Published online Jan 21, 2008. doi: 10.3748/wjg.14.421
Revised: November 2, 2007
Published online: January 21, 2008
AIM: To investigate integrin β3 mRNA and vascular endothelial growth factor (VEGF) protein expression in gastric carcinoma, and its correlation with microvascular density, growth-pattern, invasion, metastasis and prognosis.
METHODS: In situ hybridization (ISH) of integrin β3 mRNA and immunohistochemistry of VEGF and CD34 protein were performed on samples from 118 patients with gastric cancer.
RESULTS: The positive rate of integrin β3 mRNA in non-tumor gastric mucosa (20%) was significantly lower than that of the gastric cancer tissue (52.5%, χ2 = 10.20, P < 0.01). In patients of infiltrating type, stage T3-T4, vessel invasion, lymphatic metastasis, hepatic or peritoneal metastasis, the positive expression rates of integrin β3 mRNA were significantly higher than those in patients of expanding type (P < 0.01), stage T1-T2 (P < 0.01), non-vessel invasion (P < 0.01), without lymphatic metastasis (P < 0.01), without hepatic and peritoneal metastasis (P < 0.01), respectively. In patients of infiltrating type, stage T3-T4, vessel invasion, lymphatic metastasis, hepatic or peritoneal metastasis, the positive expression rates of VEGF protein were significantly higher than those in patients of expanding type (P < 0.01), stage T1-T2 (P < 0.01), non-vessel invasion (P < 0.01), without lymphatic metastasis (P < 0.01), without hepatic and peritoneal metastasis (P < 0.01), respectively. In patients of infiltrating type, stage T3-T4, vessel invasion, lymphatic metastasis, hepatic or peritoneal metastasis, the mean MVD were significantly higher than those in patients of expanding type (P < 0.01), stage T1-T2 (P < 0.01), non-vessel invasion (P < 0.01), without lymphatic metastasis (P < 0.01), without hepatic and peritoneal metastasis (P < 0.01), respectively. It was found that the positive expression rate of integrin β3 mRNA was positively related to that of VEGF protein (P < 0.01) and MVD (P < 0.05), meanwhile the positive expression rate of VEGF protein was positively related to MVD (P < 0.05). The mean survival period in patients with positive expression of integrin β3 mRNA and VEGF, and MVD ≥ 54.9/mm2 was significantly shorter than that in patients with negative expression of integrin β3 mRNA (P < 0.05) and VEGF (P < 0.01), and MVD < 54.9/mm2 (P < 0.01). Five-year survival rate in patients with positive expression of integrin β3 mRNA and VEGF, and MVD ≥ 54.9/mm2 was significantly lower than those with negative expression of integrin β3 mRNA (P < 0.05), VEGF (P < 0.05), and MVD < 54.9/mm2 (P < 0.01).
CONCLUSION: Integrin β3 and VEGF expression can synergistically enhance tumor angiogenesis, and may play a crucial role in invasion and metastasis of gastric carcinoma. Therefore, they may be prognostic biomarkers and novel molecular therapeutic targets.
- Citation: Li SG, Ye ZY, Zhao ZS, Tao HQ, Wang YY, Niu CY. Correlation of integrin β3 mRNA and vascular endothelial growth factor protein expression profiles with the clinicopathological features and prognosis of gastric carcinoma. World J Gastroenterol 2008; 14(3): 421-427
- URL: https://www.wjgnet.com/1007-9327/full/v14/i3/421.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.421
Gastric cancer is the leading cause of death in China. For patients with gastric cancer, metastasis is the most common cause of death and is a major obstacle to successful treatment[1]. The spread of tumor cells from a primary to metastatic site is a complicated and multistage process, which may include cell proliferation and migration, degradation of basement membrane, invasion, and adhesion[2]. Current clinical methods cannot accurately predict which patients will develop metastasis. To develop effective new strategies for the prediction, diagnosis and treatment of metastasis of gastric cancer, molecular mechanisms controlling metastasis must be identified.
Angiogenesis, the process leading to the formation of new blood vessels, plays a central role in cancer cell survival, local tumor growth, and development of distant metastasis. The degree of intratumoral microvascular density (MVD) is thought to influence tumor metastasis and consequently prognosis in various human cancers, including gastric cancer[3–6].
Recent investigations have shown that high expression of integrin β3 is positively correlated with invasion and metastasis of cancer cells and tumor angiogenesis[78], but only a few studies have investigated the relationship between integrin β3 and prognosis of gastric cancer. In our study, we evaluated integrin β3 mRNA and vascular endothelial growth factor (VEGF) expression in gastric carcinoma and its relationship to pathological markers such as MVD, infiltration, metastasis and prognosis of gastric cancer.
A total of 118 patients (79 male, 39 female; aged 38-80 years, median age 57.8 years) who underwent gastrectomy for gastric carcinoma at Zhejiang Provincial People’s Hospital from October 1990 to November 1998 were included in this study. Five-year follow-up data were obtained, and the follow-up ended in November 2003. According to the WHO standard classification (2002), there were 39 tubular adenocarcinomas, 19 papillary adenocarcinomas, 37 poorly differentiated adenocarcinomas 12 mucinous adenocarcinomas, and 11 signet ring cell carcinomas. These patients were also classified into well- and moderately-differentiated (G1 + G2, 70 patients), and poorly-differentiated (G3 + G4, 48 patients) types, based on the predominant differentiation mode, and classified into the expanding (51 patients) and infiltrating (67 patients) type. According to the AJCC TNM staging system (6th ed, 2002), there were 21 stage T1, 26 stage T2, 45 stage T3, and 26 stage T4; 89 patients with and 29 without vessel invasion; 84 patients with lymphatic metastasis and 34 without; 55 patients with distant metastasis (35 with peritoneal dissemination, 20 with hepatic metastasis) and 63 without. A control study was carried out in 20 samples obtained from non-tumor gastric mucosa 5 cm away from the primary tumor without hyperplasia or atypical hyperplasia.
