INTRODUCTION
The functioning of the gastrointestinal tract with its balanced microflora depends on the establishment and preservation of distinct compartments which are lined by a sheet of epithelial cells. The gastrointestinal epithelial lining consists of a monolayer of columnar cells that are held together by circumferential intercellular junctions to form a selectively permeable barrier to the luminal contents. Thus, the epithelium prevents unwanted solutes, microorganisms, and luminal antigens from entering the body[12]. Components of the intestinal mucosal barrier include luminal secretions such as mucus which is secreted on the apical surfaces of epithelial cells, followed by IECs with lipid plasma membranes, specific membrane transport systems responsible for transepithelial passage of different molecules, and the stromal compartment below the epithelial layer. The intestinal barrier has to be permeable for nutrients and macromolecules which are important for growth and development. At the same time, it has to provide an effective barrier to harmful macromolecules and microorganisms. The mechanisms that have evolved to deal with these physiological events are extremely complex. On the one hand, the structural and functional properties of the epithelium limit the amount of antigens reaching the surface of the epithelium. On the other hand, both specialized cells of the follicle-associated epithelium (M-cells) and dendritic cells sample luminal antigens that are then delivered to the cells of the mucosal immune system thereby guaranteeing permanent immunosurveillance. An intercellular junction referred to as the tight junction (TJ) is located at the apical end of the lateral intercellular space and is considered a key player that regulates paracellular movement of fluid and solutes[3]. Altered TJ structure and epithelial permeability has been observed in IBD[2]. Additionally, pathogens and bacterial toxins influence epithelial permeability by modulating TJ proteins[4–6]. Although the permeability defects could conceivably be due to the marked apoptosis that occurs during the inflammation processes, numerous studies have clearly shown that epithelial cell apoptosis alone does not account for the entire permeability deficits[7–10]. In addition, the importance of the intestinal microflora and more specifically its composition in physiological and pathophysiological processes in the human GI tract is becoming more evident. New discoveries relate to the beneficial effects of normal microflora and probiotics in preventing gastrointestinal infections[111213]. Probiotics released by bacteria may functionally modulate the intestinal epithelial barrier of the host by different mechanisms, including the competition of whole organisms for contact with the epithelial surface as well as stabilization of the cytoskeleton and barrier function and the induction of mucin expression.
Some of the most recently available data are discussed in this review. This field is changing rapidly and it is increasingly becoming accepted that immunogenetics play an important role in the predisposition, modulation and perpetuation of IBD. The role of the intestinal environment and the enteric flora in particular appears to be of greater significance than previously thought. This complex interaction of genetic, microbial and environmental factors culminates in a sustained activation of the mucosal immune system. This is facilitated by defects in the intestinal epithelial barrier and its mucosal immune system and results in active inflammation and tissue damage. We will concentrate on the role of the intestinal barrier, its regulation and modulation in IBD.
