INTRODUCTION
Daxx was first identified as a death-associated protein capable of binding the cytosolic domain of fas, an apoptosis-inducing member of the tumor necrosis factor (TNF) receptor family[1]. Daxx co-localizes with Promyelocytic Leukemia Protein (PML) within nuclear promyelocytic oncogenic domains (PODs)[23]. PML and/or POD-associated proteins may function as an important cofactor in governing nuclear hormone receptor transcriptional activity and function[45]. Recent studies[67] implied Daxx could negatively modulate androgen receptor (AR) transcriptional activity. Androgens affect lipogenic gene expression not only in tumor cells, but also in normal androgen target tissues in vivo[8]. AR can directly upregulate sterol regulatory element-binding protein (SREBP) cleavage-activating protein (SCAP) by binding an androgen response element in intron 8 of the SCAP gene[9]. Activated SREBP can increase the mRNA and protein levels of genes involved in fatty acid (fatty acid synthase and acetyl-CoA-carboxylase), and cholesterol synthesis (HMG-CoA-reductase and farnesyl diphosphate synthase)[10]. These results indicate that Daxx could possibly regulate cellular cholesterol metabolism by the SREBP pathway.
In the present study, we investigated the correlations between Daxx expression and cholesterol accumulation in liver cells. The findings herein show that overexpression of Daxx in HepG2 cells may decrease intracellular cholesterol, which may be associated with inhibition of SREBP activity related to cholesterol synthesis and an increase in caveolin-1 expression related to excretion.
MATERIALS AND METHODS
Materials
Modified Eagle medium (MEM) and fetal bovine serum were purchased from Gibco BRL. An antibody (Santa Cruz) directed against active SREBP was used to detect the activation of SREBP, and polyclonal anti-Daxx antibody or anti-caveolin-1 antibody (Santa Cruz) was used to assay the respective protein expression. The plasmids of pEGFP-C1/Daxx and pEGFP-C1 were gifts from Dr. Yanping[11]. The pEGFP-C1/Daxx contains a full-length cDNA of hDaxx in pEGFP-C1 vector. All reagents were of analysis grade.
Animal and diets
Male Sprague Dawley (SD) rats (210 g ± 10 g) were obtained from the animal laboratory of Nanhua University. The animals were individually housed in plastic cages in a temperature (23°C ± 2°C) and light (alternating 12 h periods of light and dark) controlled room. The rats were randomly divided into two groups. The control group was fed a normal diet, and another group was fed a high-fat diet (15%, lard, wt/wt, HFD) for 6 wk. Rats were allowed free access to food and deionized water throughout the test period. At the end of the experiment, the rats were anesthetized with ketamine and injected with 150 IU of heparin per kilogram of body weight. Fifteen minutes later, the rats were sacrificed by disarticulation. Blood samples were taken from the neck into glass tubes, and serum was obtained by centrifugation (2000 ×g for 10 min at 4°C). The livers were removed and rinsed with physiological saline. All samples were stored at -70°C until use.
Estimation of serum lipids
For determination of serum total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), triacylglycerol (TG) concentration, and high density lipoprotein-cholesterol (HDL-C), the corresponding diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were used according to the manufacturer’s instructions.
Cell culture and transfections
HepG2 cells, a human hepatocyte cell line, were obtained from Zhong Shan University (Guangzhou, China). The cells were maintained in RPMI 1640 medium (Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and penicillin/streptomycin, at 37°C in a humidified incubator containing 5% CO2. Cells were seeded at a density of 1 × 105 cells/well in a 24-well plate and cultured for 24 h to 60%-80% confluency. To obtain stable transfectants, HepG2 cells were transfected with the pEGFP-C1/Daxx or pEGFP-C1 plasmid using Lipofect 2000 Plus reagent (Invitrogen) in serum-free medium for 4 h at 37°C, according to the manufacturer’s recommendations. The transfection medium was removed, and fresh complete growth medium was added. After 24 h post-transfection, the cells in two wells were split into 10-cm dishes in a medium containing 500 &mgr;g/mL geneticin (G418; Amresco, Solon, USA), and the medium was changed every 3 d until G418-resistant colonies were clearly evident. Individual colonies were transferred into 6-well plates to continue incubation with G418 selection medium. Individual colonies were evaluated for Daxx expression by Immunofluorescent Microscopy, and a monoclonal cell line was used for all experiments successively.
