Published online Aug 21, 2008. doi: 10.3748/wjg.14.4938
Revised: May 2, 2008
Accepted: May 9, 2008
Published online: August 21, 2008
AIM: To investigate the feasibility of compression anastomosis clip (CAC) for gastrointestinal anastomosis proximal to the ileocecal junction.
METHODS: Sixty-six patients undergoing gastrointe-stinal anastomosis proximal to the ileocecal junction were randomized into two groups according to the anastomotic method, CAC or stapler.
RESULTS: The postoperative recovery of patients in CAC and stapled anastomosis groups was similar. No postoperative complication related to the anastomotic method was found in either group. Both upper gastrointestinal contrast radiography at the early postoperative course and endoscopic examination after a 6-mo follow-up showed a better healing at the compression anastomosis.
CONCLUSION: CAC can be used not only in colonic surgery but also in gastrointestinal anastomosis. Our result strongly suggests that CAC anastomosis is safe in various complication circumstances. However, it should be further confirmed with a larger patient sample.
- Citation: Liu PC, Jiang ZW, Zhu XL, Wang ZM, Diao YQ, Li N, Li JS. Compression anastomosis clip for gastrointestinal anastomosis. World J Gastroenterol 2008; 14(31): 4938-4942
- URL: https://www.wjgnet.com/1007-9327/full/v14/i31/4938.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4938
| Study group (n = 33) | Control group (n = 33) | |
| Sex (M:F) | 24:9 | 21:12 |
| Age (yr) | 57.0 ± 11.85 | 58.6 ± 13.23 |
| Height (cm) | 167.7 ± 7.54 | 166.4 ± 8.59 |
| Body weight (kg) | 61.7 ± 10.92 | 62.7 ± 7.49 |
| BMI (kg/m2) | 21.9 ± 3.13 | 22.7 ± 3.54 |
| Albumin (g/L) | 41.6 ± 4.44 | 39.9 ± 4.77 |
| Hemoglobin (g/L) | 125.8 ± 17.48 | 118.6 ± 16.34 |
| Prealbumin (mg/L) | 276.1 ± 105.74 | 301.9 ± 100.18 |
| Dignosis | Study group | Control group | Total |
| Malignant | |||
| Gastric cancer | 21 | 24 | 45 |
| Gastric stump cancer | 1 | 0 | 1 |
| Reflux esophagitis | 1 | 0 | 1 |
| Anastomotic stenosis | 1 | 1 | 2 |
| Intestinal tumor | 2 | 0 | 2 |
| Total | 26 | 25 | 51 |
| Benign | |||
| Intestinal adhesion | 3 | 2 | 5 |
| Intestinal tumor | 1 | 1 | 2 |
| Peptic ulcer disease | 1 | 3 | 4 |
| Annular pancreas | 1 | 0 | 1 |
| Inflammatory bowel disease | 1 | 2 | 3 |
| Total | 7 | 8 | 15 |
| Site | Study group | Control group | Total |
| Stomach-small bowel | |||
| Subtotal gastrectomy with a Billroth II reconstruction | 12 | 11 | 23 |
| Gastrojejunostomy | 6 | 3 | 9 |
| Total | 18 | 14 | 32 |
| Small bowel-small bowel | |||
| Total gastrectomy with a Roux-en-Y reconstruction | 9 | 14 | 23 |
| Enteroenterostomy | 6 | 5 | 11 |
| Total | 15 | 19 | 34 |
| Variable | Study group | Control group | P |
| (n = 33) | (n = 33) | ||
| Gases started (d) | 4.3 ± 1.24 | 4.7 ± 1.28 | 0.230 |
| Bowel movement (d) | 5.7 ± 2.36 | 6.2 ± 2.53 | 0.407 |
| Antibiotics stopped (d) | 3.8 ± 0.74 | 4.0 ± 0.83 | 0.258 |
| Duration of nasogastric drainage (d) | 2.6 ± 0.98 | 2.8 ± 0.64 | 0.314 |
| Start of oral intake (d) | 4.8 ± 1.82 | 5.4 ± 1.69 | 0.174 |
| Postoperative hospital duration (d) | 14.9 ± 6.01 | 15.1 ± 8.35 | 0.946 |
Compression anastomosis was first reported in 1826 by Denan, who conceived a sutureless bowel anastomosis that encompassed the inverting technique proposed by Lembert[1]. The idea is to compress two bowel walls together and induce a simultaneous necrosis and healing process leading to the joining of the two lumens. In 1892, Murphy introduced a mechanical device known as “Murphy’s Button”, which has been used for years[2-4].