Integrin β3 probe: oligonucleotide probe labeled with digoxigenin was obtained from Chinese Wuhan Boster Biotechnology (MK1602). The target gene mRNA sequence of human integrin β3 is: (1) 5’-GACACCTGTG AGAAGTGCCCCACCTGCCA-3’; (2) 5’-GGATGACTGTGTCGTCAGATTCCAGTACTA-3’; and (3) 5’-GCTAAATTTGAGGAAAGGCGCGCCAGAGC-3’. Immunohistochemical reagents: first antibody and EnVision kit (DAKO Denmark), mouse monoclonal antibody to VEGF (1:100) and CD34 (1:120); VEGF clone: JH121; CD34 clone: QBEnd 10.
The samples in our study were from histological sections from paraffin blocks. In situ hybridization was performed according to the manufacturer’s instructions. This experiment used RNase-free conditions and APES-treated slides, which were baked at 60°C for 2-4 h before use. The samples were cut at 4-&mgr;m intervals, dewaxed with xylene, hydrated by a series of ethanol solutions (100%, 95%, 80% and 70%); inactivated with endogenous peroxidase using 3% hydrogen peroxide for 10 min at room temperature; washed three times with distilled water, each for 5 min; digested with Pepsin Reagent for 30 min at 37°C; rinsed three times in PBS for 5 min each, rinsed once in distilled water; fixed for 10 min at room temperature using 1% paraformaldehyde plus 1/1000 DEPC, washed three times with distilled water; then incubated in 50 &mgr;L pre-hybridization solution for 4 h at 40°C; and then superfluous liquid was absorbed. Each slide was incubated with 20 &mgr;L hybridization solution (concentration of the hybridization probe was 2 ng/&mgr;L) overnight at 40°C; then washed twice with 2 × SSC at 37°C for 5 min each, once with 0.5 × SSC at 37°C for 15 min, once with 0.2 × SSC at 37°C for 15 min, and once with 0.2 × SSC at room temperature for 15 min. These sections were incubated in blocking solution for 30 min at 37°C and superfluous liquid was absorbed. Following that, the sections were incubated in mouse biotinylated anti-digoxigenin for 60 min at 37°C, washed three times with PBS for 5 min each; incubated in SABC for 30 min at 37°C; washed three times with PBS for 5 min each; incubated in biotin/peroxidase for 30 min at 37°C; washed three times with PBS for 5 min each; stained with DAB for 3 min at room temperature, and washed thoroughly. The slides were counterstained with Harris hematoxylin, washed, dehydrated in ethanol, cleared in xylene, and enveloped with neutral gum. Negative controls included: (1) hybridization solution without probe; and (2) specimens pre-treated with RNase.
The EnVision two-step method was done according to the manufacturer’s instructions. Tissue sections were cut at 4-&mgr;m intervals, dewaxed with xylene, hydrated by a series of ethanol solutions (100, 95, 80 and 70%). Method of high temperature and high pressure (CD34: 0.01 mol/L Sodium Citrate buffering Solution, pH 6.0; VEGF: 0.01 mol/L EDTA buffering solution, pH 9.0) were taken to repair the needed antigen. The sections were washed with distilled water, washed three times with PBS for 5 min each, inactivated endogenous peroxidase using 3% hydrogen peroxide for 10 min at room temperature; washed three times with PBS for 5 min each; incubated overnight with dilute first antibody at 4°C, and rinsed three times with PBS for 5 min each; incubated overnight with goat anti-mouse IgG antibody/HRP polymer for 40 min at 37°C, and washed three times with PBS for 5 min each. Visualization was achieved with DAB for 3 min. After counterstaining with Harris hematoxylin, the slides were dehydrated in 95% and 100% ethanol, cleared in xylene, and enveloped with neutral gum. The negative control included replacement of the first antibody with PBS, and the positive slides provided by the reagent kit were used as the positive control.
Brown cytoplasm was indicative of positive expression of integrin β3 mRNA. The clearly-stained areas were chosen at high power (× 400), and > 200 cells were quantified in every visual field. According to the percentage of positive tumor cells, all these cells were scored as negative (-) (< 10% or no staining); weak positive (+) (11%-50%); positive (++) (51%-75%); or strongly positive (+++) (> 75%). Positive staining for VEGF was brown in the cytoplasm and/or cell membrane. These cells were scored as negative (-) (no staining); weak positive (+) (< 25%); positive (++) (26%-50%); strongly positive (+++) (> 50%), based on the percentage of positive VEGF cells. Assessment of MVD was performed as follows. Each slide was first evaluated at low power to capture the areas with the highest vascularization. Vessels with a clearly defined lumen or well-defined linear vessel shape, but not single endothelial cells were considered for microvascular assessment. We quantified positive vessels in five chosen fields (× 200) with the highest vascularization[3]. MVD was statistically recorded as the mean ± SD. The patients were divided into a high MVD (≥ 54.9/mm2) and low MVD (< 54.9/mm2) group based on the mean MVD value (54.9/mm2) of 118 gastric cancer patients.
