Published online Jul 28, 2008. doi: 10.3748/wjg.14.4562
Revised: June 23, 2008
Accepted: June 30, 2008
Published online: July 28, 2008
AIM: To investigate the expression frequency of endocan in colorectal cancer and analyze the relationship between endocan expression and clinical parameters and to study the role of endocan in colorectal carcinogenesis.
METHODS: Expression of endocan in 72 tumor tissue samples of colorectal cancer as well as in 27 normal mucous membrane tissue samples was analyzed using in situ hybridization, immunohistochemistry on tissue microarray, Western blot and reverse-transcript polymerase chain reaction (RT-PCR).
RESULTS: The expression of endocan was higher in normal colon and rectum tissue samples than in cancerous tissue samples (mRNA = 92.6%, protein = 36%), and was lower in colorectal cancer tissue samples (mRNA = 70.4%, protein = 36.1%). No correlation was found between staining intensity and clinical parameters such as sex, age, tumor size and TNM stage. However, the expression of endocan was positively correlated with the tissue differentiation in colorectal cancer.
CONCLUSION: The expression of endocan is down-regulated in colorectal cancer and is positively correlated with the tissue differentiation in colorectal cancer, suggesting that the expression of endocan is associated with development and differentiation of colorectal cancer.
- Citation: Zuo L, Zhang SM, Hu RL, Zhu HQ, Zhou Q, Gui SY, Wu Q, Wang Y. Correlation between expression and differentiation of endocan in colorectal cancer. World J Gastroenterol 2008; 14(28): 4562-4568
- URL: https://www.wjgnet.com/1007-9327/full/v14/i28/4562.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4562
Colon and rectum cancers accounted for about 1 million new cases in 2002 (9.4% of the world total)[1], There is at least a 25-fold variation in the occurrence of colorectal cancer around the world. The incidence of colorectal cancer increases rather rapidly in countries where the overall risk was formerly low (especially in Japan, but also elsewhere in Asia)[2]. Although it has been found that many factors are correlated with genesis and development of colon and rectum cancers, it cannot explain all the clinical and pathological manifestations. It is critical to investigate new factors which are intimately correlated with initiation and development of colorectal cancer.
Endocan, previously called endothelial cell-specific molecule-1 (ESM-1)[3], is over expressed in human tumors, and its serum levels are elevated in late-stage lung cancer and experimental tumor, as measured by enzyme-linked immunoassay or by immunohistochemistry. mRNA level of endocan is also recognized as one of the most significant molecular signatures with a poor prognosis of several types of cancer including lung cancer. Over expression of this dermatan sulphate proteoglycan is also directly involved in tumor progression as observed in mouse models of human tumor xenografts. These results suggest that endocan is a biomarker of inflammatory disorders and tumor progression as well as a validated therapeutic target in cancer.
We studied the expression of endocan in colon and rectum tissue samples. The results of this study indicate that endocan expression is down-regulated in colorectal cancer and positively correlated with the differentiation of colorectal cancer. Changes in endocan expression represent an important step in development and differentiation of colorectal cancer.
Seventy-two colorectal cancer patients, who consecutively underwent radical surgical resection at Anhui Medical University Hospital from the year 2001 to 2003, were recruited into this study. Tumor and mucosa samples were embedded in paraffin after 16 h formalin fixation. None of the patients (23 males, 49 females, mean age 54 years, range 17-87 years) received any anticancer therapy. According to the TNM classification[4], 43 cases were at stages I and II, 29 cases at stages III and IV. Well- and moderately- differentiated adenocarcinoma was found in 57 patients and poorly-differentiated adenocarcinoma was observed 15 patients[5], and strong lymphoid infiltrate including lymphoid follicles with germinal centers was demonstrated in 39 patients.
cRNA probe labeling: The sequences of specific primers for endocan are as follows: sense, 5'-AGCTGGAATTCCATGAAGAG (20 bp) and antisense, 5'-TCTCTCAGAAAGCTTAGCCG (20 bp)[3]. PCR was performed to amplify endocan DNA, and the PCR product was ligated into the pGEM-T-Vector to get the recombinant plasmid pGEM-T-endocan. The recombinant plasmid was transformed into E.coli, amplified and digested with the restriction endonuclease (EcoRI and HindIII). The objective gene (V-gene) was purified using a DNA gel extraction kit to gobtain the probes for the following digoxigenin-labeling and detected according to the manufacturer's instructions.
Hybridization: All specimens were fixed in 10% neutral buffered formalin and embedded in paraffin. A series of 5-&mgr;m thick sections were cut for analysis. In situ hybridization was performed as previously described[6] with certain modifications, using digoxigenin-labeled antisense cDNA probes. Briefly, the sections were dried at 60°C for 4 h, dewaxed, rehydrated and pretreated with DEPC-treated PBS containing 100 mmol/L glycine and 0.3% Triton X-100, respectively. The sections were then permeabilized with 20 &mgr;g/mL RNase-free proteinase K (boster, Wuhuan, China) for 20 min, incubated at 37°C for at least 20 min with prehybridization buffer. Each section was overlaid with 30 &mgr;L hybridization buffer containing a 10 ng digoxigenin-labeled cDNA probe and incubated at 42°C overnight. After hybridization, the section was incubated with digoxigenin antibody (75 mU/mL) for 2 h. The positive signal for endocan mRNA was detected using DAB as a substrate. The presence of brown staining in the cytoplasm was considered positive.