Additional compression devices, such as magnetic ring, AKA-2 device, and biofragmentable anastomosis ring (BAR) were developed after almost 100 years[5-7]. Although animal experiments and clinical trials have reported the high efficacy and safety of these anastomotic devices, they are not widely accepted because of their narrow inner caliber and high expenses. Finally, these compression anastomotic devices were substituted by staplers which are used routinely at present[3,8].
However, compression anastomosis with no foreign bodies left at the anastomotic site permanently, is considered an ideal anastomotic method. Many surgeons still make effort to improve this compression device. Recently, a new device, compression anastomosis clip (CAC) emerged in 2000, was approved for its use by the FDA[9]. Clinical trials performed in Israeli Medical Center have confirmed its safety and efficacy for colonic anastomosis in laparoscopic procedures[10-12]. However, its application in gastrointestinal anastomosis proximal to the ileocecal junction is limited at an experimental level[9]. This prompts us to study its clinical effects on gastrointestinal anastomosis proximal to the ileocecal junction.
This study was approved by the institutional committee on ethics in clinical trials. Sixty-six patients at the age of 35-83 years, who underwent gastroenterostomy or enteroenterostomy in July 2005 - December 2006, were recruited into the study. Patients with an emergency surgical history or a nickel allergy history were excluded. All participants signed an informed consent form and were randomly divided into a study group or a control group according to the anastomotic method. Randomization was based on computer-generated codes that were maintained in sequentially numbered opaque envelopes. Compression anastomosis clip (CAC) (NiTi Medical Technologies, Netanya, Israel), 30 mm in diameter, was used for anastomosis in the study group (n = 33). In the control group, an anastomosis was performed with a stapler. Bowel preparation, consisting of oral magnesium sulphate, gentamycin, and metronidazole, was performed on the day prior to surgery in all patients except for seven patients with complete intestinal obstruction. There was no statistical difference in preoperative conditions between the two groups (Table 1). The main indication for underlying disease was gastric cancer in both groups (Table 2). One patient in the study group was complicated by postgastrectomy reflux esophagitis due to a previous radical gastrectomy and Brown’s reconstruction. She complained of serious nausea and vomiting after surgery and was readmitted to our hospital for reconstruction. The afferent loop was resected and a side-to-side anastomosis to distal jejunum was performed with CAC. The distribution of anastomotic sites and operative procedures are listed in Table 3. Two anastomoses were performed for the patient who underwent total gastrectomy and Roux-en-Y reconstruction during esophagojejunostomy and jejunojejunostomy. Only the difference in the latter was compared in both groups based on the different anastomotic methods.
The compression anastomosis was completed with CAC which is a shape memory nitinol double ring clip. The hollow viscera to be anastomosed were parallel to each other and two 5-mm incisions were made at both sides. The CAC mounted on a deployment device was cooled in ice-cold water for 2 min before use. After the clip was opened at an angle of approximately 30º, it was inserted into the hollow viscera through the two 5-mm incisions. The device returned to its original closed shape when it was warmed by body temperature and compressed the two visceral walls firmly. Then, the scalpel incorporated in the applier created a slit through the entrapped walls to allow free passage of air and feces through the anastomosis until the healing process ended. Finally, the applier was withdrawn and the two small incisions through which the clip was inserted were sutured. The compression anastomotic procedure was completed. The postoperative parameters were followed in three aspects.
Vital sign, recovery of bowel function, antibiotics application, duration of nasogastric drainage, time to start oral feeding and duration of postoperative hospitalization were recorded. We also recorded the time to complete the anastomosis, intraoperative and postoperative complications in all patients, and the time to expel the ring in the study group.