Statistical analyses were performed using SPSS 10.0 software. Significant differences were compared with Student’s t test. The χ2 test was performed on the numerative data. Survival analysis was carried out using the Kaplan-Meier product-limit method, and survival curves were plotted. The differences were evaluated by the log rank test. P < 0.05 was considered statistically significant.
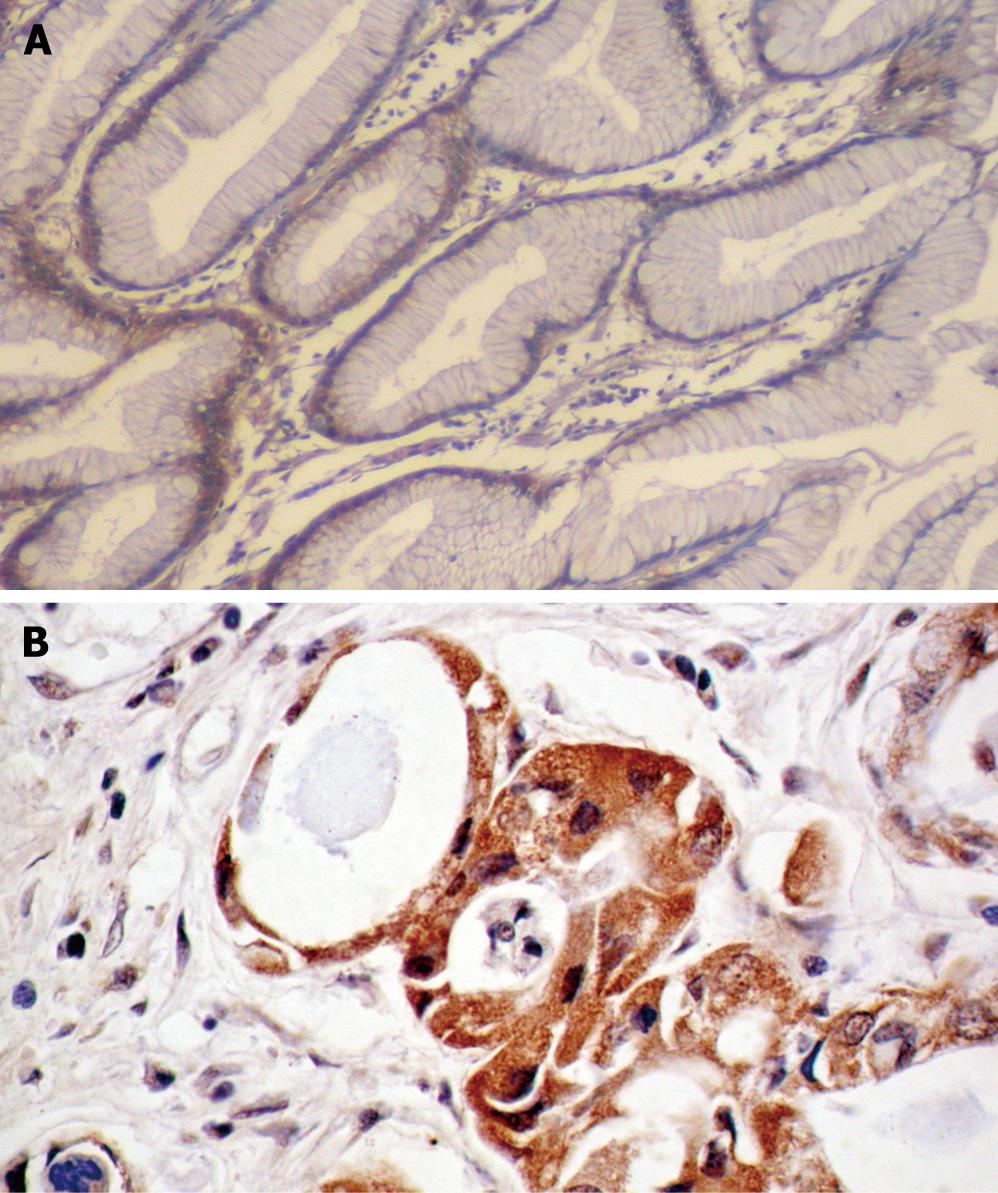
The positive expression rate of integrin β3 mRNA in non-tumor mucosa was 20% (6/30). Positive signals were distributed in the cytoplasm of non-tumor glandular cell in gastric pit epithelium and the lamina propria, and the majority appeared weakly positive or positive. However, the positive expression rate of integrin β3 mRNA in gastric cancer was 52.5% (62/118). There were significant differences between the two groups (χ2 = 10.20, P < 0.01). Positive cancer cells were stained in the cytoplasm, and most tumor cells infiltrating the muscularis mucosa, serosal layers, extra-serosal layers, and greater omentum positively expressed integrin β3 (Figure 1). In patients of infiltrating type, poorly-differentiated cases, stage T3-T4, vessel invasion, lymphatic metastasis, hepatic and peritoneal metastasis, the positive expression rates of integrin β3 mRNA was significantly higher than those of the expanding type (χ2 = 8.42, P < 0.01), well-, moderately-differentiated case (χ2 = 6.47, P < 0.01), stage T1-T2 (χ2 = 26.60, P < 0.01), non-vessel invasion (χ2 = 32.13, P < 0.01), without lymphatic metastasis (χ2 = 31.85, P < 0.01), without hepatic and peritoneal metastasis (χ2 = 20.48, P < 0.01; χ2 = 34.72, P < 0.01) (Table 1).