THE INTESTINAL BARRIER
Mucosal integrity and repair
The small and large intestine have potent mucosal defense and repair mechanisms. These mechanisms include a fast rate of cell renewal, a capable mucosal blood flow, a continuous adherent mucus layer and the presence of regulatory peptides that can stimulate repair mechanisms. The epithelial monolayer which lines the intestinal tract originates from multipotent stem cells present in the crypts[14]. Four major IECs are generated by these multipotent cells: (1) the absorptive enterocytes (reviewed in[15]), (2) the goblet cells responsible for the assembly of mucins[16] and trefoil peptides needed for epithelial growth and repair, (3) the enteroendocrine cells which export peptide hormones (reviewed in[17]) and (4) the paneth cells which secrete antimicrobial cryptidins or defensins, digestive enzymes and growth factors[18]. Injury to the epithelial lining resulting in mucosal erosion/ulceration can occur following exposure to pathogens and chemical therapeutic agents, decreased mucosal defense such as an abnormal mucus layer or reduced production of growth regulatory peptides. The GI tract epithelium has a remarkable capacity to rapidly reseal superficial erosions by migration of epithelial cells, a process referred to as restitution. Epithelial cell proliferation contributes to resealing of larger ulcers. Wound closure is influenced by a diverse array of peptides and growth factors released into the milieu of the regenerating epithelium. For example, the mucosal integrity peptide, TGFα (transforming growth factor α) directly acts on the enterocytes to stimulate proliferation and migration. TGFβ, and the pancreatic secretory trypsin inhibitor (PSTI) protect the overlying mucus layer from excessive digestion by luminal proteases and are involved in maintaining normal mucosal integrity[19]. Epidermal growth factor has been proposed as mucosal protector, playing an important role in luminal surveillance and rapid response to injury[2021]. Mucosal epithelial cells and paneth cells produce a variety of antimicrobial peptides (defensins, cathelicidins, cryptidin related sequence peptides, chemokine CCL20) and bacteriolytic enzymes (lysozyme, group IIA phospholipase A2) that protect mucosal surfaces and crypts containing intestinal stem cells against invading microorganisms[21–23]. Trefoil peptides found in the goblet cells of the intestine are a family of three small proteins (TFF1, 2 and 3) which bind to the membrane-anchored glycoproteins of the filamentous brush border glycocalyx of the IECs. These peptides promote cell migration and interact with mucins such as MUC2, suggesting