Reverse transcription-PCR
Total RNA was extracted from the cells using Trizol reagent (Gibco BRL) according to the manufacturer’s protocol. Three micrograms of total RNA were used for reverse transcription in a total volume of 20 &mgr;L with the SuperScript preamplification system (Promega, Madison, MI). Aliquots of 2 &mgr;L cDNA were subsequently amplified in a total volume of 25 &mgr;L using the GeneAmp PCR kit (Promega) following conditions recommended by the manufacturer. The sense and antisense primers for Daxx were 5'-TGGCGCTCTATGTGGCAGAGATC-3' and 5'-CTGCATCTGTTCCAGATCCTCCT-3' (829 bp); the sense and antisense primers for actin that were used as an internal control were 5’-GGTGGCACCTGTGGTCCACCT-3’ and 5’-CTTCACTTGTGGCCCAGATAG-3’ (420 bp), respectively. The cycling conditions were as follows: 94°C for 5 min, followed by 28 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min, and a final extension of 72°C for 10 min. PCR products were separated on the 1.5% agarose gel viewed by ethidium bromide staining. These data were acquired with Alpha Imager 2200 software.
Lipid analysis by high performance liquid chromatography (HPLC)
Cells were scraped from culture flasks into 0.9% NaCl (1 mL per 50 cm2 flask) and homogenized by sonication for 10 s on ice. The protein concentration of cell lysate was determined by a bicinchoninic acid (BCA) kit. An equal volume of freshly prepared cold (-20°C) KOH in ethanol (150 g/L) was added. The cell lysate was repeatedly vortexed until clear. An equal volume of hexane-isopropanol 3:2 (v/v) was then added. The mixture was vortexed for 5 min, followed by centrifugation at 800 ×g (15°C for 5 min). The extraction procedure was repeated twice. The combined organic phase was transferred to clean tapered glass tubes and thoroughly dried under nitrogen at 40°C. The tubes were allowed to cool to room temperature. One hundred &mgr;L of isopropanol-acetonitrile 20:80 (v/v) was added. The sample was solubilized in an ultrasound water bath at room temperature for 5 min. After centrifugation at 800 ×g for 5 min, the samples were introduced into the HPLC device using an Agilent 1100 series. Cholesterol was eluted at a flow rate of 1 mL/min, temperature of 40°C using an eluent consisting of isopropanol-acetonitrile 20:80 (v:v), and detected by UV-absorption at 206 nm[12].
Western blot analysis
Liver tissues excised from rats were analyzed by western blot with an antibody directed against Daxx. HepG2 cells were washed with PBS, and then 0.5 mL of TME lysis buffer (10 mmol/L Tris, pH 7.5, 5 mmol/L MgCl2, 1 mmol/L EDTA, and 25 mmol/L NaF) containing fresh 100 &mgr;mol/L Na3VO4, 20 &mgr;g/mL leupeptin, 1 &mgr;g/mL pepstatin A, 4 &mgr;g/mL aprotinin, and 1 mmol/L DTT were added. Cell lysates were prepared by freezing and thawing of the cells on ice, and subsequent scraping and sonicating for 30 s. The cell lysates were centrifuged for 30 min at 15 000 ×g. Protein concentrations in the supernatants were determined by a BCA protein assay kit, and the samples were stored at -80°C. For western blot analysis, 20 &mgr;g of protein was subjected to SDS-PAGE under reducing conditions, and proteins were then transferred to polyvinylidene difluoride membrane as described previously[13]. The membrane was blocked for 2 h at room temperature with a commercial blocking buffer from Life Technologies, Inc. The blots were incubated for 1 h at room temperature with the respective primary antibody (1:2000 dilution), which was followed by 1 h incubation with a secondary antibody (horseradish peroxidase-conjugated, 1:4000 dilution). Target proteins were visualized by a chemiluminescent assay (Amersham-Pharmacia Biotech).
