Published online Aug 21, 2008. doi: 10.3748/wjg.14.4880
Revised: August 12, 2008
Accepted: August 17, 2008
Published online: August 21, 2008
AIM: To investigate the clinical significance of BMP and activin membrane-bound inhibitor (BAMBI) which is a pseudoreceptor of transforming growth factor-beta (TGF-β) type I receptors and acts as a negative regulator of TGF-β signaling and expression aberrantly elevated in colorectal cancers (CRCs). We studied BAMBI expression in CRCs.
METHODS: We studied BAMBI expression in 183 surgically resected CRCs by immunochemical and immunoblotting analyses using a generated monoclonal anti-BAMBI antibody. Commercially available anti-β-catenin and anti-p53 antibodies were also applied for immunochemical analyses as a comparison control.
RESULTS: Immunohistochemical analysis revealed that BAMBI expression was observed in 148 (80.8%), and strong BAMBI expression was observed in 46% of the CRCs. Strong BAMBI expression was positively correlated with histological type, depth of invasion, lymph node metastases, and tumor node metastasis (TNM) stage (P < 0.05). Clear associations were found between BAMBI and β-catenin (P = 0.035) and p53 (P = 0.049) expression. In curatively resected CRC, 5-year recurrence-free survival was 51.9% (P = 0.037) for strong BAMBI expression compared to 79.8% for weak BAMBI expression. In the Cox’s multivariate analysis, lymph node metastases (RR 6.685; P < 0.001) and depth of invasion (RR 14.0; P = 0.013) were significant indicators for recurrence, and strong BAMBI expression (RR 2.26; P = 0.057) tended to be significant.
CONCLUSION: BAMBI was linked to a potentially aggressive tumor phenotype and predicted tumor recurrence and cancer-related death in CRC. BAMBI expression might be applicable in the routine clinical setting of CRC.
- Citation: Togo N, Ohwada S, Sakurai S, Toya H, Sakamoto I, Yamada T, Nakano T, Muroya K, Takeyoshi I, Nakajima T, Sekiya T, Yamazumi Y, Nakamura T, Akiyama T. Prognostic significance of BMP and activin membrane-bound inhibitor in colorectal cancer. World J Gastroenterol 2008; 14(31): 4880-4888
- URL: https://www.wjgnet.com/1007-9327/full/v14/i31/4880.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4880
| Characteristic | Category | Data | |
| Age | Mean (yr) | 66 | |
| Range | 27-95 | ||
| Gender | Male | n = 115 | 62.8% |
| Female | n = 68 | 37.2% | |
| Location1 | Right side | 61 | 33.3 |
| Left side | 52 | 28.4 | |
| Rectum | 70 | 38.3 | |
| Tumor size (mm) | Mean | 45.1 | |
| Range | 10-110 | ||
| ≤ 39 | 74 | 40.4 | |
| 40-59 | 62 | 33.9 | |
| ≥ 60 | 47 | 25.7 | |
| Depth of invasion2 | T0 | 7 | 3.8 |
| T1 | 18 | 9.9 | |
| T2 | 22 | 12.0 | |
| T3 | 129 | 70.5 | |
| T4 | 7 | 3.8 | |
| Histology3 | G1 | 51 | 27.9 |
| G2 | 116 | 63.4 | |
| G3 | 16 | 8.7 | |
| TNM stage | I | 40 | 21.8 |
| II | 51 | 27.9 | |
| III | 53 | 29.0 | |
| IV | 39 | 21.3 |
| BAMBI expression | ||||||
| Weak | Strong | P1 | ||||
| n | % | n | % | |||
| Gender | Male | 41 | 26.1 | 11 | 7.1 | |
| Female | 69 | 43.9 | 36 | 22.9 | NS | |
| Age | 27-59 | 60 | 38.2 | 24 | 15.4 | |
| 60-95 | 50 | 31.8 | 23 | 14.6 | NS | |
| Location | Right side | 37 | 23.6 | 12 | 7.6 | |
| Left side | 27 | 17.2 | 16 | 10.2 | ||
| Rectum | 46 | 29.3 | 19 | 12.1 | NS | |
| Tumor size (mm) | ≤ 39 | 50 | 31.8 | 19 | 12.1 | |
| 40-59 | 35 | 22.3 | 15 | 9.6 | ||
| ≥ 60 | 25 | 15.9 | 13 | 8.3 | NS | |
| Depth of invasion | Tis, T1, T2 | 38 | 24.2 | 9 | 5.7 | |
| T3, T4 | 72 | 45.9 | 38 | 24.2 | 0.054 | |
| Histology | G1 | 36 | 22.9 | 9 | 5.7 | |
| G2 | 67 | 42.7 | 32 | 20.4 | ||
| G3 | 7 | 4.5 | 6 | 3.8 | 0.043 | |
| LN metastases2 | No | 59 | 37.6 | 22 | 14.0 | |
| Yes | 51 | 32.5 | 25 | 15.9 | NS | |
| TNM stage | I | 33 | 21.0 | 7 | 4.5 | |
| II | 35 | 22.3 | 16 | 10.2 | ||
| III | 35 | 22.3 | 18 | 11.4 | ||
| IV | 7 | 4.5 | 6 | 3.8 | 0.035 | |
| Metastatic site | ||||||
| Liver | No | 2 | 28.6 | 5 | 83.3 | |
| Yes | 5 | 71.4 | 1 | 16.7 | NS | |
| Lung | No | 6 | 85.7 | 6 | 100 | |
| Yes | 1 | 14.3 | 0 | 0 | NS | |
| Distant lymph nodes | No | 7 | 100 | 6 | 100 | |
| Yes | 0 | 0 | 0 | 0 | NS | |
| Characteristics | Category | Univariate analysis | Multivariate analysis | ||||
| Hazard ratio | P | 95% CI | Hazard ratio | P | 95% CI | ||
| Depth of invasion | Tis, T1, T2/T3, T4 | 14.773 | 0.008 | 2.011-108.548 | 14.008 | 0.013 | 1.741-112.694 |
