mL were prepared in duplicate. Immunomagnetic beads (CELLection® Epithelial Enrich; Dynal AS; 4 × 108 beads/mL), were washed in PBS + 0.1% (w/v) BSA, and added to half the tubes in the ratio of 20 beads to 1 HT-29 cell. The remaining preparations constituted controls and contained no immunomagnetic beads. All tubes were placed in a rotator (15 r/min) at 4°C for 30 min, to allow maximal tumor cell-bead contact and capture. Treated tubes were placed in the magnetic particle concentrator provided by the manufacturer for 2 min, the supernatant transferred to new tubes and the process repeated. One mL from each sample was collected after treatment for RT-PCR analysis to assess the efficacy of immunomagnetic bead-mediated tumor-purging.
Male Balb/C athymic nude mice (Animal Resource Centre, Perth, WA, Australia) were housed and fed under specific pathogen-free conditions according to study protocols approved by the Animal Care & Ethics Committee of UNSW (approval No. 02/103). The athymic mouse was chosen due to its minimal cellular immunity, so as to minimize risk of rejection of human hepatocytes and facilitate tumor engraftment. The main aims were to study (a) the tumor load required for tumorigenesis following intraperitoneal transplantation, (b) the effect of co-transplantation of human hepatocytes on tumorigenesis and (c) the effect of our immunomagnetic purging protocol on tumorigenesis. The experimental protocol is described below in Table 2.
Hepatocytes and HT-29 cells were prepared as per the in vitro arm, separately and in combination to produce suspensions containing the cell numbers required per mL PBS (cf Table 2). Two hundred μL samples, containing 20% of the cell number in each 1 mL inoculation, were collected for negative control, positive control and immunomagnetic bead groups, before and after purging for RT-PCR analysis.
Mice were monitored for 28 d post-transplantation after which they were sacrificed with a lethal 6 mg dose of pentobarbital sodium. The abdomen, pelvis and thorax were examined visually for the presence of tumor.
The total viable hepatocyte yield averaged at 9.3 × 108 cells per isolation (range 2.0 × 10-36.3 × 108; mean ± SD viable hepatocyte yield 9.33 × 106± 6.0 × 106 cells/g digested liver tissue), with the five most recent isolations each yielding over 1.0 × 109 cells. The mean viability of freshly isolated hepatocytes was 70.5% ± 8.1%. Mean warm ischemic time was 31 ± 19 min (range, 25-60 min); mean cold ischemic time was 1.5 ± 0.6 h (range, 0.5-16 h).
RT-PCR analysis demonstrated clear single bands at the 515 bp position, indicating Ep-CAM detection, in representative samples of all hepatocyte/HT-29 cell suspensions transplanted into mice belonging to Positive Control 2 and pre-treatment Bead groups. No detectable bands were seen in the samples transplanted into the Negative Control and post-treatment Bead groups (gel images not shown), indicating in the latter case the removal of Ep-CAM positive cells (including HT-29 cells) to below the detection limit of 1 tumor cell in 1 million hepatocytes (Figure 1) and thus, a maximum tumor purging efficacy of immunomagnetic bead treatment of at least 400 000 fold (Figure 2).
In the control groups, all mice injected with 100 000 HT-29 cells and below showed no tumor, except for one animal inoculated with 100 000 HT-29 cells only. The mouse developed a small (0.1 g) skin nodule at the injection site and had no evidence of intra-abdominal tumor on detailed examination. This is probably due to an inadvertent subcutaneous rather than intraperitoneal injection of cells, and could thus be excluded on the basis of technical error. All except two mice inoculated with at least 500 000 HT-29 cells (83%) developed tumor. There was no significant difference in tumor expression between mice injected with or without hepatocytes (Tables 2 and 3). These results would suggest that the minimum tumor load required for engraftment and growth was between 100 000 and 500 000.
There was a complete absence of tumor development in any mouse injected with HT-29 cell-spiked hepatocyte suspensions treated with immunomagnetic beads. All mice survived until day 28 without any significant weight loss/gain or signs of malaise or distress. Where intraperitoneal tumors occurred, these were seen only intra-abdominally, expressed as distinct, scirrhous, white nodules adherent to the parietal or visceral peritoneum, without evidence of metastasis.
Our results show that clinically relevant numbers of hepatocytes can be recovered from macroscopically normal liver unavoidably removed during hepatic resection for neoplasia. Further, when isolated and subjected to an immunomagnetic cell separation technique, the resulting hepatocytes can be safely trans-planted intra-peritoneally in athymic mice without increased risk of development of tumor. Only patients requiring anatomical resection were included in this study. Our technique may be less useful in the case of patients undergoing small, non-anatomical resection; in this situation the viable hepatocyte yield would be less.