Statistical analysis
The values are expressed as the mean ± SE. The correlation between cholesterol of serum and Daxx was analyzed by SPSS. Statistical analysis of the data was performed using student’s t test or ANOVA. Values with P < 0.05 were considered statistically significant.
RESULTS
Correlation of Daxx and cholesterol
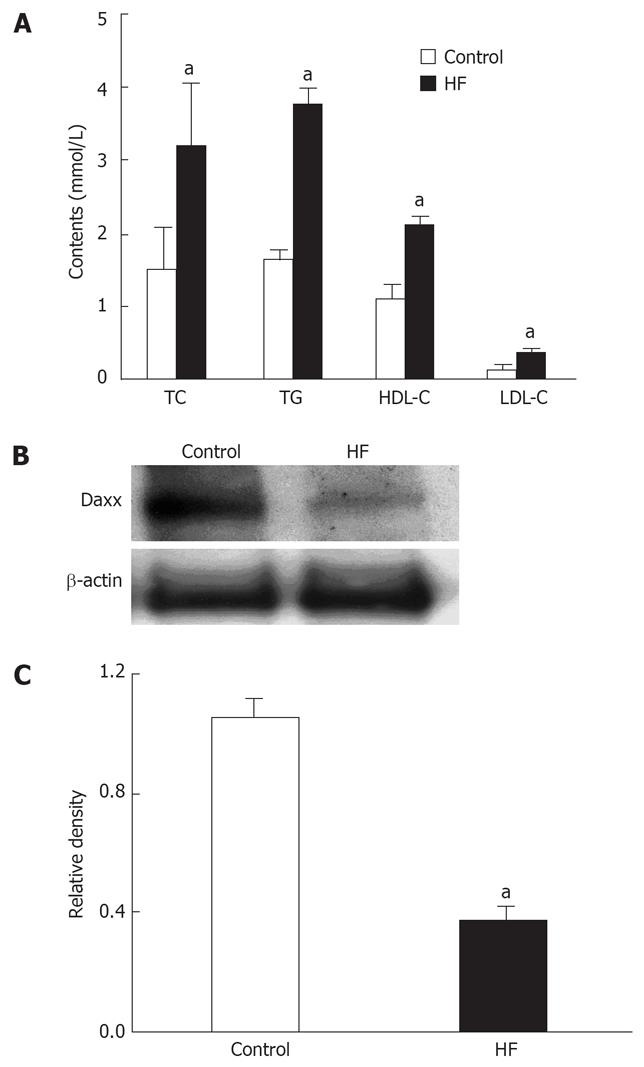
HFD feeding for 6 wk resulted in the development of hyperlipidemia in experimental rats, as shown in Figure 1A. Significant increases in TC (211%), TG (231%), HDL-C (197%), and LDL-C (246%) contents were observed in HFD-fed rats compared with those in control rats. At the same time, hepatic Daxx expression in the HFD-fed rats was decreased to one third of the control (Figure 1B and C). These data suggested that Daxx might have possible association with the change of blood lipid content as determined by correlation analysis. Further analysis revealed that there were negative correlations between Daxx and LDL-C (γ = -7.56, P = 0.018) and between Daxx and TC (γ = -9.07, P = 0.01), respectively.
Figure 1 The correlation between Daxx expression of Hepatic tissues and serum cholesterol.
A: The effect of control or high fat (HF) food on serum cholesterol and triglycerides in rats; B: Hepatic Daxx expression of rats as estimated by western blotting; C: Quantitative data of Daxx expression, results were normalized to β-actin. Data are the mean ± SE of three independent experiments. Control: normal food. HF: Added high fat to normal food. TC: Total cholesterol; TG: Triglyceride; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol. aP < 0.05 vs control.