| Clinicopathological parameters | n | Integrinβ3 mRNA | VEGF | MVD | ||||||||
| - | + - +++ | χ2 | P | - | + - +++ | χ2 | P | n/mm2 | t | P | ||
| Growth pattern | 118 | 8.42 | < 0.01 | 10.44 | < 0.01 | 3.92 | < 0.01 | |||||
| Expansive | 51 | 32 | 19 | 32 | 19 | 49.45 ± 21.72 | ||||||
| Infiltrative | 67 | 24 | 43 | 22 | 45 | 64.06 ± 18.76 | ||||||
| Histological grade (G) | 6.47 | < 0.05 | 3.49 | 0.062 | 1.25 | > 0.05 | ||||||
| G1 + G2 | 70 | 40 | 30 | 37 | 33 | 55.72 ± 21.56 | ||||||
| G3 + G4 | 48 | 16 | 32 | 17 | 31 | 60.70 ± 20.73 | ||||||
| Invasive depth | 26.60 | < 0.01 | 34.19 | < 0.01 | 6.41 | < 0.01 | ||||||
| T1-T2 | 47 | 36 | 11 | 37 | 10 | 44.42 ± 19.96 | ||||||
| T3-T4 | 71 | 20 | 51 | 17 | 54 | 66.56 ± 17.23 | ||||||
| Vessel invasion | 32.13 | < 0.01 | 39.96 | < 0.01 | 7.96 | < 0.01 | ||||||
| No | 29 | 27 | 2 | 28 | 1 | 35.68 ± 14.04 | ||||||
| Yes | 89 | 29 | 60 | 26 | 63 | 64.93 ± 18.07 | ||||||
| Lymph node metastasis | 31.85 | < 0.01 | 34.71 | < 0.01 | 8.45 | < 0.01 | ||||||
| No | 34 | 30 | 4 | 30 | 4 | 37.60 ± 15.73 | ||||||
| Yes | 84 | 26 | 58 | 24 | 60 | 66.24 ± 17.24 | ||||||
| Distant metastasis | < 0.01 | |||||||||||
| No | 63 | 48 | 15 | 50 | 13 | 42.81 ± 17.23 | ||||||
| Liver metastasis | 20 | 4 | 16 | 20.48a | < 0.01a | 3 | 17 | 27.25a | < 0.01a | 73.40 ± 8.07 | 7.65a | < 0.01a |
| Peritoneal dissemination | 35 | 4 | 31 | 34.72b | < 0.01b | 1 | 34 | 52.77b | < 0.01b | 75.79 ± 9.48 | 10.46b | < 0.01b |
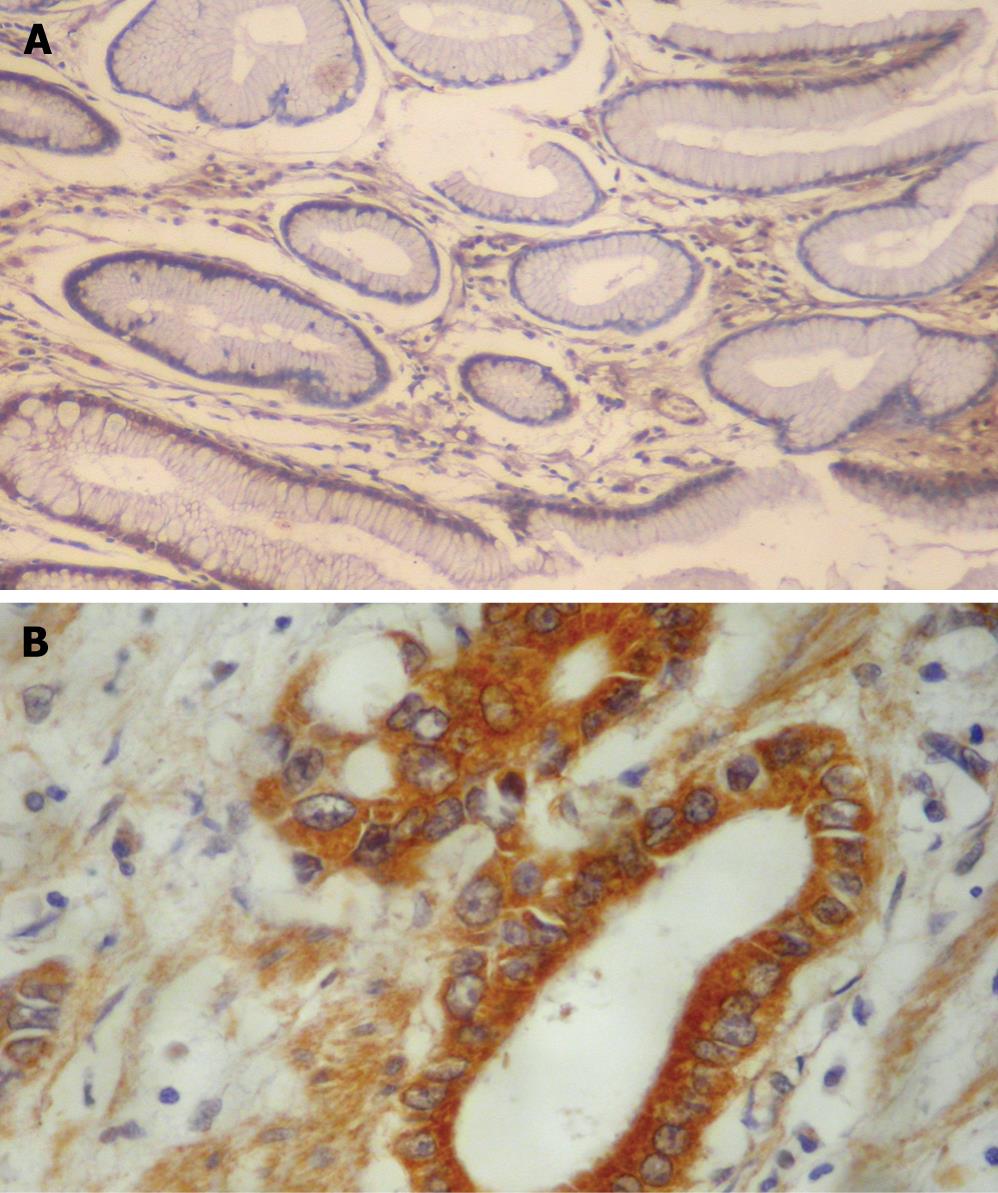