| LN metastases | Yes/No | 8.937 | < 0.001 | 3.100-25.768 | 6.685 | < 0.001 | 2.242-19.927 |
| TNM stage | 0/I/II/III/IV | 4.633 | < 0.001 | 2.838-7.563 | |||
| BAMBI | Weak/Strong | 2.109 | 0.041 | 1.029-4.324 | 2.265 | 0.057 | 0.952-5.385 |
| β-Catenin | Low/High | 1.944 | 0.108 | 0.864-4.377 | |||
| p53 | Negative/Positive | 1.702 | 0.147 | 0.830-3.489 | 0.937 | 0.871 | 0.427-2.057 |
Activation of the Wnt/β-catenin pathway is a critical process in the malignant transformation of colonic epithelium[1-5]. Wnt signaling promotes the stabilization and accumulation of β-catenin, which in turn interacts with the T cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors and activates the transcription of downstream genes such as c-Myc, cyclin D1, and Axin2[6-10]. Constitutive activation of β-catenin-TCF-mediated transcription is believed to be a critical step in the tumorigenesis of various cancers[11-13]. Conversely, the transforming growth factor-beta (TGF-β) pathway participates in tumor suppressor activities such as growth inhibition and apoptosis, and this negative regulation is lost during colorectal carcinogenesis, with mutations in the type II receptor, SMAD2, and SMAD4[14-17]. TGF-β also mediates tumor-promoting effects such as growth stimulation, as well as increases in motility, invasion, and metastasis through either differential effects on tumor and stromal cells or a fundamental alteration in the TGF-β responsiveness of the tumor cells themselves[18-20].
The TGF-β and Wnt/β-catenin signaling pathways cross talk to regulate tumor biology[20]. Axin, a negative regulator of the Wnt pathway, activates TGF-β signaling through Smad3 binding[21], while TCF/LEF interacts directly with Smad3 to regulate the expression of their target genes[22]. It is also known that β-catenin and TCF/LEF both interact directly with Smad4 to regulate target genes during development[23].
BMP and activin membrane-bound inhibitor (BAMBI) is a transmembrane glycoprotein induced by BMP signaling[24] that is related to TGF-β-type I receptors, but lacks an intracellular kinase domain[25]. BAMBI inhibits TGF-β signaling by forming a heterodimer with TGF-β-type II receptors[25]. Previously, we found that both Wnt/β-catenin signaling and TGF-β signaling activate transcription of BAMBI and that BAMBI expression is aberrantly elevated in most colorectal cancers (CRCs)[26]. To analyze the clinical significance of BAMBI, we studied its expression in CRC using immunohistochemical staining. We show that BAMBI overexpression is correlated with aggressive tumor phenotypes and predicts tumor recurrence and cancer-related death in CRC. BAMBI may be usable as a target for diagnostic and antibody medicine.
Colorectal tumor tissues were obtained from 183 consecutive patients who underwent to surgical resection between January 1995 and July 2006 at Gunma University Hospital. All of the patients underwent to radical colorectal resection intended to obtain clear pathological margins and regional lymphadenectomy, even in Stage IV. The clinicopathological features of the patients are shown in Table 1. The subjects were 115 males and 68 females with a mean age of 66 years (range 27-95). Of these, 113 were colon and 70 were rectal cancers. The tumor tissues were staged pathologically according to the American Joint Committee on Cancer Tumor-Node-Metastasis (TNM) classification. The tumors were categorized according to the World Health Organization (WHO) classification as well differentiated (G1; 51 cases, 27.9%), moderate (G2; 116 cases, 63.4%), and poor (G3; 16 cases, 8.7%).
Anti-BAMBI antibodies were generated by immunizing mice with a fragment comprising either amino acids 45-147 or 177-241. Known methods were used to prepare the anti-BAMBI antibody, collect antibody-producing cells, obtain cell fusion, select and clone hybridomas, collect and purify monoclonal antibodies[26]. Twenty-six mouse monoclonal anti-BAMBI antibodies were generated at the first screening. To check the utility of immunostaining for paraffin sections, these monoclonal antibodies were further screened using various immunochemistry methods, using formalin-fixed and paraffin-embedded colon cancers. Finally, one monoclonal antibody was selected and its specificity was confirmed in an absorbed test using excess GST-BAMBI protein and in the immunoblotting analysis[26].