Location and expression of Daxx in HepG2 hepatocytes
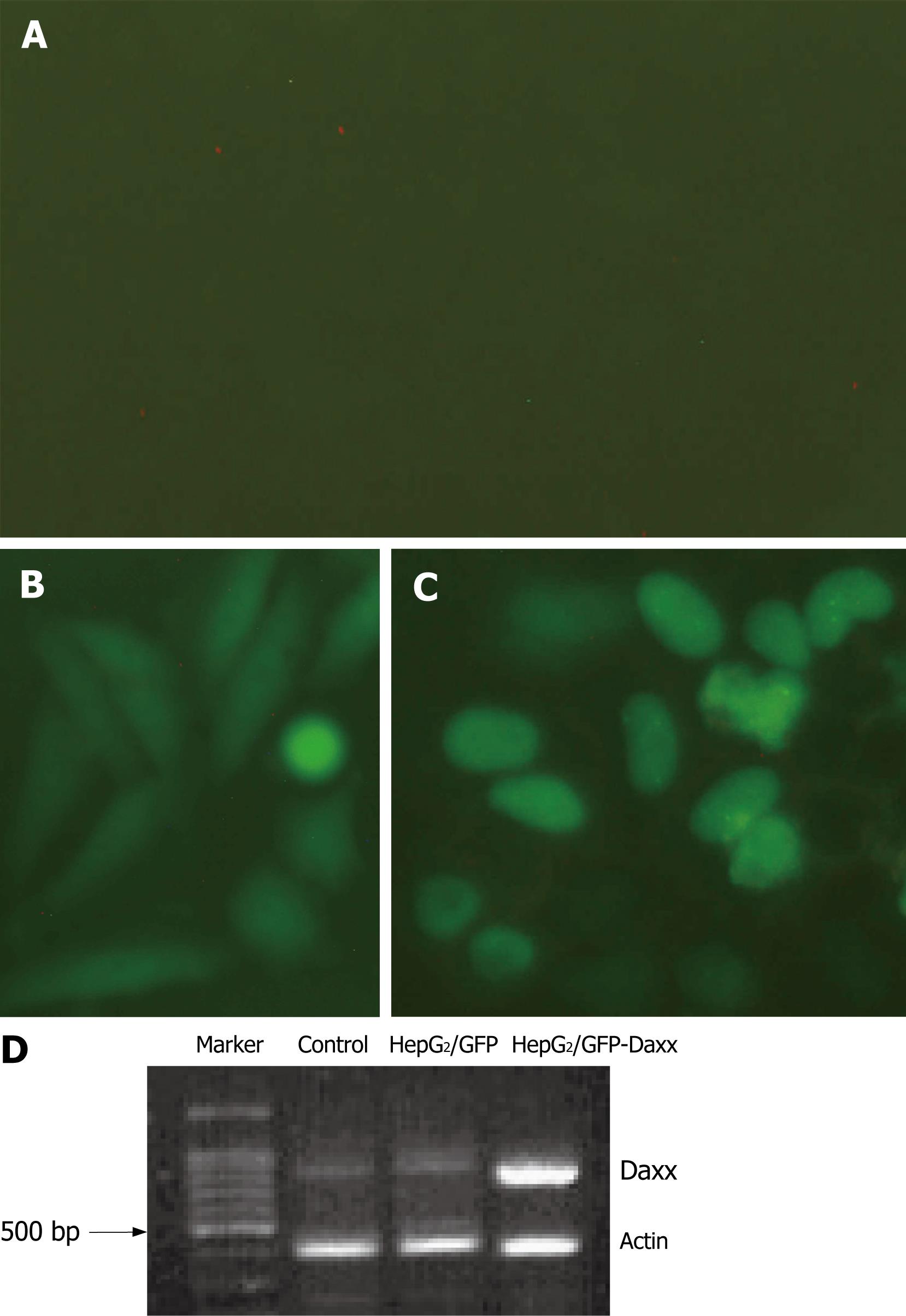
HepG2 cells were transfected with pEGFP-C1-Daxx or pEGFP-C1 plasmid. Daxx was mostly located in the nucleus of HepG2 cells (Figure 2A). Because the efficiency of transient-transfection was low, we screened out G418-resistant colonies. The majority of the colonies had fluorescence. PT-PCR analysis indicated that Daxx mRNA expression was significantly increased in HepG2/GFP-Daxx cells when compared with control or HepG2/GFP cells (Figure 2B).
