Published online Jan 21, 2008. doi: 10.3748/wjg.14.484
Revised: October 10, 2007
Published online: January 21, 2008
Aorto-duodenal fistulae (ADF) are the most frequent aorto-enteric fistulae (80%), presenting with upper gastrointestinal bleeding. We report the first case of a man with a secondary aorto-duodenal fistula presenting with a history of persistent occlusive syndrome. A 59-year old man who underwent an aortic-bi-femoral bypass 5 years ago, presented with dyspepsia and biliary vomiting. Computed tomography scan showed in the third duodenal segment the presence of inflammatory tissue with air bubbles between the duodenum and prosthesis, adherent to the duodenum. The patient was submitted to surgery, during which the prosthesis was detached from the duodenum, the intestine failed to close and a gastrojejunal anastomosis was performed. The post-operative course was simple, secondary ADF was a complication (0.3%-2%) of aortic surgery. Mechanical erosion of the prosthetic material into the bowel was due to the lack of interposed retroperitoneal tissue or the excessive pulsation of redundantly placed grafts or septic procedures. The third or fourth duodenal segment was most frequently involved. Diagnosis of ADF was difficult. Surgical treatment is always recommended by explorative laparotomy. ADF must be suspected whenever a patient with aortic prosthesis has digestive bleeding or unexplained obstructive syndrome. Rarely the clinical picture of ADF is subtle presenting as an obstructive syndrome and in these cases the principal goal is to effectively relieve the mechanical bowel obstruction.
- Citation: Geraci G, Pisello F, Volsi FL, Facella T, Platia L, Modica G, Sciumè C. Secondary aortoduodenal fistula. World J Gastroenterol 2008; 14(3): 484-486
- URL: https://www.wjgnet.com/1007-9327/full/v14/i3/484.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.484
Aorto-duodenal fistulae (ADF) are the most frequent aorto-enteric fistulae (80%). The most frequent presenting sign of ADF is upper gastrointestinal bleeding (UGI). We report the first case of a man with a secondary aortoduodenal fistula presenting with a history of persistent occlusive syndrome, which was treated with surgical exploration.