Frozen samples (25 CRCs and 5 tubular adenomas were picked at random) kept at -80°C were thawed, cut into small pieces, and homogenized in SDS lysis buffer (Sigma-Aldrich, St. Louis, MO). The homogenate was then centrifuged at 10 000 g for 15 min at 4°C, and the protein concentration of the supernatant was estimated using the BCA protein assay kit (Pierce, Rockford, IL). Twenty micrograms of protein from each supernatant was electrophoresed on 7.5% SDS polyacrylamide gel under reducing conditions and electrotransferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA). After treatment with blocking solution, the membrane was incubated with anti-BAMBI antibody (dilution 1:1000) overnight at 4°C. The membrane was then treated with goat anti-mouse IgG conjugated with horseradish peroxidase (Dako, Carpentaria, CA, dilution 1:200 000) for 1 h at room temperature, and the enhanced chemiluminescence kit (ECL Advance detection kit, Amersham, Piscataway, NJ) was used for development according to the manufacturer’s instructions. The luminescence image was captured and digitized using Lumi Analyst 3.0v (Boehringer Mannheim GmbH, Germany), and the integrated densities of each band were quantified using densitometry software (ImageJ 1.37v, National Institutes of Health, Bethesda, MD). To examine the equality of protein loading, the total amount of protein loaded in each lane was examined by staining with Coomassie blue.
For the immunohistochemical study, 3 μm thick sections were cut from formalin-fixed, paraffin-embedded tissue blocks containing representative tumor histology. The sections were de-waxed in xylene, rehydrated with graded ethanol, and subjected to an antigen retrieval step. For antigen retrieval, the slides for β-catenin and p53 were placed in 0.01 mol/L citrate buffer, 20% ZnSO4 solution, and heated in a microwave processor (Energy Beam Science, East Granby, CT) at 95°C for 20 min. They were then cooled and rinsed in 0.01 mol/L phosphate-buffered saline (PBS), pH 7.4. The antigen retrieval was not performed on the immunohistochemistry for BAMBI. The slides were then reduced with nonspecific antibody binding by incubating the sections with 10% normal serum in PBS for 30 min at room temperature. After decanting the excess serum, the sections were incubated overnight at 4°C with each monoclonal antibody for BAMBI (1:2000 dilution), β-catenin (1:1600 dilution), and p53 (1:50 dilution) in humidified chambers. After washing thoroughly with PBS, the sections were set in a Ventana Nexus Automated Stainer (Ventana Medical Systems, Tucson, AZ) for immunostaining. The automated protocol was performed according to the manufacturer’s instructions, which were based on the labeled streptavidin–biotin method and used the I-VIEW DAB universal kit (Ventana Medical Systems); the kit included a universal biotinylated IgG secondary antibody (anti-mouse), avidin horseradish peroxidase, DAB, 0.03% H2O2, and 0.5% CuSO4. After immunostaining, the sections were lightly counterstained with hematoxylin, and then mounted in a carousel inside the staining module and run to completion. To standardize and confirm the immunohistochemical evaluation of BAMBI, 26 samples of colon carcinoma with tubular adenoma were collected and were tentatively subjected to immunohistochemistry using paraffin sections. Immunohistochemical staining intensity was categorized blindly by two pathologists (T.N, S.S). Most tubular adenomas showed weak immunoreactivity compared to the cancer component.
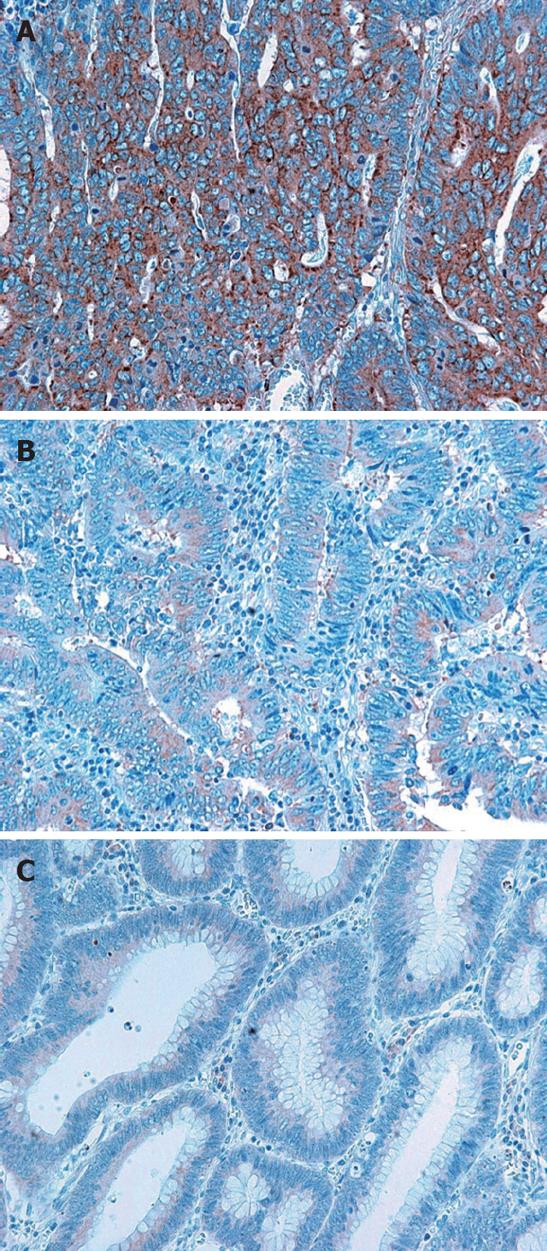
Therefore, diffuse BAMBI immunoreactivity, which was observed in the cytoplasm and cell membrane, but not in the nucleus, was evaluated as weak expression if the positivity was similar to that of tubular adenomas. If the BAMBI immunoreactivity was far stronger than that of tubular adenomas, it was evaluated as strong expression (Figures 1 and 2). No BAMBI immunoreactivity was evaluated as negative.