cooperation between the two in epithelial cell protection[24] and preservation of mucosal integrity[25]. Such peptides are rapidly up-regulated at sites of injury and inflammation. Recent experimental data has shown that the physiological role of TFF2, a member of the gastrointestinal trefoil factor family is associated with modulation of the immune system[25] and that the TFF2 rhythm is impaired in cohorts of individuals known to suffer from gastric symptoms, like H pylori infection and sleep deprivation[26].
KEY PLAYERS OF THE INTESTINAL BARRIER
Components
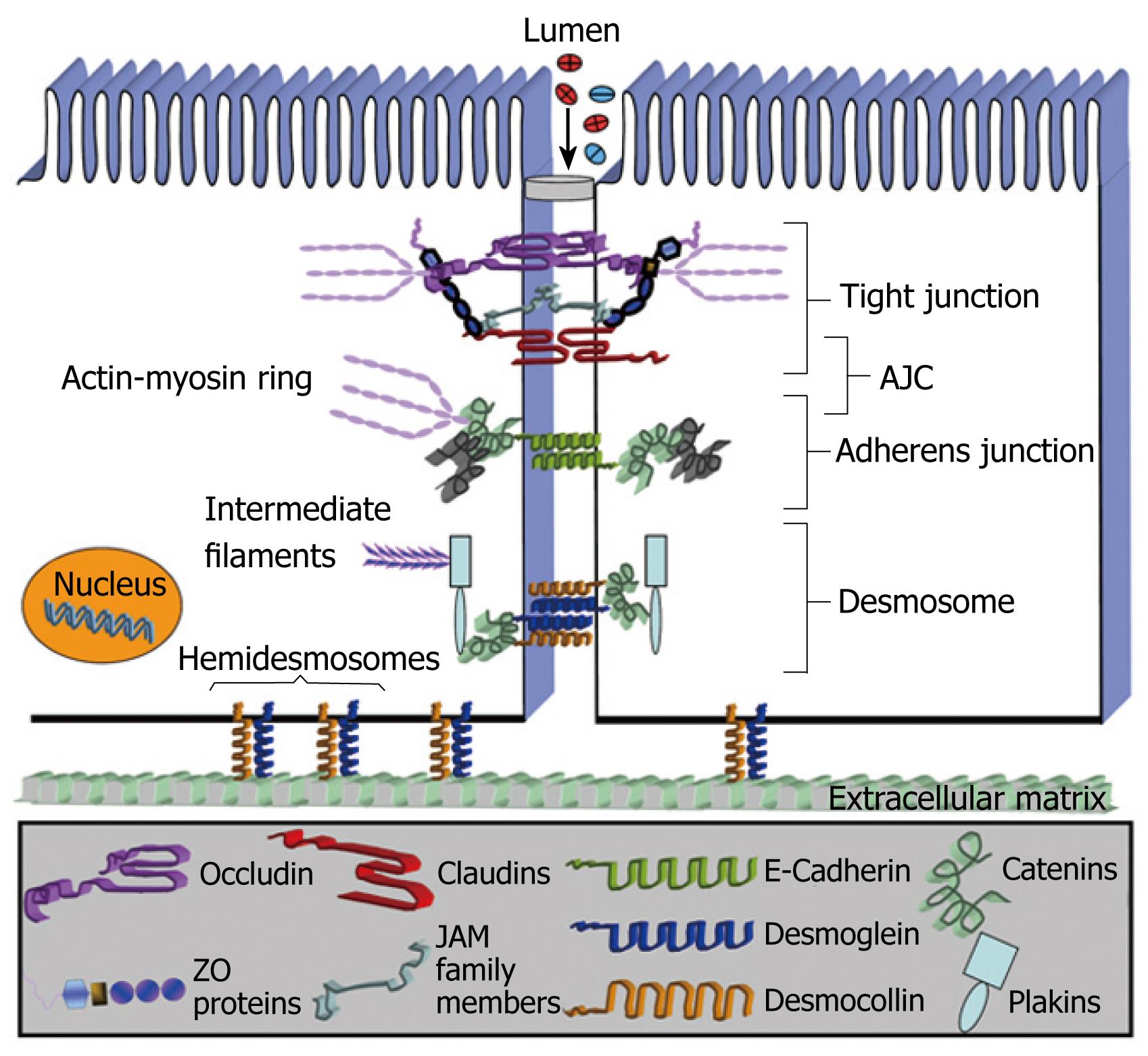
Intercellular junctions in epithelial cells play a vital role in regulating mucosal barrier properties. Specifically, the epithelial AJC consisting of the TJ and adherens junction (AJ) (Figure 1) is important in regulating cell-cell adhesion and paracellular movement of fluids and solutes. TJs are continuous, circumferential belt-like structures that form a permeability barrier at the apical end of the intercellular space. TJs regulate vectorial transport of water and electrolytes across the intestinal epithelium and prevent leakage of macromolecules from the gut lumen[2728]. Additionally, TJs restrict the diffusion of lipids and proteins between the apical and basolateral plasma membranes (fence function) thereby preserving cellular polarity and, in combination with transcellular vectorial transport processes, generate distinct environments in the opposing compartments across the epithelium. Lastly, TJ proteins play an important role in the overall epithelial differentiation of cells, and their deregulation has been observed in epithelial cancers[29–31]. By freeze-fracture electron microscopy, TJs are viewed as a series of anastomosing intramembranous strands, the complexity of which correlates with barrier properties of the epithelium[3233]. The TJ is a highly dynamic structure that regulates physiologic processes such as glucose absorption[3] and undergoes rapid regulatory changes in response to inflammation[34]. The AJs reside immediately subadjacent to TJs and play an important role in cell recognition and in mediating intercellular associations[3536]. Both the TJ and AJ are intimately linked in their regulation and function and have therefore been collectively referred to as the AJC. Lastly, subadjacent to the AJC are spot-like intercellular junctions referred to as desmosomes (DMs). Although DMs create strong intercellular associations important in the integrity of stratified epithelia such as the epidermis, the function of DMs in the intestinal epithelium is poorly understood.
Figure 1 Intestinal epithelial intercellular junctions.