Our mean viable yield of 9.33 × 106/g is a significant improvement from our preliminary study[13] and marginally exceeds that reported by Richert et al[22]. To achieve this, the original isolation protocol was altered to include sealing of cannula points and cut surfaces prior to perfusion and ensure a cold ischemic time of less than 5 h; both of these changes have been independently identified to positively influence viable yield[22,23].
The number of hepatocytes transplanted in clinical experiences to date has ranged between 2 × 108 to 4 × 109 cells[24]. Given a mean viable yield of 9.3 × 108 cells per isolation, our technique offers the possibility of one hepatocyte transplant for every hepatocyte isolation. In the order of 80-100 liver resections are performed annually in our unit, of which approximately half will be suitable for hepatocyte isolation. Thus, a minimum of forty hepatocyte transplants could potentially be performed annually.
A comparison of viable hepatocytes yield from resection specimens versus cells obtained from explanted organs rejected for OLT shows that the resected specimens have a consistently higher viable yield[22]. Further, recovery from cryopreservation of hepatocytes derived from normal resected liver is significantly higher compared to that of cells obtained from organs rejected for liver transplantation[25], thus improving the potential quality and quantity of bankable cells for use on-demand. Such factors, combined with the opportunity to utilize a hitherto untapped hepatocyte source, further enhance the potential application of hepatocyte transplantation as a clinically relevant treatment modality.
An important concern regarding the use of hepatocytes isolated from liver resections performed for malignancy has been the possibility of co-transplanting contaminating tumor cells. Although immunomagnetic cell separation has been widely utilized to enhance detection of tumor cells in different body compartments, purging has been mainly limited to ex vivo removal of tumor cells from autologous stem cell transplants[18,19,26,27]. To our knowledge, our centre is the first to propose immunomagnetic purging of any residual, contaminating tumor cells from isolated hepatocyte suspensions.
The Ep-CAM cell-surface antigen is consistently present on both HT-29 colorectal cancer cells and most colorectal metastases[28], the pathology in the majority of our liver resection specimens in this study. The antigen is not expressed by mature hepatocytes[20] making differential separation of Ep-CAM-expressing tumor cells by immunomagnetic beads (coated with Ber-EP4) possible. Various other carcinomas, including all the types from our patient cohort, also express Ep-CAM[29]. As Ep-CAM has been shown consistently to be absent in hepatocellular carcinoma, patients with such tumors, along with other Ep-CAM-negative lesions, were excluded from our study to avoid undetectable tumor contamination. Whilst hepatocytes were harvested only from patients whose tumors expressed Ep-CAM in this study, the targeting of additional molecular markers such as CK-20 and CEA enhanced detection of any potential tumour cells without an Ep-CAM phenotype[30,31]. Additional surface antigens are currently being studied to potentially increase the tumor-purging efficacy by multiplying the number of target molecules per cell.
In our study, immunomagnetic purging was shown to remove tumor cells by a factor greater than 400 000. This compares favorably with similar large-scale experiments involving breast cancer cells in blood stem cell harvests[32]. It also represents a substantial improvement on our preliminary experience[13], attributable to optimization of the sample purging treatment and bead to cell ratio employed. The development of an RT-PCR detection assay, in addition to standard immunohistochemical methods, has enabled a more efficient and sensitive detection of tumor contamination (1 tumor cell per 1 million hepatocytes) enabling confidence that in a typical human hepatocyte transplant of 1 billion cells, a maximum tumor load of no more than 1000 cells could be present in the preparation. This is significantly below the 100 000-500 000 cell threshold for tumor engraftment and growth demonstrated in our athymic mouse model in this study. Further, no additional growth potential was conferred to tumor cells by the co-transplantation of hepatocytes, an important observation that indicates that the presence of hepatocytes does not magnify the risk of tumor cell engraftment.
We have demonstrated that with the use of an optimized cell-isolation protocol, liver resection specimens obtained from patients undergoing resection for neoplasia can offer sufficient viable hepatocytes to potentially provide clinically-relevant liver support. We have further shown both in vitro and in a suitable in vivo animal model that immunomagnetic purging can confer safety from the potential of tumor contamination of hepatocyte suspensions. We therefore propose that liver resection specimens, by a simple purging step, may provide a safe, alternative hepatocyte source for clinical transplantation.