The intensity of nuclear β-catenin immunostaining was graded semiquantitatively into four categories: negative, weakly positive, moderately positive, and strongly positive[27]. This evaluation was grouped into two categories: low expression (negative and weakly positive) and high expression (moderately to strongly positive). To evaluate the p53 immunostaining, the nuclear immunoreactivity for p53 was counted in five random tumor areas at high magnification (× 400), and the average percentage of these positive cells was calculated. The p53 immunoreactivity was evaluated as negative or positive expression if the percentage of nuclear positivity was less than or more than 70%, respectively[28].
Statistical analysis was performed using SPSS 11.5 software (SPSS Japan, Tokyo, Japan). The Mann-Whitney U-test, Kruskal-Wallis test, and Spearman’s coefficient of rank correlation were used to examine the correlation of BAMBI with other protein and pathological factors. Recurrence-free and overall survival curves were generated using the Kaplan-Meier method, and the log-rank test was used to compare the curves. Deaths without recurrence were included as censored observations. The relative risk and confidence limits were estimated for each variable using the Cox univariate model, using the most suitable prognostic category as the referent group. A multivariate Cox proportional hazard model was also developed using stepwise regression (forward selection) with predictive variables. The limits for entry and removal were both P < 0.5. Statistical significance was set at P < 0.05.
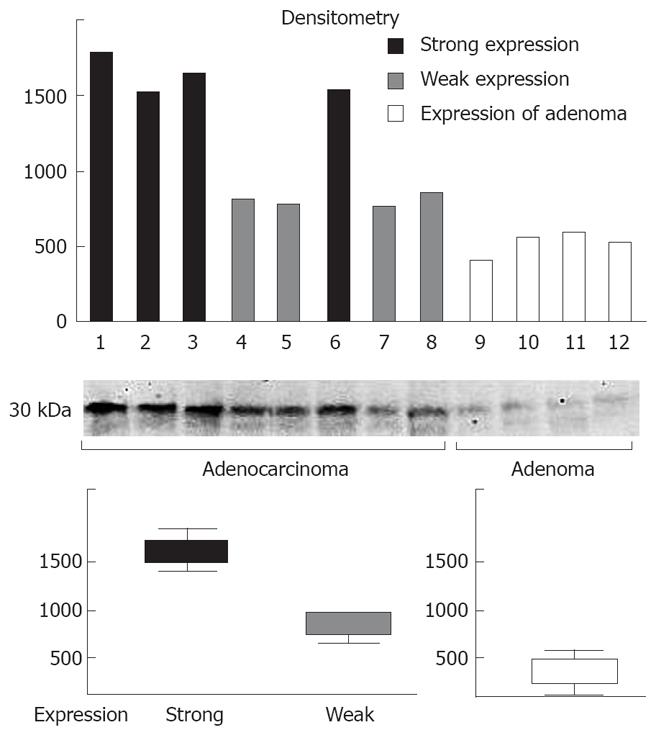
Immunoblotting analysis using anti-BAMBI monoclonal antibody detected BAMBI as a 30-kDa protein in all of the CRC and tubular adenoma specimens. The levels of BAMBI expression in CRCs were higher than those in tubular adenomas (Figure 1). By contrast, no specific band was detected in normal colonic epithelium (data not shown). The quantitative analysis of the bands revealed that the band intensity was divided into two groups: strong expression and weak expression was three and two times higher than that of tubular adenoma, respectively (Figure 1). Furthermore, CRC tissues with strong expression in immunoblotting were strongly positive for BAMBI immunohistochemistry, whereas other CRC tissues with weak expression revealed weak immunoreactivity to BAMBI (Figure 2). These results indicate that the immunohistochemical expression level correctly reflected the level of BAMBI protein expressed in CRC tissues.