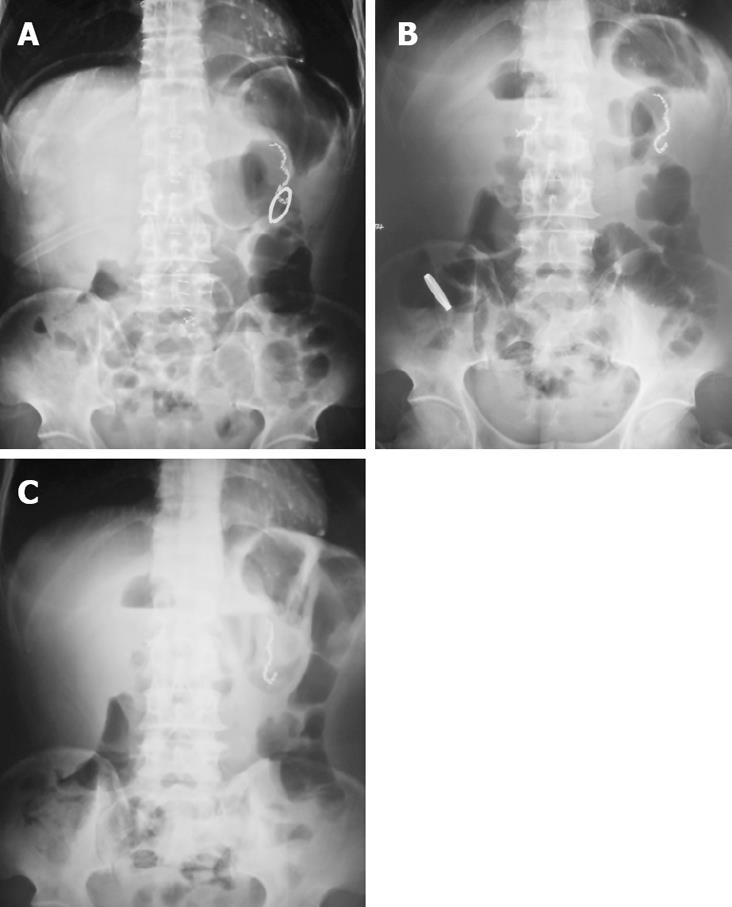
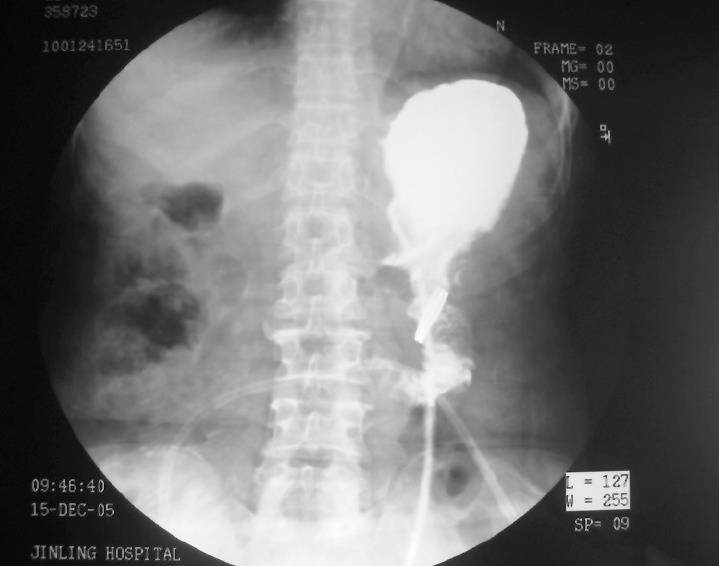
Four days after surgery, patients in the CAC group received abdomen plain X-ray examination to locate the ring in body. In addition, the integrity of anastomosis was examined by upper gastrointestinal contrast radiography with dilute gastrografin 7 d after operation.
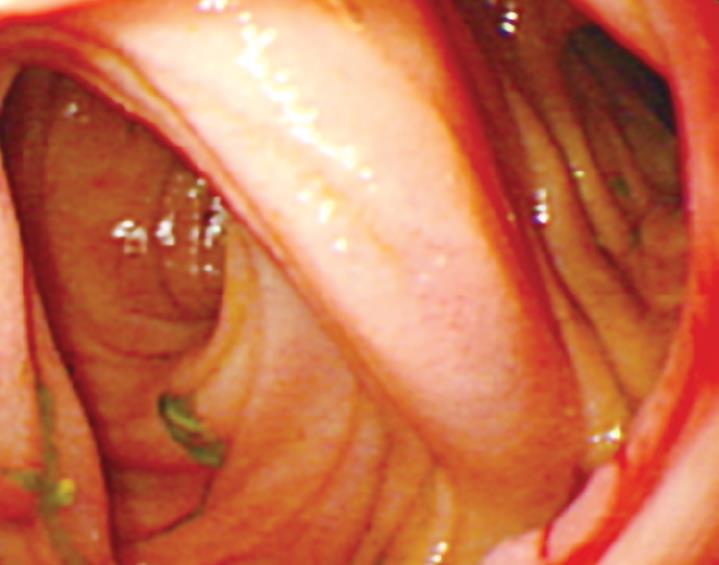
After a six-month follow-up period, endoscopy was performed to evaluate the healing of anastomosis.