Paraffin-fixed tissue was cut into 50 5-&mgr;m thick sections for protein extraction and mounted onto plain glass slides. Three 5-&mgr;m thick sections for protein extraction were deparaffinized in xylene, rehydrated in graded ethanol, immersed in distilled water, and air-dried. To exclusively collect 5 mm × 5 mm cancer tissues, the targeted areas were cut microscopically with a fine needle for observation of the morphology of HE-stained sections under a microscope. After the tissue sections on the glass slide were immersed in distilled water, only the targeted areas of cancer tissue were separated from the glass slide and recovered. Adenoma tissue was also cut into sections and collected in the same manner. Normal mucosa was recovered from 5 cm-long sections of full-depth colorectal wall with a fine needle as previously described[78].
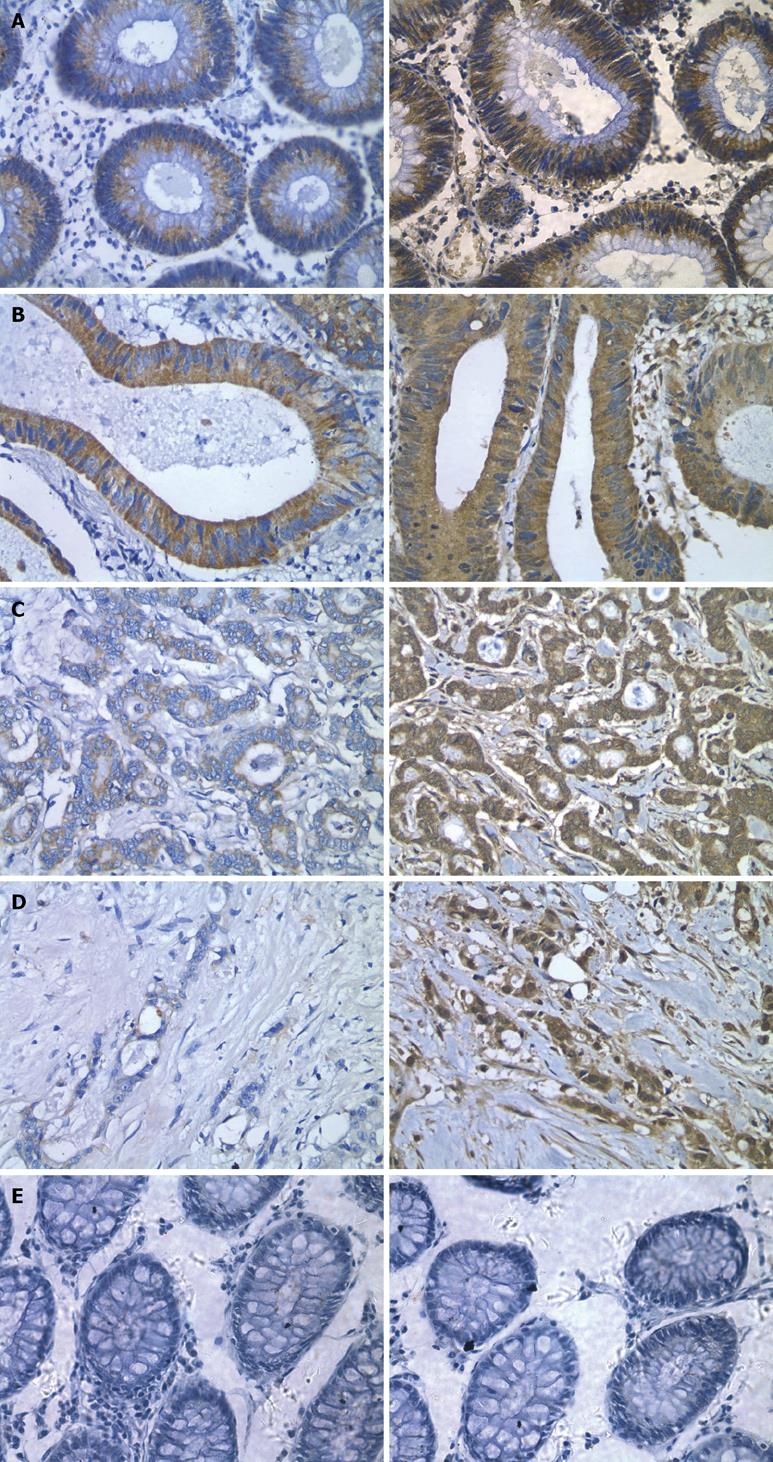
The pathology of colorectal carcinoma was performed on 5-&mgr;m thick sections of 10% formalin-embedded samples with a S-P kit. Slides were boiled in 10 mmol/L citrate buffer (pH 6.0) for 10 min to allow antigen retrieval before a 12-h incubation at 4°C with primary antibody against endocan (Santa Cruz). The mean percentage of positive tumor cells was determined in ten areas at a high magnification (× 400) and graded from 0 to 4 (0 ≤ 5% positive cells, 1 = 6%-25%, 2 = 26%-50%, 3 = 51%-75%, and 4 = 76%-100%, 0 = negative, 1-4= positive). Negative controls were obtained by omitting the primary antibody. Each normal mucosa sample, as an internal positive control, was simultaneously analyzed. Slides were read by two observers blinded to the clinical data.