Immunohistochemically, no BAMBI expression was detected in normal colonic epithelium. In contrast, diffuse BAMBI immunoreactivity was observed in the cytoplasm and cell membrane of tubular adenoma and CRC cells (Figure 3). We observed BAMBI immunoreactivity in 148 (80.8%) of 183 CRC tissues, and weak and strong BAMBI expression was observed in 80 (54.1%) and 68 (45.9%) CRCs, respectively.
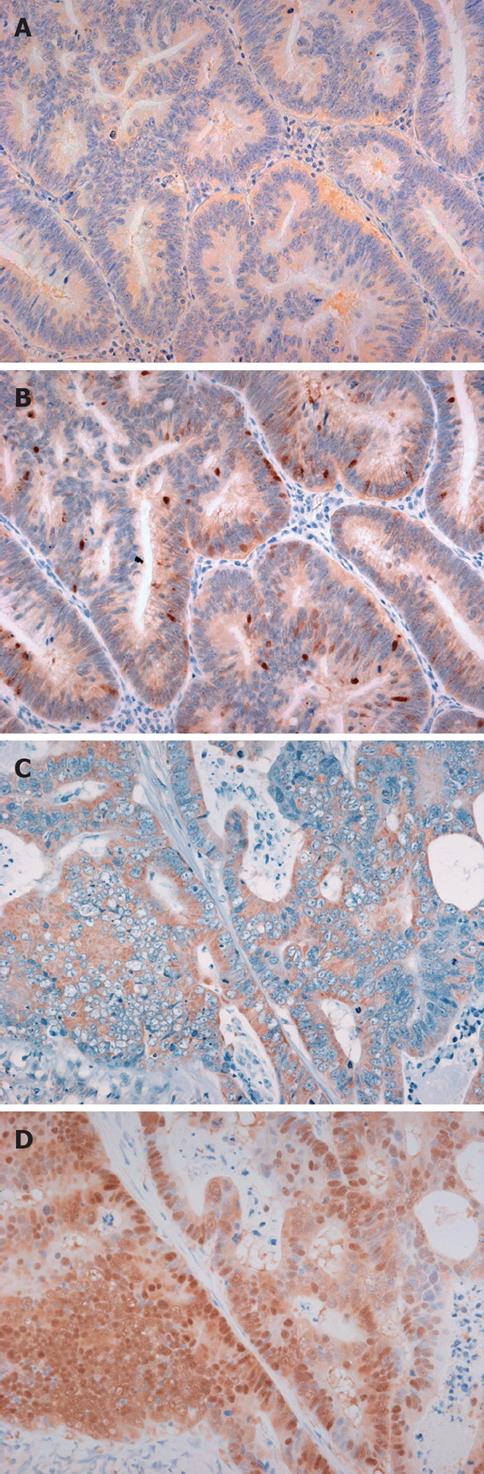
Nuclear β-catenin immunostaining was not observed in normal colonic mucosa. Nuclear β-catenin immunoreactivity was observed in 154 (84.1%) of 183 CRCs. Nuclear accumulation of p53 was observed in 74 (40.4%) of 183 CRCs (data not shown).
Strong immunohistochemical expression of BAMBI was positively correlated with histological type (P = 0.023), depth of invasion (P = 0.021), the presence or absence of lymph node metastases (P = 0.035), and TNM staging (P < 0.001), whereas no correlations were found with gender, age, location, or tumor size (data not shown).
We evaluated the correlation of immunohistochemical BAMBI expression with immunohistochemical β-catenin and p53 expression. Strong expression of BAMBI was significantly associated with β-catenin (P = 0.035) and p53 (P = 0.049) expression (Table 2).
The Stage IV patients who underwent to macroscopic resection of metastasis with no residual disease were excluded from the recurrence-free and overall survival groups and multivariate analysis. The patients who died from non-cancer-related causes were excluded from the recurrence-free survival and overall survival groups. In all, 157 of 183 patients were analyzed with a mean follow-up period of 33.7 mo (range, 2-137). In the background analysis, histological type (P = 0.043) and TNM staging (P = 0.035) were significant in strong BAMBI expression compared to weak BAMBI expression. The tumor depth (P = 0.054) tended to be significant in strong BAMBI expression (Table 3). The cancer recurred in 30 patients, and 20 patients died of cancer-related causes.