Results were expressed as mean ± SD. Differences in means between the two groups were calculated for significance with independent-samples t test using SPSS 10.0. P < 0.05 was considered statistically significant.
All patients recovered from operation uneventfully. The parameters collected during their early postoperative course were similar in both groups (Table 4). No difference was observed in the duration of operation between the two groups (139.4 ± 41.39 min vs 143.2 ± 40.53 min). No intraoperative and postoperative complications were found, including anastomotic leakage, bleeding, and stenosis, which were related to the anastomotic methods. However, postoperative complications unrelated to the anastomosis occurred in two patients with compression anastomosis. One patient who underwent subtotal gastrectomy and Billroth II reconstruction showed delayed emptying of the stomach. The intestinal adherence distant from the compression anastomosis was observed at the second operation. The patient recovered finally. Another gastric cancer patient with pyloric obstruction underwent a palliative gastrojejunostomy and was refed with normal diet gradually. However, the patient’s condition deteriorated suddenly and the patient died on day 35 after operation because of cardiopulmonary decompensation.
In the CAC group, all of the anastomosis clips were expelled with stool except two, and none complained of discomfort or tenesmus. The mean time of ring expulsion was 15.1 ± 6.04 d (5-29 d). One patient who died 35 d after surgery did not expel the ring. The anastomotic ring in another patient with adhesive ileus was taken out by gastroscopy on the 16th postoperative day.
Using the radiological examination, we were able to locate the anastomosis ring in the patient’s body dynamically (Figure 1). The upper gastrointestinal contrast radiography showed that the anastomoses in both groups were intact. No stenosis was observed in all patients, even with the CAC left at the anastomotic site (Figure 2).
After a 6-mo follow-up period, the anastomotic healing was evaluated in all patients by gastroscopy or enteroscopy. No relevant stenosis was detected in either group. All anastomoses showed good healing without scar. The mucosa of compression anastomosis was smooth (Figure 3). However, slight edema and congestion occurred in three of the stapled mucosa.
Compression anastomosis is very close to the sutureless anastomosis in the absence of permanent foreign bodies at the anastomotic site[2]. Although the technique of compression anastomosis was introduced more than 180 years ago and the theoretical advantages were appreciated, the technique has not been accepted as a standard for gastrointestinal anastomosis, which could be explained by its narrow inner caliber, difficult application and assemblage, and high cost[3,8]. It seems that these barriers can be tackled well with the new device, compression anastomosis clip.
The mechanism of CAC is similar to that of Murphy’s button. The walls of hollow organs are placed between coils of the ring, pressed and clamped together. Its continuous pressure creates gradual and controlled necrosis of the tissue in the area limited by the CAC coil’s perimeter while the external edges of this area become sealed, forming a smooth tight homogeneous anastomosis. The device is biofragmented or detached from the anastomotic site and is excreted from the body. The innovation of CAC is the shape memory nitinol double ring that becomes flexible in cool water and resumes its programmed shape when warmed by body temperature.
Technical failure rates of a new technique must be reduced by eliminating the learning curve on a suitable animal model[13]. In our study, no intraoperative problems were observed and no additional supportive measures were needed, suggesting that the increasing experience with our previous animal experiments may significantly shorten the duration of technique learning. The learning process of this new technique is short, indicating that this technique is easy to learn and handle. We also confirmed that CAC could be used not only for colonic anastomosis but also for gastrointestinal anastomosis and enteroenterostomy. In addition, we also performed gastrojejunostomy and enteroenterostomy with CAC for patients with intestinal adhesion, peptic ulcer, or inflammatory bowel disease. Almost all the patients in the CAC group passed the ring without discomfort only with two exceptions. No obstruction due to anastomotic device was observed, indicating that the air and feces are able to pass through the temporary passage of anastomosis during the healing process of anastomosis. The CAC, 30 mm in diameter and 8 mm in inner caliber, could pass through the ileocecal junction successfully.