VEGF was rarely expressed in normal gastric mucosa (Figure 2A). Of 118 gastric carcinoma patients, 64 positively expressed VEGF (54.2%), and VEGF staining was mainly located in the cytoplasm of tumor cells and usually observed at the tumor margins (Figure 2B). In patients of infiltrating type, stage T3-T4, vessel invasion, lymphatic metastasis, hepatic and peritoneal metastasis, the positive expression rates of VEGF protein were significantly higher than those of the expanding type (χ2 = 10.44, P < 0.01), stage T1-T2 (χ2 = 34.19, P < 0.01), non-vessel invasion (χ2 = 39.96, P < 0.001), without lymphatic metastasis (χ2 = 34.71, P < 0.01), without hepatic and peritoneal metastasis (χ2 = 27.25, P < 0.01; χ2 = 52.77, P < 0.01). However, no correlation was found between the different histological types (Table 1).
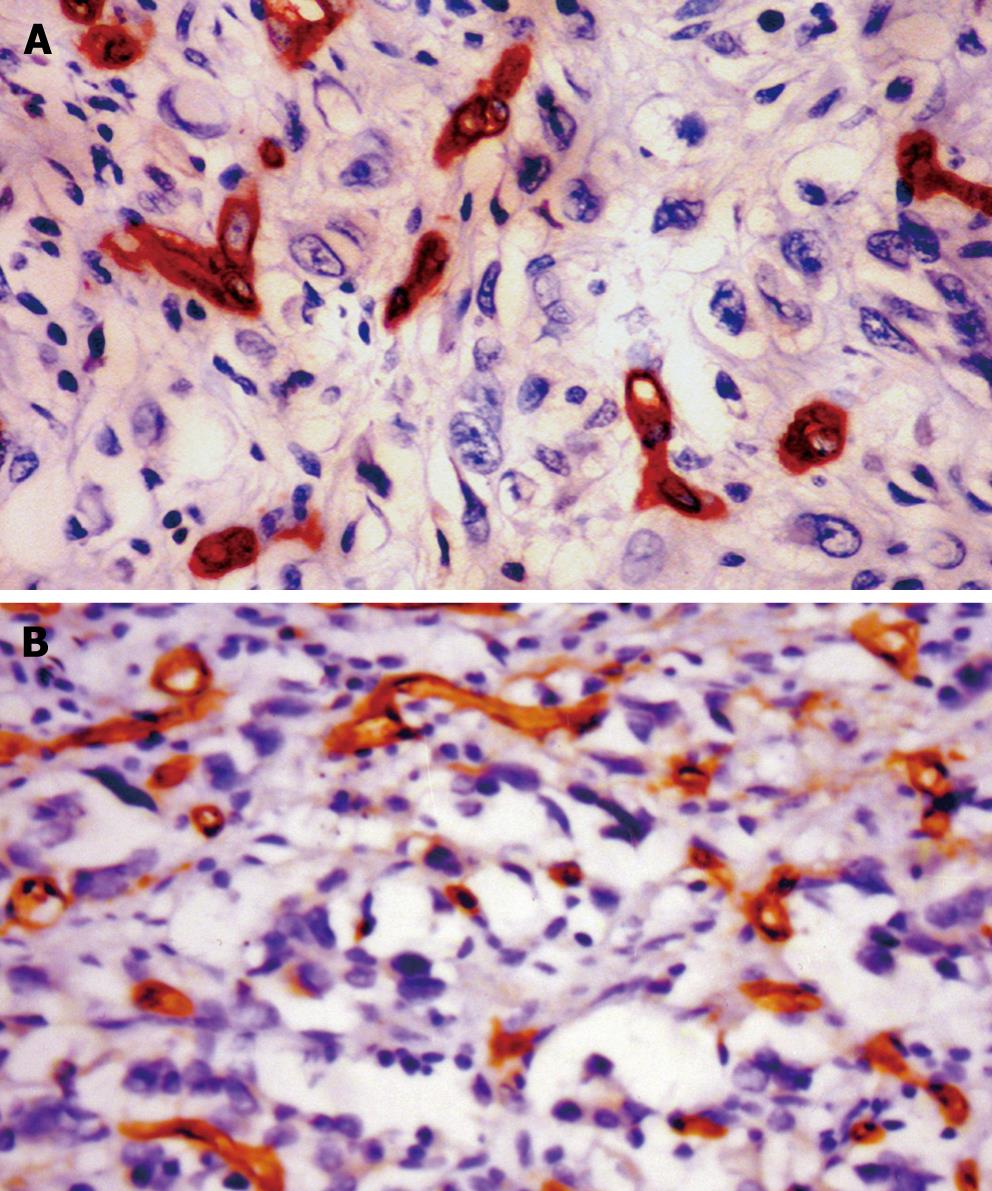
CD34 staining was positive in vascular endothelial cells. Most microvessels in cancer tissue and surrounding carcinoma were brown or dark brown, strongly stained (Figure 3). In patients of infiltrating type, stage T3-T4, vessel invasion, lymphatic metastasis, hepatic and peritoneal metastasis, the mean MVD were significantly higher than those of the expanding type (t = 3.92, P < 0.001), stage T1-T2 (t = 6.41, P < 0.01), non-vessel invasion (t = 7.96, P < 0.01), without lymphatic metastasis (t = 8.45, P < 0.001), without hepatic and peritoneal metastasis (t = 7.65, P < 0.01; t = 10.46, P < 0.01), respectively. But no correlation was found within the different histological types (P > 0.05) (Table 1).