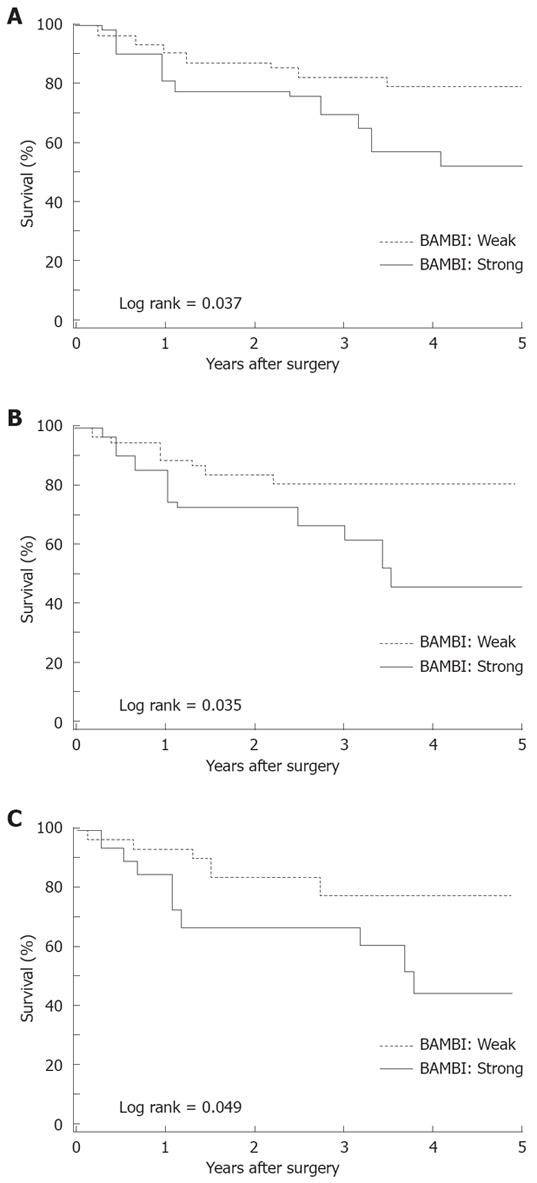
No significant differences were observed in the recurrence-free and overall survival at each stage according to the BAMBI expression level. However, the 5-year recurrence-free survival rate differed significantly (P = 0.037); it was 51.9% with strong BAMBI expression compared to 79.8% for weak BAMBI expression (Figure 4A). The 5-year overall survival rate was 61.0% for strong BAMBI expression and 75.0% for weak BAMBI expression (P = 0.495). No significant differences were detected in recurrence-free survival according to β-catenin (low vs high: 77.2% vs 65.9%, P = 0.109) and p53 (negative vs positive: 75.8% vs 61.1%, P = 0.417) expression.
Furthermore, we evaluated the impact of the coexpression of BAMBI, β-catenin, and p53 on recurrence-free survival. With high β-catenin expression, the 5-year recurrence-free survival was 46.9% for strong BAMBI expression compared to 80.8% for weak BAMBI expression (P = 0.035; Figure 4B). Weak β-catenin expression did not affect the survival rate according to the BAMBI expression level (weak vs strong: 75.0% vs 79.2%, P = 0.6294). With positive p53 expression, the 5-year recurrence-free survival rate was 43.4% for the strong BAMBI expression group compared to 78.2% for the weak BAMBI expression group (P = 0.049; Figure 4C). With negative p53 expression, the 5-year recurrence-free survival rate was 62.4% with strong BAMBI expression versus 80.8% for weak BAMBI expression (P = 0.582).
The relative risk and confidence limits for recurrence-free survival were estimated for each variable using the Cox univariate model. TNM staging (relative risk 4.63; 95% CI 2.84-7.56; P < 0.001), presence or absence of lymph node metastases (relative risk 8.937; 95% CI 3.10-25.77; P < 0.001), depth of invasion (relative risk 14.77; 95% CI 2.01-108.55; P = 0.008), and strong BAMBI expression (relative risk 2.109; 95% CI 1.029-4.323; P = 0.041) were significant. In the multivariate Cox proportional hazard model, TNM stage (relative risk 4.35; 95% CI 2.65-7.14; P < 0.001) was the only significant indicator for recurrence. According to Spearman’s coefficient of rank correlation, BAMBI expression was significantly correlated with TNM staging (P = 0.038) and the nuclear expression of β-catenin (P = 0.01). In the model excluding TNM stage and the nuclear expression of β-catenin, lymph node metastases (relative risk 6.685; 95% CI 2.24-19.93; P < 0.001), depth of invasion (relative risk 14.01; 95% CI 1.74-112.69; P = 0.013), and strong BAMBI expression (relative risk 2.26; 95% CI 0.95-5.38; P = 0.057) were significant indicators of recurrence (Table 4).
BAMBI was expressed in 80% of CRC tumors, and strong BAMBI expression was observed in 46% of CRC tumors. The region with BAMBI expression matched the region of β-catenin expression, and the expression of BAMBI and β-catenin were correlated, consistent with the fact that β-catenin is responsible for the aberrant expression of BAMBI[26]. In addition, the association of BAMBI expression and p53 expression is consistent with a previous report that p53 induces Siah-1, which mediates β-catenin degradation[29]. The overexpression of BAMBI inhibits the response of tumor cells to TGF-β signaling, and interferes with TGF-β-mediated growth arrest in vitro[25,26]. These results indicate that BAMBI expression is one of the critical early genetic events in CRC tumorigenesis.