At a structural level, all the above intercellular junctions consist of transmembrane proteins that affiliate with the cytoskeleton via cytoplasmic plaque proteins. The association of AJC proteins with an underlying perijunctional filamentous actin (F-actin) ring plays an important structural and regulatory role in the AJC[37]. Key integral membrane proteins of the TJ include occludin, claudin family members, junction adhesion molecule-A (JAM-A) and the coxsackie adenovirus receptor (CAR). These proteins are believed to interact in a heterotypic or homotypic fashion in the extracellular space to create the selectively permeable epithelial barrier[37–39]. The intracytoplasmic domains of these TJ proteins interact with scaffolding proteins like the zonula occludens proteins (ZO), which contain several protein-protein interaction domains such as the PDZ domains (PSD 95, Discs large, ZO-1) important in mediating interactions of AJC-associated proteins. E-cadherin is a major transmembrane protein in AJs that associates with the apical perijunctional F-actin ring via cytoplasmic proteins in the catenin family[283640]. The intestinal epithelial intercellular junctions are not static extracellular domains. Their adaptive mechanisms can undergo rapid regulatory changes in response to inflammation processes affiliated with IBD. Moreover the appropriate function of the AJC is coordinated by a complex array of signaling proteins which include the Rho and Rap family of GTPases, kinases and phosphatases[91028].
INTESTINAL BARRIER AND REGULATION OF PERMEABILITY IN IBD
Most current treatments for IBD are aimed at reducing disease severity and at prolonging periods of disease-free remission by pharmaceutical suppression of inflammation. It appears that the pathophysiology of IBD is multifactorial. The enormous complexity of IBD patho-physiology therefore requires a systematic approach to identify the molecular events that cause and sustain the chronic, recurring inflammation. It has been suggested that the continuous stimulation of the mucosal immune system due to an increased permeability of the IECs may be the primary defect in patients suffering from IBD, whereas a healthy epithelium provides an effective barrier against luminal antigens. Increased paracellular permeability has been documented in the epithelial lining from both the acutely inflamed and chronically damaged areas of the intestine[4142]. Animal studies support the tendency for development of inflammation in areas of the intestine lying beneath the permeability defect[43] or even for an increased permeability prior to the onset of a spontaneous intestinal inflammation[44]. It is well known that intestinal permeability is regulated directly through alteration of TJ proteins, or indirectly through effects on the cytoskeleton. A broad array of inflammatory cytokines have been reported to regulate TJs and barrier function by recruiting additional inflammatory cells into the intestinal wall and by re-distribution of TJ proteins in IECs and endothelial cells[45].
CROHN’S DISEASE
Besides genetic linkage like CARD15/NOD2 mutations on chromosome 16[4647], OCTN1 and 2 mutations on chromosome 5 and DLG5 mutations on chromosome 10[48], abundant evidence indicates that increased intestinal permeability is implicated in the pathogenesis of CD[49]. The integrity of the intestinal barrier in patients with Crohn’s disease is known to be compromised. Moreover, first degree relatives of patients with CD have been documented to have increased enteric permeability[50–52]. Interestingly, spouses of patients with CD suffered from increased intestinal permeability[53], which suggests that environmental factors may play a role. It has not been investigated whether these individuals develop CD or other gastrointestinal disorders. However, the debate continues if the increased enteric permeability is to blame for the development of CD or if the barrier defect is a consequence of an existing immune or inflammatory response. Animal models mimicking CD such as the SAMP1/Yit model, showed increased paracellular permeability across intestinal epithelial cells at an early stage of disease (three week old mice), prior to the onset of inflammation[54]. Transgenic animal models revealed the importance of E-cadherin in maintaining the epithelial barrier by demonstrating that dysfunction of AJ proteins contributed to an IBD-like process[55]. In addition, a number of studies have shown a potential role for inflammatory cytokines like TNF-κ and