Figure 2 Daxx expression in HepG2 hepatocytes.
Cultured HepG2 cells were untransfected (control, A) or transfected with pEGFP-C1-Daxx or pEGFP-C1 vectors (B, C). Images show the location and expression of Daxx in HepG2 cells, which were taken at 400 × magnitude. (D) RT-PCR of Daxx mRNA expression.
Effect of Daxx on cholesterol content in HepG2 hepatocytes
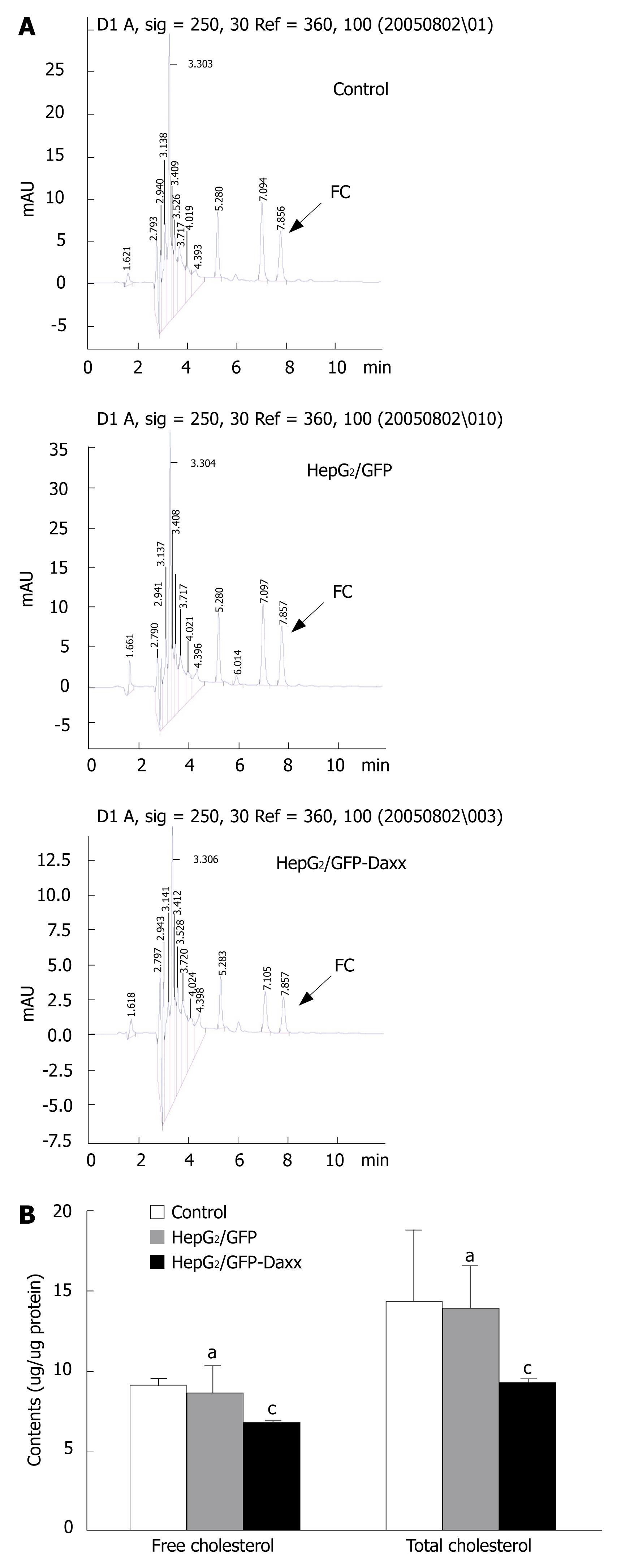
Figure 3 shows the effect of Daxx on cholesterol concentration in HepG2 cells as analyzed by HPLC. The arrows show the area of apices which represent the contents of free cholesterol. The area of apices before the arrow represents the total cholesterol (Figure 3A). There was a significant decrease (P < 0.05) of the cholesterol concentration in HepG2/GFP-Daxx cells compared with other samples. The free cholesterol in HepG2/GFP-Daxx cells was 6.74 ± 0.13 (&mgr;g/&mgr;g protein), whereas those of control and HepG2/GFP cells were 9.21 ± 0.37 and 8.66 ± 1.72, respectively. The total cholesterol in HepG2/GFP-Daxx cells was lower than that of control or HepG2/GFP cells (9.28 ± 0.19 vs 14.36 ± 4.45 or 13.94 ± 2.62, both P < 0.05). The empty vectors did not show obvious effects on cholesterol concentrations in HepG2 cells (Figure 3B).