Strong BAMBI expression was more frequently associated with deep tumor penetration, lymph node metastases, and advanced TNM stage. Patients with strong BAMBI expression had shorter recurrence-free and overall survival, and strong BAMBI expression was an independent factor predicting a lower recurrence-free survival in the multivariate analysis. The nuclear accumulation of β-catenin contributes to the transactivation of target genes, which encode regulators of differentiation and effectors supporting invasion and metastasis (CD44, laminin-52, matrix metalloproteinase MMP-7 and MT1-MMP) and angiogenesis (vascular endothelial growth factor), among others[5]. In fact, the overexpression of nuclear β-catenin is reported to be an independent predictor of short patient survival time[30-33]. Inconsistent with these reports, we showed that nuclear β-catenin accumulation alone does not affect survival and is not a prognostic factor in the univariate and multivariate analyses. However, we found that patients with both strong BAMBI expression and the overexpression of β-catenin have poorer survival compared to those without β-catenin expression. Smad activation or expression is lost in approximately 10% of CRC occurrences, and these patients have a poor prognosis because of its association with advanced disease and the presence of lymph node metastases at diagnosis[34]. BAMBI expression allows tumor cells to escape from TGF-β signaling and activate oncogenic processes such as growth stimulation, increases in motility, and invasion. Therefore, BAMBI plays an important role in the invasiveness and metastatic potential of colon cancers through cross-talk with the Wnt and TGF-β signaling pathways.
Although the available data on the correlation of p53 status and the prognosis of colorectal cancers are controversial[35], we found that p53 expression status did not influence the recurrence-free and overall survival and was not a prognostic factor in the univariate and multivariate analyses. Nevertheless, patients with both strong BAMBI expression and p53 overexpression had lower survival compared to those without p53 expression. We speculate that target genes induced by BAMBI overexpression may complement or cooperate with those induced by p53 inactivation and thereby contribute to poorer survival.
Although CRC prognosis is stage and grade dependent, cancers with similar clinicopathological features may have major differences in outcome. A large proportion of patients with Stage II disease and some with Stage III disease can be cured by surgery alone and do not derive any benefit from adjuvant therapy[36]. Therefore, the identification of robust molecular prognostic markers to supplement conventional pathological staging systems is highly desirable. Several molecular markers are promising prognostic predictors for the outcome in CRC, including deleted in colorectal cancer (DCC), microsatellite instability (MSI), and loss of heterozygosity at 18q[37,38]. CRC patients with strong BAMBI expression had more aggressive disease and their recurrence-free survival was markedly shorter. In contrast, patients with weak BAMBI expression had less aggressive disease and significantly longer recurrence-free survival. Strong BAMBI expression was tending to be an independent molecular predictor for survival secondary to lymph node metastasis and depth of tumor in the Cox hazard model. Therefore, strong BAMBI expression might be applicable in clinical practice to predict recurrence in additional to lymph node metastasis and depth of tumor.
In clinical scenarios, targeting the TGF-β signaling pathway for chemoprevention and the treatment of human cancers should decrease or abrogate TGF-β signaling, particularly for advanced or metastatic disease[20]. Given that the TGF-β signaling pathway has a important role in tumor-induced immunosuppression[39], inhibitors of this pathway may be used to improve natural immunosurveillance of tumor cells or to enhance the effectiveness of active or passive immunotherapy strategies. Our findings suggest that the development of a new active monoclonal anti-BAMBI antibody may offer a great improvement in survival of CRC patients and might also serve as a diagnostic tool for CRC prognosis.
In generally, BMP and activin membrane-bound inhibitor (BAMBI) expression is aberrantly elevated in most colorectal cancers (CRCs). However, few studies are reported on BAMBI expression in colorectal tissue. To analyze the clinical significance of BAMBI, authors studied its expression in CRC using immunohistochemical staining. They show that BAMBI overexpression is correlated with aggressive tumor phenotypes and predicts tumor recurrence and cancer-related death in CRC. This study was to map BAMBI expression in colorectal tissue and analyze the relationship between BAMBI expression and CRC prognosis.
To analyze the clinical significance of BAMBI, authors studied its expression in CRC using immunohistochemical staining. They show that BAMBI overexpression is correlated with aggressive tumor phenotypes and predicts tumor recurrence and cancer-related death in CRC. BAMBI may be usable as a target for diagnostic and antibody medicine.
The results of this study show that BAMBI expression plays a role in the pathogenesis of colorectal cancer.
The expression level of BAMBI plays an important role in the pathogenesis of colorectal cancer. The development of a new active monoclonal anti-BAMBI antibody may offer a great improvement in survival of CRC patients and might also serve as a diagnostic tool for CRC prognosis.
This is an extremely well written and researched paper. It discovers yet another marker of prognosis for CRC.