IFN-γ in directly increasing intestinal epithelial permeability. In vitro model systems have demonstrated that such pro-inflammatory cytokines influence barrier function by inducing disassembly of TJs in epithelial cells[791056]. Furthermore, a role for TNF-κ in both apoptosis independent disruption of epithelial barrier function via alteration of TJs and upregulation of epithelial apoptosis (reversible by anti-TNF-κ antibody treatment) in the absence of changes in the expression of TJ proteins was reported, suggesting that TNF-κ may be one of the major links between the leaky bowel and Crohn’s disease[5758]. Soderholm et al[59] reported that non-inflamed ileac mucosa from patients with CD showed increased epithelial permeability and that increased endosomal uptake of antigens in ileac CD may be mediated by TNF-κ[60]. A recently published study pertaining to the expression of TJ proteins (occludin and zonula occludens), alpha2-smooth muscle actin, TGF-κ with a cytoskeletal protein (F-actin) in the intestinal epithelium of patients with inflammatory bowel disease showed that latent dislocation of TJ proteins, without disturbance of the cytoskeleton in the inactive mucosa of patients with CD, may permit the invasion of gut antigens secondary to the functional disruption of TJs, that in turn could initiate an altered immune response[61]. Some reports have demonstrated that IFN-γ increases permeability across model intestinal epithelial cell lines[756]. Recently published data suggest that normally poorly invasive enteric bacteria may, in situations of inflammatory stress, exploit lipid raft-mediated transcytotic pathways to cross the intestinal epithelium because of INF-γ-induced disruption of TJs[62]. Although the mechanisms by which IFN-γ induces permeability changes across the epithelium are still incompletely understood, our data demonstrated that the permeability changes occur secondary to endocytosis of TJ transmembrane proteins occludin, JAM-A and claudin-1[9]. Furthermore, we believe that such endocytosis of TJ proteins is initiated by activation of the Rho GTPases and subsequent downstream activation of Rho kinase and actin-myosin II contraction[10].
ULCERATIVE COLITIS
UC is characterized by diffuse mucosal chronic inflammatory disease in the colon. Analogous chronic mucosal inflammation has been observed in animal models such as the mdr1a-/- mice. In this mdr1a-/- animal model a link between epithelial barrier dysfunction and development of colitis has been proposed[63]. Moreover, UC is associated with a mutation of the Toll-like receptor (TLR)-4 gene in humans, resulting in impaired lipopolysaccharide (LPS) signaling[64]. LPS comprises the major cell wall component of gram-negative bacteria and is mainly recognized via TLR-4. Stimulation of TLR-4 with bacterial LPS or other ligands[65] leads to activation of the NF-κB signaling system and subsequent induction of inflammatory responses[66]. Kiechl et al[67] showed that carriers of these mutations displayed an increased risk of gram-negative infections stressing that the predisposition of UC is associated with a genetic background. Besides these genetic factors, it has been demonstrated that an activated mucosal immune system leads to impaired epithelial barrier function and tissue destruction in patients suffering from UC[668]. In contrast to patients with CD, where the cytokines IFN-γ and TNF-κ play a central role in altering the epithelial barrier function, it still remains unclear which panel of cytokines regulates inflammation and induces epithelial barrier dysfunction in UC. Recently published data has shown that IL-13 produced by CD1-reactive natural killer T cells (NKT) played a central role in a murine model of colitis[69]. The IL-13 producing cells were also found in patients suffering from UC which suggests that this cytokine is one of the key mediators in the intestinal pathology of UC. Interestingly Heller et al showed that IL-13 mediates a drop of transepithelial resistance without any induction of necrosis in model IECs by increasing the paracellular permeability stressing the profound effect of IL-13 on epithelial barrier function[34]. Furthermore, it has been shown that IL-13 is produced in large amounts in the lamina propria of patients with UC. This is accompanied by increased expression of the pore-forming tight junction molecule claudin 2, which leads to the development of impaired barrier function for small cations and is thought to be responsible for the diarrhea in UC[70].