The mean MVD (70.45 ± 14.33/mm2) in gastric carcinoma specimens with positive expression of integrin β3 mRNA was higher than that of the negative expression group (43.68 ± 18.77/mm2; t = 8.78; P < 0.01). Similarly, the mean MVD in gastric carcinoma specimens with positive expression of VEGF (72.96 ± 1.38/mm2) was higher than that of the negative expression group (37.76 ± 1.84/mm2; t = 8.78; P < 0.01). In addition, the rate of VEGF in carcinoma tissues with positive expression of integrin β3 mRNA was 87.1% (54/62), which was significantly higher than that in the integrin β3 mRNA negative expression group (21.4%, 12/56; χ2 = 51.48; P < 0.01). Therefore, there was a positive expression relationship between integrin β3 mRNA, VEGF protein and MVD.
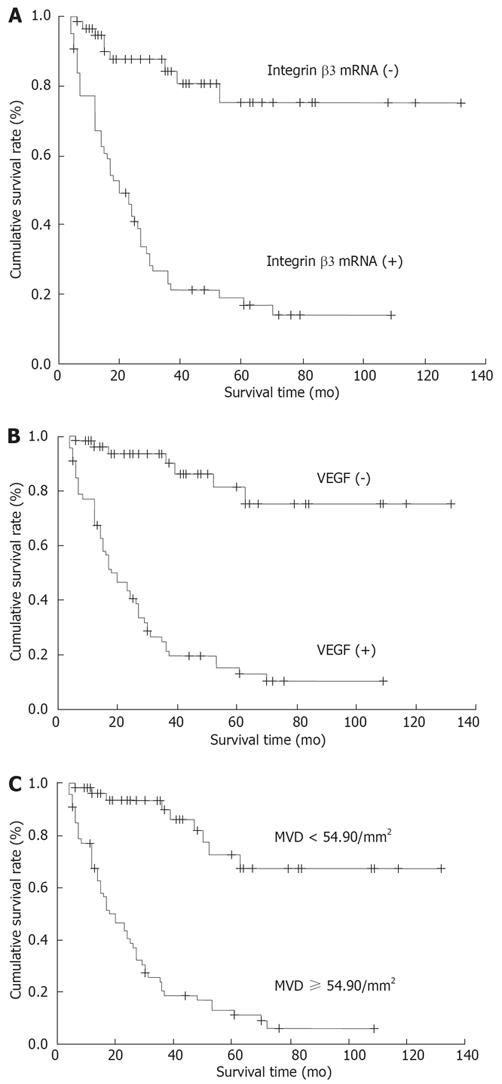
The survival time in patients with positive expression of integrin β3 mRNA, VEGF protein and MVD ≥ 54.9/mm2 was significantly shorter than that in patients negative for expression of integrin β3 mRNA (P < 0.05), VEGF protein (P < 0.01) and MVD < 54.9/mm2 (P < 0.01). The five-year survival rate in patients with positive expression of integrin β3 mRNA, VEGF and MVD ≥ 54.9/mm2 was significantly lower than that of patients negative for expression of integrin β3 mRNA (P < 0.05), VEGF protein (P < 0.05) and MVD < 54.9/mm2 (P < 0.01) (Figure 4A-C; Table 2).