With a world wide shortage of liver donor organs, adjunct treatment regimes to support patients to orthotopic liver transplantation (OLT) or to replace when OLT is contra-indicated are becoming increasingly important. Hepatocyte transplantation is one such, however, a major limitation to its clinical application is the availability of primary human hepatocytes. Hepatocytes isolated from macroscopically normal liver removed during hepatic resection for neoplasia could provide an additional source of hepatocytes, given the development of strategies to detect and remove residual malignant cells.
The liver margins of neoplasia patients, an increasingly common procedure, are normally discarded. The aim was to discover whether clinically relevant numbers of healthy hepatocytes could be recovered from these waste pieces and used for transplantation, thereby adding another source to the traditional sources of hepatocytes.
It was found that clinically relevant numbers of hepatocytes can be recovered from macroscopically normal liver removed during neoplasia hepatic resection.A protocol based on current immunomagnetic bead technology was developed to capture and remove cancerous cells from hepatocytes in concert with a novel RT-PCR assay for detection of the tumour cells.
An additional source of hepatocytes for transplantation boosts both ongoing research into the efficacy of hepatocyte transplantation and clinically, increases the number of treatable patients. Additionally, hepatocytes prepared correctly can be stored until required, divided amongst multiple patients, or combined with hepatocytes from other donors, increasing further the number of patients that can be treated.
The authors evaluated the efficacy of Ep-CAM-antibody-coated magnetic beads in tumor cell removal from hepatocyte suspensions. This is an interesting work that normal liver resected for neoplasia may be potential as another clinically useful source of hepatocytes for transplantation.
Peer reviewer: Ana J Coito, Associate Professor, Department of Surgery, The Dumont UCLA Transplant Center, 77-120 CHS BOX 90095-7054, Los Angeles 90095, United States
S- Editor Zhong XY L- Editor Li M E- Editor Lin YP
| 1. | Eguchi S, Rozga J, Lebow LT, Chen SC, Wang CC, Rosenthal R, Fogli L, Hewitt WR, Middleton Y, Demetriou AA. Treatment of hypercholesterolemia in the Watanabe rabbit using allogeneic hepatocellular transplantation under a regeneration stimulus. Transplantation. 1996;62:588-593. |
| 2. | Moscioni AD, Rozga J, Chen S, Naim A, Scott HS, Demetriou AA. Long-term correction of albumin levels in the Nagase analbuminemic rat: repopulation of the liver by transplanted normal hepatocytes under a regeneration response. Cell Transplant. 1996;5:499-503. |
| 3. | Kocken JM, Borel Rinkes IH, Bijma AM, de Roos WK, Bouwman E, Terpstra OT, Sinaasappel M. Correction of an inborn error of metabolism by intraportal hepatocyte transplantation in a dog model. Transplantation. 1996;62:358-364. |
| 4. | Vogels BA, Maas MA, Bosma A, Chamuleau RA. Significant improvement of survival by intrasplenic hepatocyte transplantation in totally hepatectomized rats. Cell Transplant. 1996;5:369-378. |
| 5. | Sutherland DE, Numata M, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation in acute liver failure. Surgery. 1977;82:124-132. |
| 6. | Sommer BG, Sutherland DE, Matas AJ, Simmons RL, Najarian JS. Hepatocellular transplantation for treatment of D-galactosamine-induced acute liver failure in rats. Transplant Proc. 1979;11:578-584. |
| 7. | Eguchi S, Lilja H, Hewitt WR, Middleton Y, Demetriou AA, Rozga J. Loss and recovery of liver regeneration in rats with fulminant hepatic failure. J Surg Res. 1997;72:112-122. |
| 8. | Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, McGavran L, Ostrowska A, Durham J. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000;6:32-40. |
| 9. | Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951-952. |
| 10. | Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559-569. |
| 11. | Strom SC, Chowdhury JR, Fox IJ. Hepatocyte trans-plantation for the treatment of human disease. Semin Liver Dis. 1999;19:39-48. |