Anastomotic leakage is a serious postoperative complication of gastrointestinal anastomosis. However, surgical procedure is one of the important factors that affect the healing of anastomosis[14]. Our data are consistent with a parallel study in Israel[10-12]. No anastomotic leakage was observed in our study, which may be partially explained by a constant stress plateau, which is exerted by coils at about 400 Mpa and up to 6% perfect recoverable strain[8]. The constant pressure makes the ring detached from the anastomotic site at an appropriate time. The unique physical properties of nitinol, mostly the constancy of stress, enable the device to exert a relatively constant pressure irrespective of the bowel wall thickness.
Ischemia, inflammation, and fibrosis due to anastomosis leakage are the factors for anastomotic stenosis[15,16]. No sign of anastomotic stenosis at compression anastomotic site was observed either in upper gastrointestinal contrast radiography early postoperatively or in the endoscopy after a long- term follow-up period. The compression anastomosis showed a smooth and intact healing. In contrast, edema and congestion occurred in three of the stapled anastomoses. These discrepancies may be explained by the following. First, the absence of foreign bodies at CAC anastomotic site may greatly decrease inflammatory stimuli and formation of fibrous tissue. Second, the compression anastomotic diameter is determined by the outer diameter of the CAC device, as opposed to staplers, which is determined by its inner diameter. Thus, the CAC is capable of creating a larger anastomosis using a small device. Finally, the raw surface at the edge of the stapler line after firing may also increase the possibility of stricture.
The mean time of expulsion of anastomosis clip was 15.1 ± 6.04 d after surgery. However, significant variations were found in different individuals. Half of the patients in the CAC group expelled the anastomotic clip in about 2 wk. The patients who passed the ring within about 1 wk were always younger and more likely to have benign diseases. They recovered from surgical stress faster and started activities earlier. In contrast, the elder patients with malignant disease often had complex conditions and malnutrition before surgery. They were bed-ridden for a longer time after surgery and took a longer time to expel the anastomotic ring, suggesting that many factors including anastomotic healing, enterokinesia, and distance of pathway, etc, can affect the duration of the ring expelling. Therefore, we hold that the duration for the ring to detach from the anastomotic site is more important. Further study is needed to confirm our findings.
The characteristics of postoperative recovery were similar in patients with CAC or stapled anastomosis. The anastomotic device did not influence the normal diet intake. None of the patients complained of abdominal pain due to dietary. However, Thiede and colleagues conducted a multicenter prospective trial of biofragmentable anastomosis ring and found that a low fiber diet until the evacuation of ring fragments from the bowel could prevent obstruction with no discomfort[16].
The recovery course of 7 patients with a compression anastomosis who had complete intestinal obstruction before surgery, was uneventful and similar to those without complete intestinal obstruction before operation, which might be related to the characteristics of the CAC. When a CAC anastomosis was performed, the coils were inserted into the bowel lumens that were to be anastomosed through the small incisions and the compression anastomosis was completed after closure of the incisions. However, performing a stapled anastomosis needed a relatively larger surgical field and had more chances to contaminate the abdominal cavity when the applier was withdrawn. In addition, application of the CAC may simplify the gastrointestinal bypass operation and shorten the operative time. Therefore, surgical injury to the patient could be minimized and the bowel function could recover earlier. Moreover, previous studies demonstrated that persistent foreign bodies may be responsible for anastomotic recurrence of cancer and inflammatory bowel disease and affect radiological examination[17,18]. Although no recurrence at anastomotic site was found in our study during the 6-mo follow-up period, further study is needed.
In conclusion, CAC is an alternative method for gastrointestinal anastomosis under different disease circumstances. Further study with a larger patient sample and a longer follow-up period is needed to confirm our findings before CAC application in emergency and laparoscopic surgery.
Gastrointestinal anastomosis is a common surgical procedure in abdominal surgery. Compression anastomosis, which was first reported in 1826 by Denan, is one of the methods. Because there is no foreign body left at the anastomotic site permanently, it is considered an ideal anastomotic method. However, the compression anastomotic devices have been substituted by staplers due to their narrow inner caliber and high cost.
Recently, a new device, compression anastomosis clip (CAC), which was emerged in 2000, was approved by the FDA. The mechanism of CAC is similar to that of Murphy’s button. The innovation of CAC is the shape memory nitinol double ring that becomes flexible in cool water and resumes its programmed shape when warmed by body temperature. Clinical trials performed in Israeli Medical Center confirmed the safety and efficacy of CAC for colonic anastomosis in laparoscopic procedures.