| Groups | n | Mean survival period (mo) | t | P | 5-yr survival rate (%) | χ2 | P | |
| Integrin β3 mRNA | - | 56 | 38.46 ± 29.20 | 2.27 | < 0.05 | 71.4 (40/56) | 5.60 | < 0.05 |
| + - +++ | 62 | 27.18 ± 24.73 | 11.3 (7/62) | |||||
| VEGF | - | 54 | 41.70 ± 30.62 | 3.40 | < 0.01 | 70.4 (16/54) | 6.52 | < 0.05 |
| + - +++ | 64 | 24.80 ± 21.78 | 10.9 (7/64) | |||||
| MVD (n/mm2) | < 54.90 | 47 | 44.70 ± 32.05 | 3.84 | < 0.01 | 63.8 (17/47) | 13.85 | < 0.01 |
| ≥ 54.90 | 71 | 24.48 ± 20.40 | 8.5 (6/71) |
Integrins are a major family of cell adhesion molecules, which mediate cell-cell adhesion or cell-extracellular matrix (ECM) adhesion, and affect signal transduction, cell proliferation, differentiation, survival and apoptosis[9]. Integrins are essential for invasion and metastasis of carcinoma cells. Integrins are transmembrane αβ heterodimers that require divalent cations for their non-covalent association. To date, 19α and 8β subunits have been identified, and these can combine to form at least 25 different integrins. The ligands of integrins are type I and type IV collagen, laminin, fibronectin, vitronectin and fibrinogen. Integrins can bind to the ligands in ECM through the specific amino acid sequence in the ligands, which is a tripeptide that features in the integrin-interaction site of many ECM proteins, known as arginine-glycine-aspartic acid (RGD)[1011]. Different adhesion receptors play separate roles in intercellular homotypic or heterotypic adhesion, and the connection between cancer cells and ECM[12]. The connection between integrin αvβ3 and fibronectin enables membrane type-matrix metalloproteinase (MT-MMP) to be activated in human endothelial cells, and can also enforce cell invasion[13]. Ria et al[14] have found that integrin β3 interacts with fibronectin and vitronectin, which play a role in enhancing proliferation and migration of tumor cells.
Hosotani et al[15] have indicated integrin αvβ3 expression is elevated in pancreatic cancer, and expression in patients with lymphatic metastasis is significantly higher than that in patients without lymphatic metastasis. Furthermore, some studies have shown that, in malignant melanoma, ovarian, breast and prostate cancer, the invasive ability of tumor cells with positive integrin β3 expression is powerful, which suggests that integrin β3 is closely related to cancer invasion and metastasis[16–18].
Our study showed that integrin β3 mRNA expression in gastric carcinoma samples was stronger than that in non-tumor gastric mucosa, and the positive expression rate were significantly higher in the infiltrating type group, poor differentiation, perforating serosa layer, lymphoid node invasion, hepatic metastasis and peritoneal dissemination. This result was consistent with previous studies, and showed that increased expression of integrin β3 in gastric cancer influenced the adhesion between tumor cells and ECM. Moreover, it may influence signal transduction, thereby changing the biological behavior of tumor cells, and enhancing the potency of infiltration and migration[19].
Angiogenesis not only accelerates tumor growth, but also increases the opportunity of tumor cells for invading the vasculature, hence it promotes tumor metastasis. As the bridge between vascular endothelial cells and ECM, integrins play an important role in angiogenesis[820]. Previous studies have shown that integrin αvβ3 is minimally expressed on resting or normal blood vessels, but is significantly up-regulated in vascular cells within human tumors, and integrin αvβ3 has been implicated in tumor-induced angiogenesis[2122].
CD34 protein, an endothelial-specific marker, was immunohistochemically stained, and MVD was determined in our study. Statistical analysis showed that β3 integrin mRNA expression ratio was positively correlated with MVD and VEGF. β3 integrin mRNA, VEGF and MVD were all associated with tumor clinicopathological features such as growth pattern, depth of invasion, vessel invasion, and lymph node and distant metastasis, which indicates that VEGF and β3 integrins contribute greatly to the progress of gastric cancer, by modulating angiogenesis. VEGF, the most specific and most potent regulator of angiogenesis, can stimulate the activation of endothelial cells, degradation of the matrix membrane, and migration and proliferation of cells to form new blood vessels[23]. Meanwhile, integrins and ECM are necessarily involved in proliferation and migration of endothelial and vascular smooth muscle cells[24–26]. Integrin β3 may enable VEGF to stimulate endothelial cell proliferation and capillary angiogenesis through activating VEGF receptor-2 (Flk-1) and VEGF receptor-3 (Flt-4)[2728]. Therefore, integrin β3 and VEGF can enhance synergistically tumor angiogenesis, and lead to proliferation, infiltration and migration of tumor cells.
This study also demonstrated the relationship between integrin β3 mRNA, VEGF protein expression, MVD, survival period, and 5-year survival rate of gastric carcinoma patients. The results suggest positive integrin mRNA and VEGF protein expression, and MVD ≥ 54.9, are indicative of poor prognosis.
In summary, integrin β3 and VEGF synergistically enhance tumor angiogenesis, and may play crucial roles in invasion and metastasis of gastric carcinoma. Therefore, they can be used as biomarkers for diagnosis and prognosis, and as novel molecular therapeutic targets[2930].
Metastasis is the most common cause of death and is a major obstacle to the successful treatment of gastric cancer. It is necessary to develop effective new strategies for the prediction, diagnosis and treatment of gastric cancer metastasis. Angiogenesis plays a central role in tumor growth and development.
Recent investigations have shown that integrin β3 expression is elevated in pancreatic and breast cancer. High expression of integrin β3 is positively related to cancer cell invasion and metastasis and tumor angiogenesis, but few studies have investigated integrin β3 expression in gastric cancer, and the impact of integrin β3 and VEGF on prognosis.
Integrin β3 and VEGF expression can synergistically enhance tumor angiogenesis, and may play a crucial role in invasion and metastasis of gastric carcinoma.