| 12. | Baccarani U, Sanna A, Cariani A, Sainz-Barriga M, Adani GL, Zambito AM, Piccolo G, Risaliti A, Nanni-Costa A, Ridolfi L. Isolation of human hepatocytes from livers rejected for liver transplantation on a national basis: results of a 2-year experience. Liver Transpl. 2003;9:506-512. |
| 13. | Haghighi KS, Woon WW, Akhter J, Marr PJ, Bolton E, Riordan S, Morris DL. A new source of hepatocytes for transplantation. Transplant Proc. 2004;36:2466-2468. |
| 14. | Mitry RR, Hughes RD, Aw MM, Terry C, Mieli-Vergani G, Girlanda R, Muiesan P, Rela M, Heaton ND, Dhawan A. Human hepatocyte isolation and relationship of cell viability to early graft function. Cell Transplant. 2003;12:69-74. |
| 15. | Alexandre E, Cahn M, Abadie-Viollon C, Meyer N, Heyd B, Mantion G, Cinqualbre J, David P, Jaeck D, Richert L. Influence of pre-, intra- and post-operative parameters of donor liver on the outcome of isolated human hepatocytes. Cell Tissue Bank. 2002;3:223-233. |
| 16. | Ringe B, Braun F, Moritz M, Zeldin G, Soriano H, Meyers W. Safety and efficacy of living donor liver preservation with HTK solution. Transplant Proc. 2005;37:316-319. |
| 17. | Seglen PO. Preparation of rat liver cells. I. Effect of Ca 2+ on enzymatic dispersion of isolated, perfused liver. Exp Cell Res. 1972;74:450-454. |
| 18. | Kielhorn E, Schofield K, Rimm DL. Use of magnetic enrichment for detection of carcinoma cells in fluid specimens. Cancer. 2002;94:205-211. |
| 19. | Flatmark K, Bjornland K, Johannessen HO, Hegstad E, Rosales R, Harklau L, Solhaug JH, Faye RS, Soreide O, Fodstad O. Immunomagnetic detection of micrometastatic cells in bone marrow of colorectal cancer patients. Clin Cancer Res. 2002;8:444-449. |
| 20. | de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999;188:201-206. |
| 21. | Sakaguchi M, Virmani AK, Ashfaq R, Rogers TE, Rathi A, Liu Y, Kodagoda D, Cunningham HT, Gazdar AF. Development of a sensitive, specific reverse transcriptase polymerase chain reaction-based assay for epithelial tumour cells in effusions. Br J Cancer. 1999;79:416-422. |
| 22. | Richert L, Alexandre E, Lloyd T, Orr S, Viollon-Abadie C, Patel R, Kingston S, Berry D, Dennison A, Heyd B. Tissue collection, transport and isolation procedures required to optimize human hepatocyte isolation from waste liver surgical resections. A multilaboratory study. Liver Int. 2004;24:371-378. |
| 23. | LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, Richert L. Isolation and culture of primary human hepatocytes. Methods Mol Biol. 2005;290:207-229. |
| 24. | Fox IJ, Chowdhury JR. Hepatocyte transplantation. Am J Transplant. 2004;4 Suppl 6:7-13. |
| 25. | Terry C, Mitry RR, Lehec SC, Muiesan P, Rela M, Heaton ND, Hughes RD, Dhawan A. The effects of cryopreservation on human hepatocytes obtained from different sources of liver tissue. Cell Transplant. 2005;14:585-594. |
| 26. | Zborowski M, Chalmers JJ. Magnetic cell sorting. Methods Mol Biol. 2005;295:291-300. |
| 27. | Pedrazzoli P, Lanza A, Battaglia M, Da Prada GA, Zambelli A, Perotti C, Ponchio L, Salvaneschi L, Robustelli della Cuna G. Negative immunomagnetic purging of peripheral blood stem cell harvests from breast carcinoma patients reduces tumor cell contamination while not affecting hematopoietic recovery. Cancer. 2000;88:2758-2765. |
| 28. | Shetye J, Frodin JE, Christensson B, Grant C, Jacobsson B, Sundelius S, Sylven M, Biberfeld P, Mellstedt H. Immunohistochemical monitoring of metastatic colorectal carcinoma in patients treated with monoclonal antibodies (MAb 17-1A). Cancer Immunol Immunother. 1988;27:154-162. |
| 29. | Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM). J Mol Med. 1999;77:699-712. |
| 30. | Chausovsky G, Luchansky M, Figer A, Shapira J, Gottfried M, Novis B, Bogelman G, Zemer R, Zimlichman S, Klein A. Expression of cytokeratin 20 in the blood of patients with disseminated carcinoma of the pancreas, colon, stomach, and lung. Cancer. 1999;86:2398-2405. |
| 31. | Martin-Henao GA, Picon M, Limon A, Carmona M, Amill B, Azqueta C, Lopez R, Gonzalez-Barca E, Granena A, Brunet S. Immunomagnetic bone marrow (BM) and peripheral blood progenitor cell (PBPC) purging in follicular lymphoma (FL). Bone Marrow Transplant. 1999;23:579-587. |
| 32. | Tyer CL, Vredenburgh JJ, Heimer M, Peters WP, Bast RC Jr. Breast cancer cells are effectively purged from peripheral blood progenitor cells with an immunomagnetic technique. Clin Cancer Res. 1996;2:81-86. |