A 59-year old male patient who underwent an aortic-bi-femoral bypass 5 years ago, was admitted to the Emergency Room for a 5-day history of persistent occlusive syndrome with dyspepsia and biliary vomiting. Physical examination revealed a distended abdominal pain. Full blood examination was unremarkable.
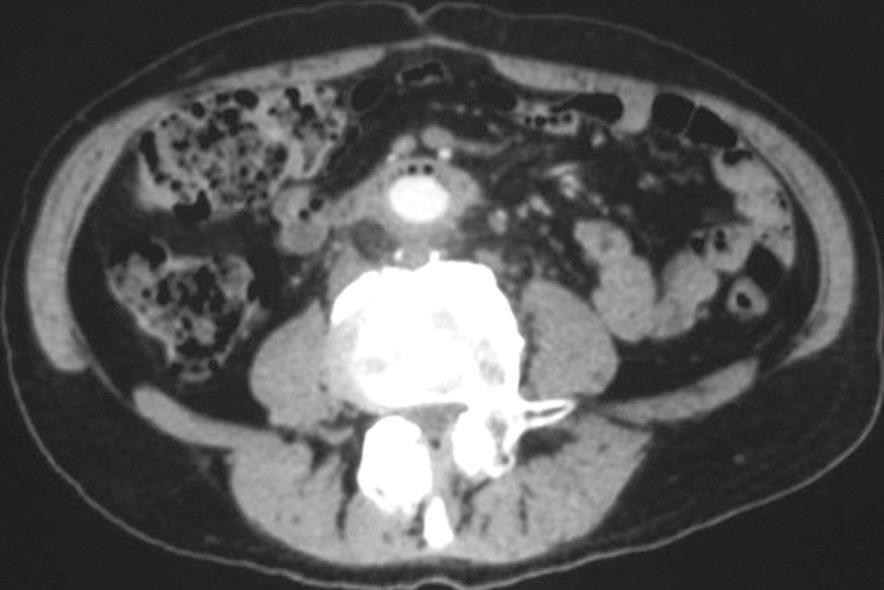
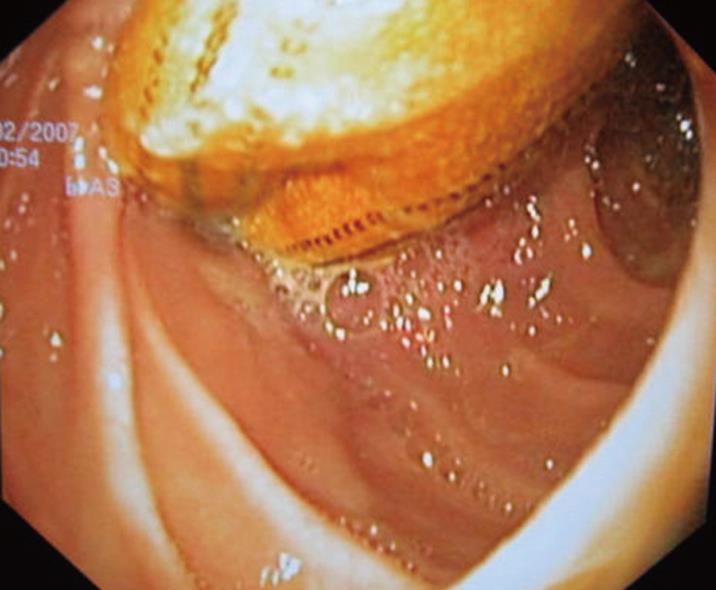
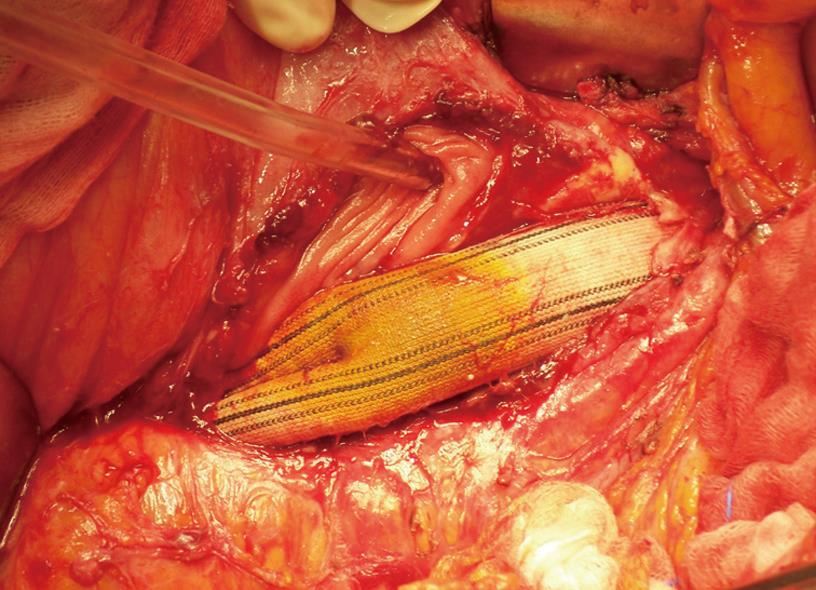
Computed tomography (CT) scan showed in the third duodenal segment the presence of an area with the characteristics of inflammatory tissue including air bubbles between the duodenum and aortic-bi-femoral prosthesis adherent to the third duodenal portion (“comma sign”) (Figure 1). Microbiological cultures and scintigraphy were unremarkable. Esophago-gastro-duodenoscopy showed the aortic prosthesis crossing the third segment of the duodenal wall occluding the intestinal lumen (Figure 2). At laparotomy, after viscerolisis, the prosthesis was detached from the duodenal wall and the intestine failed to close transversely (Figure 3). To protect the intestinal wall, a pedunculated fragment of the greater omentum was placed between the duodenum and aortic bypass. Furthermore, a gastrojejunal Roux anastomosis was employed. The prosthesis was not changed because there were no local or systemic signs of infection. The post-operative course was uneventful. The patient was discharged 10 days after operation and received a regular follow-up for 6 mo.
ADF are the most frequent aorto-enteric fistulae (80%)[1]. They may be primary due to a spontaneous communication between the lumen of aortic aneurysm and intestinal loop, or secondary due to surgical repair of aneurysms with prosthetic implants. The most frequent presenting sign of ADF is UGI bleeding. Clinical suspicion is essential in the diagnosis of ADF and the most commonly used techniques for its diagnosis are esophago-gastro-duodenoscopy (EGDS) and CT.