The application of CAC in gastrointestinal anastomosis proximal to the ileocecal junction was evaluated in this study. Besides, it can also be applied in selected patients who suffered from inflammatory bowel disease, peptic ulcer disease and intestinal obstruction, etc.
CAC can be used for gastrointestinal anastomosis proximal to the ileocecal junction.
This study elucidated the safety of CAC for gastrointestinal anastomosis under different disease circumstances, which may be an alternative method for abdominal surgery and decrease the anastomotic recurrence of cancer and inflammatory bowel disease. The study was well designed and the findings were reliable and significant.
Peer reviewer: Dr. Kalpesh Jani, Sigma, Abhishek House 102, Opp Tulsidham Appt, Manjalpur, Vadodara 390011, India
S- Editor Zhong XY L- Editor Wang XL E- Editor Ma WH
| 1. | Amat C. Appareils a sutures: les viroles de Denans; les points de Bonnier; les boutons de Murphy. Arch Med Pharmacie Millitaires Paris. 1985;25:273-285. |
| 2. | Wullstein C, Gross E. Compression anastomosis (AKA-2) in colorectal surgery: results in 442 consecutive patients. Br J Surg. 2000;87:1071-1075. |
| 3. | Rosati R, Rebuffat C, Pezzuoli G. A new mechanical device for circular compression anastomosis. Preliminary results of animal and clinical experimentation. Ann Surg. 1988;207:245-252. |
| 4. | Hardy TG Jr, Pace WG, Maney JW, Katz AR, Kaganov AL. A biofragmentable ring for sutureless bowel anastomosis. An experimental study. Dis Colon Rectum. 1985;28:484-490. |
| 5. | Jansen A, Brummelkamp WH, Davies GA, Klopper PJ, Keeman JN. Clinical applications of magnetic rings in colorectal anastomosis. Surg Gynecol Obstet. 1981;153:537-545. |
| 6. | Cahill CJ, Betzler M, Gruwez JA, Jeekel J, Patel JC, Zederfeldt B. Sutureless large bowel anastomosis: European experience with the biofragmentable anastomosis ring. Br J Surg. 1989;76:344-347. |
| 7. | Lifshitz D, Michowitz M, Raban M, Jellin A, Lelcuk S. Biofragmentable anastomotic ring (BAR) in colonic surgery: a prospective randomized clinical study. Coloproctolo. 1993;5:306-310. |
| 8. | Song C, Frank T, Cuschieri A. Shape memory alloy clip for compression colonic anastomosis. J Biomech Eng. 2005;127:351-354. |
| 9. | Nudelman IL, Fuko VV, Morgenstern S, Giler S, Lelcuk S. Gastrointestinal anastomosis with the nickel-titanium double ring. World J Surg. 2000;24:874-877. |
| 10. | Nudelman IL, Fuko V, Greif F, Lelcuk S. Colonic anastomosis with the nickel-titanium temperature-dependent memory-shape device. Am J Surg. 2002;183:697-701. |
| 11. | Nudelman I, Fuko V, Rubin M, Lelcuk S. A nickel-titanium memory-shape device for colonic anastomosis in laparoscopic surgery. Surg Endosc. 2004;18:1085-1089. |
| 12. | Nudelman I, Fuko V, Waserberg N, Niv Y, Rubin M, Szold A, Lelcuk S. Colonic anastomosis performed with a memory-shaped device. Am J Surg. 2005;190:434-438. |
| 13. | Aggarwal R, Darzi A. Compression anastomoses revisited. J Am Coll Surg. 2005;201:965-971. |
| 14. | Fielding LP, Stewart-Brown S, Blesovsky L, Kearney G. Anastomotic integrity after operations for large-bowel cancer: a multicentre study. Br Med J. 1980;281:411-414. |
| 15. | Chung RS, Hitch DC, Armstrong DN. The role of tissue ischemia in the pathogenesis of anastomotic stricture. Surgery. 1988;104:824-829. |
| 16. | Thiede A, Geiger D, Dietz UA, Debus ES, Engemann R, Lexer GC, Lunstedt B, Mokros W. Overview on compression anastomoses: biofragmentable anastomosis ring multicenter prospective trial of 1666 anastomoses. World J Surg. 1998;22:78-86; discussion 87. |