Peer reviewer: Burton I Korelitz, MD, Department of Gastroenterology, Lenox Hill Hospital, 100 East 77th Street, 3 Achelis, New York NY 10021, United States
S- Editor Li DL L- Editor Alpini GD E- Editor Lin YP
| 1. | Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286-3305. |
| 2. | Akiyama T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273-282. |
| 4. | Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606-1609. |
| 5. | Brabletz T, Jung A, Kirchner T. Beta-catenin and the morphogenesis of colorectal cancer. Virchows Arch. 2002;441:1-11. |
| 6. | Ahnen DJ, Feigl P, Quan G, Fenoglio-Preiser C, Lovato LC, Bunn PA Jr, Stemmerman G, Wells JD, Macdonald JS, Meyskens FL Jr. Ki-ras mutation and p53 overexpression predict the clinical behavior of colorectal cancer: a Southwest Oncology Group study. Cancer Res. 1998;58:1149-1158. |
| 7. | Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422-426. |
| 8. | Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc Natl Acad Sci USA. 2001;98:14973-14978. |
| 9. | Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184-1193. |
| 10. | Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem. 2002;277:21657-21665. |
| 11. | Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence beta-catenin in vivo and promotes hyperphosporylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol. 1996;16:4088-4094. |
| 12. | Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787-1790. |
| 13. | Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245-250. |
| 14. | Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257-261. |
| 15. | Datto MB, Li Y, Panus JF, Howe DJ, Xiong Y, Wang XF. Transforming growth factor beta induces the cyclin-dependent kinase inhibitor p21 through a p53-independent mechanism. Proc Natl Acad Sci USA. 1995;92:5545-5549. |
| 16. | Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295-309. |
| 17. | Miyazono K, ten Dijke P, Heldin CH. TGF-beta signaling by Smad proteins. Adv Immunol. 2000;75:115-157. |
| 18. | Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117-129. |
| 19. | Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002;12:22-29. |
| 20. | Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J Clin Oncol. 2005;23:2078-2093. |
| 21. | Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y, Kikuchi A, Miyazono K, Kato M. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol. 2001;21:5132-5141. |
| 22. | Labbe E, Letamendia A, Attisano L. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-beta and wnt pathways. Proc Natl Acad Sci USA. 2000;97:8358-8363. |
| 23. | Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW. Interaction between Wnt and TGF-beta signalling pathways during formation of Spemann's organizer. Nature. 2000;403:781-785. |
| 24. | Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999;401:598-602. |
| 25. | Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480-485. |
| 26. | Sekiya T, Adachi S, Kohu K, Yamada T, Higuchi O, Furukawa Y, Nakamura Y, Nakamura T, Tashiro K, Kuhara S. Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-beta signaling, as a target of the beta-catenin pathway in colorectal tumor cells. J Biol Chem. 2004;279:6840-6846. |
| 27. | Nakashima M, Meirmanov S, Naruke Y, Kondo H, Saenko V, Rogounovitch T, Shimizu-Yoshida Y, Takamura N, Namba H, Ito M. Cyclin D1 overexpression in thyroid tumours from a radio-contaminated area and its correlation with Pin1 and aberrant beta-catenin expression. J Pathol. 2004;202:446-455. |
| 28. | King-Yin Lam A, Ong K, Ho YH. Colorectal mucinous adenocarcinoma: the clinicopathologic features and significance of p16 and p53 expression. Dis Colon Rectum. 2006;49:1275-1283. |
| 29. | Matsuzawa SI, Reed JC. Siah-1, SIP, and Ebi collaborate in a novel pathway for beta-catenin degradation linked to p53 responses. Mol Cell. 2001;7:915-926. |
| 30. | Gunther K, Brabletz T, Kraus C, Dworak O, Reymond MA, Jung A, Hohenberger W, Kirchner T, Kockerling F, Ballhausen WG. Predictive value of nuclear beta-catenin expression for the occurrence of distant metastases in rectal cancer. Dis Colon Rectum. 1998;41:1256-1261. |
| 31. | Hugh TJ, Dillon SA, Taylor BA, Pignatelli M, Poston GJ, Kinsella AR. Cadherin-catenin expression in primary colorectal cancer: a survival analysis. Br J Cancer. 1999;80:1046-1051. |
| 32. | Cheah PY, Choo PH, Yao J, Eu KW, Seow-Choen F. A survival-stratification model of human colorectal carcinomas with beta-catenin and p27kip1. Cancer. 2002;95:2479-2486. |
| 33. | Wong SC, Lo ES, Lee KC, Chan JK, Hsiao WL. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10:1401-1408. |
| 34. | Xie W, Rimm DL, Lin Y, Shih WJ, Reiss M. Loss of Smad signaling in human colorectal cancer is associated with advanced disease and poor prognosis. Cancer J. 2003;9:302-312. |
| 35. | Allegra C, Sargent D. Molecular diagnostics: assays, tissues, progress, and pitfalls. J Clin Oncol. 2003;21:395-396. |
| 36. | Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Goodman PJ, Ungerleider JS, Emerson WA, Tormey DC, Glick JH. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352-358. |
| 37. | Watanabe T, Wu TT, Catalano PJ, Ueki T, Satriano R, Haller DG, Benson AB 3rd, Hamilton SR. Molecular predictors of survival after adjuvant chemotherapy for colon cancer. N Engl J Med. 2001;344:1196-1206. |
| 38. | Popat S, Houlston RS. A systematic review and meta-analysis of the relationship between chromosome 18q genotype, DCC status and colorectal cancer prognosis. Eur J Cancer. 2005;41:2060-2070. |
| 39. | Kirkbride KC, Blobe GC. Inhibiting the TGF-beta signalling pathway as a means of cancer immunotherapy. Expert Opin Biol Ther. 2003;3:251-261. |