Secondary ADF is an uncommon (0.3%-2%) and life-threatening long-term complication of aortic reconstructive surgery[2]. Although the exact pathogenesis remains speculative, mechanical erosion of the prosthetic material into the adjacent bowel may be due to the lack of interposed retroperitoneal tissue or the excessive pulsation of redundantly placed grafts or septic procedures (low-grade, Staphylococcus epidermidis “biofilm” infection). Finally, some authors have described cases of inadequate prosthetic materials[23]. In our case, ADF formation was related to graft pulsation on the duodenal wall (in this patient, the blood pressure was stably high and not controlled by drugs).
The time between the first intervention and develop-ment of fistula can vary from months to years[34]. The presentation is often subtle with herald bleeding followed by a period of grace, or catastrophic bleeding, or rarely an episode of intestinal obstruction. The third or fourth duodenal segment is the most frequently involved site. In Dacron prosthesis patients, fistula develops in the proximal graft tract opening in the third segment of the duodenum[1]. The right diagnosis of ADF can be difficult. It needs an early and correct suspicion index to establish the diagnosis of ADF in all patients who have undergone aortic graft surgery and present with gastrointestinal haemorrhage, sometimes fever, abdominal pain or mass or obstructive syndrome[56].
Though difficult, diagnosis can be achieved through EGDS or CT scan. Additional diagnostic procedures are often not useful. EGDS, the main diagnostic procedure, is able to demonstrate the fistula and rule out other possible causes of obstruction, but the most important tool to achieve diagnosis is clinical suspicion[36]. Because of the high mortality and morbidity, associated with secondary aorto-enteric fistula, surgical treatment is always recommended. Explorative laparotomy is the treatment of choice. Surgical procedures help guide the diagnosis and constitute the main part of treatment with suture of the duodenum and evaluation of vascular prosthesis.
In the case of non-treated aortic-enteric fistula presenting with massive UGI-bleeding, the mortality rate is near to 100%. While the morbidity (limb loss in 10%-40%) and mortality related to treated ADF are also high (75%) and require preventive measures, including more particularly delicate surgery and antibiotic therapy in case of any episode of infection. Several surgical procedures are possible[47].
ADF must be suspected whenever a patient with aortic prosthesis has digestive bleeding or unexplained obstructive syndrome[8]. More rarely, the clinical picture of ADF is subtle presenting as an obstructive syndrome and in these cases the principal goal is to effectively relieve the mechanical bowel obstruction. Further study is necessary to establish the more effective diagnostic mode.
| 1. | Connolly JE, Kwaan JH, McCart PM, Brownell DA, Levine EF. Aortoenteric fistula. Ann Surg. 1981;194:402-412. |
| 2. | Armstrong PA, Back MR, Wilson JS, Shames ML, Johnson BL, Bandyk DF. Improved outcomes in the recent management of secondary aortoenteric fistula. J Vasc Surg. 2005;42:660-666. |
| 3. | Galloro G, De Palma GD, Siciliano S, Amato B, Catanzano C. Secondary aortoduodenal fistula. Rare endoscopic finding in the course of digestive hemorrhage. Hepatogastroenterology. 2000;47:1585-1587. |
| 4. | Socrate AM, Rosati L, Locati P. Surgical treatment of aorto-enteric fistulas. Minerva Cardioangiol. 2001;49:37-45. |
| 5. | Quilez Ivorra C, Massa Dominguez B, Amillo Marques M, Moya Garcia MI, Arenas Jimenez J, Gomez Andres A. Aortoenteric fistulas: clinical presentation and helical computed tomography findings. Gastroenterol Hepatol. 2005;28:378-381. |
| 6. | Limani K, Place B, Philippart P, Dubail D. Aortoduodenal fistula following aortobifemoral bypass. Acta Chir Belg. 2005;105:207-209. |
| 7. | Kavanagh DO, Dowdall JF, Younis F, Sheehan S, Mehigan D, Barry MC. Aorto-enteric fistula: changing management strategies. Ir J Med Sci. 2006;175:40-44. |
| 8. | Constans J, Midy D, Baste JC, Demortiere F, Conri C. Secondary aortoduodenal fistulas: report of 7 cases. Rev Med Interne. 1999;20:121-127. |