Figure 3 Effect of Daxx overexpression on cholesterol accumulation in HepG2 hepatocytes.
A: Representative change of intracellular cholesterol levels in HepG2 cells, as determined by HPLC; B: The contents of free and total cholesterol in HepG2 cells. FC: Free cholesterol. aP > 0.05 vs control; cP < 0.05 vs control or HepG2/GFP cells.
Effect of Daxx on SREBP and caveolin-1 protein expression in HepG2 hepatocytes
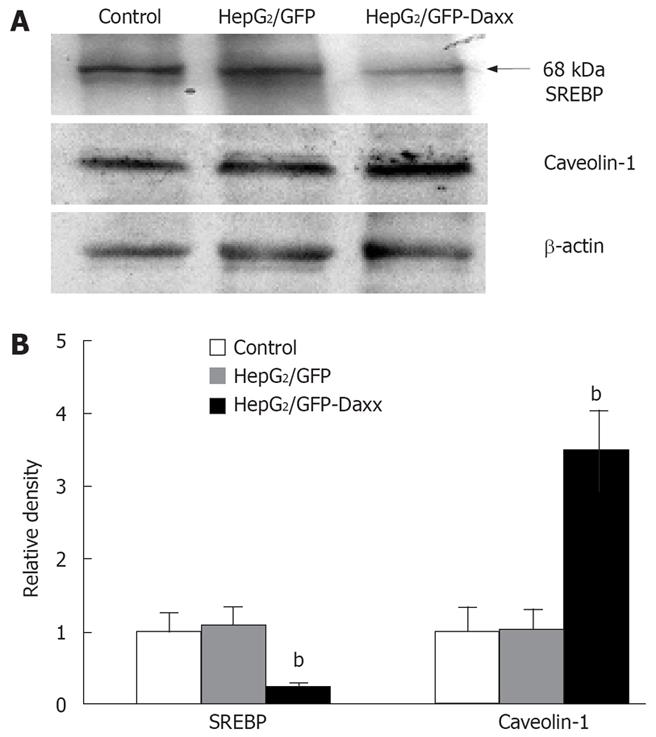
Overexpression of Daxx in HepG2 cells significantly decreased the expression of activated-SREBP from 1 ± 0.23 to 0.21 ± 0.05. Likewise, caveolin-1 expression increased nearly 3.5 times compared to the control (1 ± 0.31 to 3.48 ± 0.56). The empty vectors did not show any effect on the proteins of HepG2 cells (Figure 4A and B).
Figure 4 Effect of Daxx on the expression of SREBP and caveolin-1 proteins in HepG2 hepatocytes.
A: Representative western blot data showing the effects of Daxx on SREBP and caveolin-1 proteins in HepG2 cells; B: Quantitative data of the Daxx effect on SREBP and caveolin-1 expression. Data are the mean ± SE of three independent experiments. bP < 0.01 vs control or HepG2/GFP cells.
DISCUSSION
In the animal experiment, we observed that expression of Daxx in hepatic tissues was negatively correlated with hyperlipidemia. The liver is a very important organ for maintaining the physical balance of lipids, and has many proteins (such as SREBP and caveolin) responsible for mediating cholesterol synthesis and excretion[14–16]. It has been reported that hepatic cells predominantly express Daxx[17], but the direct relation between Daxx and cholesterol still remains unclear. Recently, Daxx has been shown primarily to function as a transcriptional regulator[18–20]. These results indicate that Daxx could possibly affect cholesterol accumulation in hepatic cells.