This study cannot only define further the mechanism of metastasis of gastric cancer, but can also aid the development of tumor angiogenesis inhibitor. Integrin β3 and VEGF can be used as prognostic biomarkers and as novel molecular therapeutic targets.
Angiogenesis is the process that leads to the formation of new blood vessels, and it plays a central role in cancer cell survival, local tumor growth, and development of distant metastasis.
The authors studied integrin β3 mRNA and VEGF protein expression in gastric carcinoma by in situ hybridization and immunohistochemistry. They concluded that high expression of integrin β3 mRNA and VEGF protein were related to invasion and metastasis, and poor prognosis of gastric cancer.
| 1. | Ma JL, Liu WD, Zhang L, Feng GS, Zhao HJ, You WC. Analysis of mortality trend of cancer from 1980 to 2002 in Linqu County Shandong Province. Zhonghua Yufang Yixue Zazhi. 2006;40:405-408. |
| 2. | Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624-1636. |
| 3. | Zhang L, Zhao ZS, Ru GQ, Ma J. Correlative studies on uPA mRNA and uPAR mRNA expression with vascular endothelial growth factor, microvessel density, progression and survival time of patients with gastric cancer. World J Gastroenterol. 2006;12:3970-3976. |
| 4. | Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C, Sculier JP. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694-701. |
| 5. | Foote RL, Weidner N, Harris J, Hammond E, Lewis JE, Vuong T, Ang KK, Fu KK. Evaluation of tumor angiogenesis measured with microvessel density (MVD) as a prognostic indicator in nasopharyngeal carcinoma: results of RTOG 9505. Int J Radiat Oncol Biol Phys. 2005;61:745-753. |
| 6. | Zhao HC, Qin R, Chen XX, Sheng X, Wu JF, Wang DB, Chen GH. Microvessel density is a prognostic marker of human gastric cancer. World J Gastroenterol. 2006;12:7598-7603. |
| 7. | Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91-100. |
| 8. | Hwang R, Varner J. The role of integrins in tumor angiogenesis. Hematol Oncol Clin North Am. 2004;18:991-1006, vii. |
| 9. | Moschos SJ, Drogowski LM, Reppert SL, Kirkwood JM. Integrins and cancer. Oncology (Williston Park). 2007;21:13-20. |
| 10. | Humphries MJ. Integrin structure. Biochem Soc Trans. 2000;28:311-339. |
| 12. | Hato T, Pampori N, Shattil SJ. Complementary roles for receptor clustering and conformational change in the adhesive and signaling functions of integrin alphaIIb beta3. J Cell Biol. 1998;141:1685-1695. |
| 13. | Ip YC, Cheung ST, Leung KL, Fan ST. Mechanism of metastasis by membrane type 1-matrix metalloproteinase in hepatocellular carcinoma. World J Gastroenterol. 2005;11:6269-6276. |
| 14. | Ria R, Vacca A, Ribatti D, Di Raimondo F, Merchionne F, Dammacco F. Alpha(v)beta(3) integrin engagement enhances cell invasiveness in human multiple myeloma. Haematologica. 2002;87:836-845. |
| 15. | Hosotani R, Kawaguchi M, Masui T, Koshiba T, Ida J, Fujimoto K, Wada M, Doi R, Imamura M. Expression of integrin alphaVbeta3 in pancreatic carcinoma: relation to MMP-2 activation and lymph node metastasis. Pancreas. 2002;25:e30-e35. |
| 16. | Trikha M, Timar J, Zacharek A, Nemeth JA, Cai Y, Dome B, Somlai B, Raso E, Ladanyi A, Honn KV. Role for beta3 integrins in human melanoma growth and survival. Int J Cancer. 2002;101:156-167. |
| 17. | Liapis H, Adler LM, Wick MR, Rader JS. Expression of alpha(v)beta3 integrin is less frequent in ovarian epithelial tumors of low malignant potential in contrast to ovarian carcinomas. Hum Pathol. 1997;28:443-449. |
| 18. | Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67:61-73. |
| 19. | Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124-138. |
| 21. | Cooper CR, Chay CH, Pienta KJ. The role of alpha(v)beta(3) in prostate cancer progression. Neoplasia. 2002;4:191-194. |
| 22. | Reinmuth N, Liu W, Ahmad SA, Fan F, Stoeltzing O, Parikh AA, Bucana CD, Gallick GE, Nickols MA, Westlin WF. Alphavbeta3 integrin antagonist S247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079-2087. |
| 23. | Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242-248. |
| 24. | Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207-238. |
| 25. | Ross RS. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res. 2004;63:381-390. |
| 26. | Mahabeleshwar GH, Feng W, Reddy K, Plow EF, Byzova TV. Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res. 2007;101:570-580. |
| 27. | Reynolds AR, Reynolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, Bodary SC, Hodivala-Dilke KM. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64:8643-8650. |
| 28. | Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593-599. |
| 29. | Gasparini G, Longo R, Toi M, Ferrara N. Angiogenic inhibitors: a new therapeutic strategy in oncology. Nat Clin Pract Oncol. 2005;2:562-577. |
| 30. | Belvisi L, Riccioni T, Marcellini M, Vesci L, Chiarucci I, Efrati D, Potenza D, Scolastico C, Manzoni L, Lombardo K. Biological and molecular properties of a new alpha(v)beta3/alpha(v)beta5 integrin antagonist. Mol Cancer Ther. 2005;4:1670-1680. |