PROBIOTICS AND BARRIER FUNCTION IN UC AND ANIMAL MODELS OF COLITIS
Data from different in vitro and in vivo models support the involvement of luminal bacteria in mucosal inflammation and alteration of the intestinal barrier function especially in UC. In contrast to CD, ulcerative colitis is a disease involving mucosa only. Intestinal inflammation is accompanied by direct adherence of bacteria to this mucosal surface while the protective function of the mucus layer seems to be disrupted[71]. Studies have shown that bacteria like E. coli, lactobacilli, bifidobacteria and streptococci are able to interact with immunocompetent cells, using the mucosal interface and locally modulate the production of proinflammatory cytokines[72]. They may also directly change the structure of the intestinal epithelial barrier. Although the treatment of CD with probiotics has not demonstrated any sufficient results yet[73–75], the treatment of UC has shown encouraging data[76–81]. Generally the mechanisms by which probiotic microbial agents contribute to the protection of the inflamed intestinal epithelium involve two main categories: (1) competition for binding sites and inhibition of pathogen growth as well as epithelial attachment or invasion[8283] and (2) stimulation of the mucosal immune system including the stimulation of anti-inflammatory cytokine levels and enhancement of the barrier function. Recent work provided evidence that protective effects of probiotic microorganisms in a DSS model of experimental colitis are mediated by DNA, which was recognized by the mucosal TLR9 receptor and this interaction consequently lead to an increased production of β-defensins. Defensins are known to be responsible for the destabilization and disruption of microorganism cell membranes, leading to an increase in permeability and leakage of small molecules[8485]. To combat invading pathogens, phagocytes need to be recruited to sites of bacterial entry. Leukocyte recruitment occurs along gradients of chemotactic factors, including chemokines and defensin chemoattractants at nanomolar concentrations[8687]. In addition, it has been shown that the probiotic compound VSL #3 was effective as primary therapy in a colitis model of IL-10 gene-deficient mice. A direct effect on epithelial barrier function was described. The treatment resulted in a normalization of colonic physiologic function and barrier integrity along with a reduction in mucosal levels of proinflammatory cytokines and a significant improvement in histological disease by secretion of soluble factors enhancing the barrier integrity[88]. This stabilization of the intestinal barrier function is an important target in the treatment of intestinal inflammatory disorders. Otte et al[89] reported that the treatment of cultured IECs with VSL #3 lead to an increase of the transepithelial electrical resistance (TEER). In addition, this probiotic mixture was found to diminish Salmonella-induced alterations in the cellular cytoskeleton[9091], including the distribution of the tight junction protein ZO-1. The stabilization of the cytoskeleton by regulation of tight junctions is important in the preservation of the epithelial architecture and, thereby, in the maintenance of the intestinal barrier, which might be affected by the MAPK pathway as postulated by the authors[89]. Interestingly, the incubation of IECs with these probiotics also induced the expression of several mucins, leading to less adhesion of microorganisms and its compounds like LPS to the epithelial surface. Together these housekeeping mechanisms might be responsible for the homeostasis of the intestinal barrier function during inflammatory disorders like UC.
CONCLUSION AND PERSPECTIVES
The pathophysiology of intestinal inflammation is multifactorial. Whatever the trigger, increased epithelial permeability plays a central role in the inflammatory process. The permeability changes and inflammation are intimately linked and play a central role in perpetuating the chronic mucosal damage observed in IBD. TJs do not merely represent static junctions between epithelial cells but are multifunctional protein complexes involved in numerous vital and diverse functions of epithelial cells. It is becoming evident that the TJs are extremely dynamic structures involved in developmental, physiological, and pathological conditions like CD and UC, where defects in mucosal integrity and repair remain key elements in initiation and perpetuation of the disease. Future studies will be focused on the mechanisms by which the epithelial barrier can be made “less leaky”. The mucosal immune system is the central effector of intestinal inflammation and injury, with cytokines playing a central role in modulating inflammation and epithelial barrier function. Cytokines are, therefore, logical targets for IBD therapy. Finally, preliminary clinical trials of probiotic therapies in IBD may offer a valuable tool for prevention and control of IBD. Understanding the function and action of these probiotics will lead to the selection of useful probiotic strains for clinical application. As more work is directed at the function and modulation of the intestinal barrier, further potential therapeutic targets will provide more options to combat CD and UC.
Supported by Grants from the German Research Foundation (Deutsche Forschungsgemeinschaft La 2359/1-1 to M.L.), National Institutes of Health (DK 55679, DK 59888 to A.N.) and Crohn’s and Colitis Foundation of America (to A.N.)