In cultured HepG2 cells, Daxx overexpression decreased the levels of FC and TC compared to those untransfected or transfected with GFP, which indicated that Daxx could affect cholesterol homeostasis of hepatic cells. HMG-CoA-reductase is a key enzyme of cholesterol synthesis and is regulated by sterol regulatory element binding proteins (SREBPs)[2122]. SREBP-1 represents an important protein of the transcription regulator family (SREBP-1a, -1c, and -2) controlling lipid homeostasis in cells[2324]. SREBP-1 are synthesized as 125-kDa inactive precursor proteins, and inserted into the membranes of the endoplasmic reticulum where they form tight complexes with SCAP. SREBP is proteolytically cleaved and activated by SCAP when the complex translocates to the Golgi apparatus[25]. The active 68-kDa SREBP fragment migrates to the nucleus and increases the transcription of sterol-responsive element (SRE) that contains many genes encoding lipogenic enzymes belonging to the pathways of cholesterol synthesis[26]. Daxx can inhibit AR transcriptional activity[67], which can down-regulates the activity of SCAP[9]. Thus, we checked the activity of SREBP-1 and detected that Daxx has the potential to decrease the expression of active SREBP-1. This also revealed that Daxx might mediate intracellular cholesterol accumulation by inhibiting cholesterol synthesis.
SREBP-1 represses caveolin expression by the SRE/SREBP pathway. In this case, SREBP inhibits caveolin gene transcription in contrast to its stimulating effect on other gene promoters[2728]. Caveolin-1, a type of free cholesterol-binding protein, is another significant protein involved in cholesterol homeostasis[29]. Transfection of cells with full-length caveolin-1 cDNA resulted in the expression of morphologically authentic caveolae structure and FC efflux[30]. The expression of caveolin may represent a mechanism, by which FC excretion became facile[31]. The results of experiments showed that Daxx promoted the expression of caveolin-1. These findings show the possibility of Daxx mediating intracellular cholesterol accumulation presumably by increasing cholesterol excretion.
In conclusion, our results confirm that Daxx is likely to decrease the intracellular cholesterol accumulation by regulating cholesterol synthesis and excretion. One of the main future challenges will be the generation of suitable animal models to be used to dissect Daxx function in cholesterol homeostasis.
COMMENTS
Background
Death-associated protein (Daxx) could negatively modulate androgen receptor (AR) transcriptional activity. Androgens affect lipogenic gene expression not only in tumor cells but also in normal androgen target tissues in vivo. AR can directly upregulate sterol regulatory element-binding protein (SREBP) cleavage-activating protein (SCAP) by binding an androgen response element in intron 8 of SCAP gene. Activated SREBP can increase the mRNA and protein levels of genes involved in fatty acid, and cholesterol synthesis. These results indicate that Daxx could possibly regulate cellular cholesterol metabolism by the SREBP pathway.
Research frontiers
Hypercholesrerolemia is mainly a pathologic feature of cardiovascular diseases. The liver is a very important organ for maintaining the physical balance of cholesterol. In liver SREBPs are very important proteins which have been responsible for mediating cholesterol metabolism. Some studies have shown Daxx can regulate SREBP indirectly, but no evidences have suggested Daxx can affect cholesterol balance.
Innovations and breakthroughs
In the present study, we investigated the correlations between Daxx expression and cholesterol accumulation in liver cells. The findings herein show that overexpression of Daxx in HepG2 cells may decrease intracellular cholesterol, which may be associated with inhibition of SREBP activity related to cholesterol synthesis and increase in caveolin-1 expression related to excretion.
Applications
Our study will provide academic value in finding a new function of Daxx, and experimental reference for clinical treatment of hypercholesterolemia.
Peer review
The authors demonstrated the inverse relationship between Daxx expression and cholesterol accumulation in hepatocytes. It appears that the effect of Daxx on cholesterol level in liver cells may be associated with inhibition of SREBP activity and an increase in caveolin-1 expression. The study was well performed and the data are clearly presented.