Two micrograms of total RNA was prepared from colon and rectal tissues, randomly primed, and reverse transcribed with Superscript II (Gibco). The sequences of specific primers used for endocan are as follows: sense, 5'-CTCAGGCATGGATGGCATGAAGTG-3'; antisense, 5'-GAGACCCGGCAGCATTCTCTT TCA-3'; and β-actin: sense: 5'-ACTCTTCCAGCCTTC CTTC-3' and antisense: 5'-ATCTCCTTCTGCATC CTGTC-3'. After a hot start at 94°C, 35 PCR cycles were performed, each cycle consisting of annealing at 57°C for 45 s and extension at 72°C for 45 s.
Twenty micrograms of protein was incubated in a loading buffer (125 mmol/L Tris-HCl, pH 6.8, 10% β-mercapto-ethanol, 4.6% SDS, 20% glycerol and 0.003% bromophenol blue) for 5 min at 100°C, separated by sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and electroblotted to PVDF membrane (BioRad). After non-specific binding sites were blocked for 1 h with 5% nonfat milk in TPBS (PBS contained 0.05% Tween 20), the membrane was incubated overnight at 4°C with primary antibody. After washing 3 times in TPBS, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG for 2 h at room temperature, and washed twice with TPBS. Immunoblot was detected by autoradiography using an enhanced chemoluminescence detection kit.
Chi-square test and F-test were used to compare the categorical data. SPSS 11.0 was used to analyze the data.
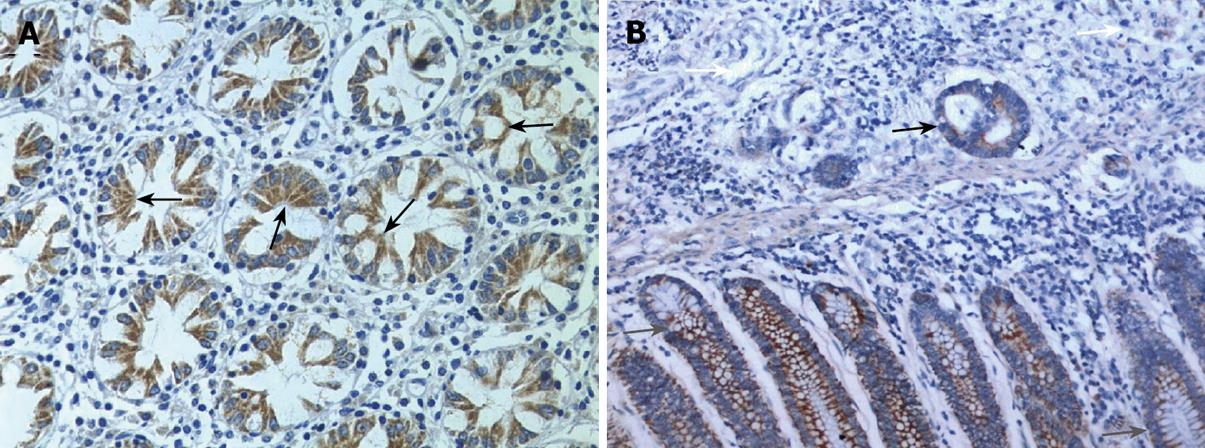
In situ hybridization analysis showed that endocan mRNA was expressed in the cytoplasm of epithelial cells in mucous membrane of colon and rectum and in well- and moderately-differentiated colorectal cancer. However, endocan mRNA expression was down-regulated in poorly-differentiated colorectal cancer (Figures 1 and 2).
Meanwhile, we performed immunohistochemical staining for endocan protein with a monoclonal antibody against human endocan. The endocan protein expression was concordantly regulated by mRNA.
The statistical results demonstrated that endocan was differentially expressed in normal colon mucosa and carcinoma tissue samples. The expression rate of endocan was 92.6% (25/27) in normal colon mucosa tissue samples and 36.1% (24/72) in colorectal cancer tissue samples, and was significantly lower in cancerous tissue samples than in normal tissue samples (P = 0.001, Table 1). Endocan protein was identically expressed as mRNA; The expression rate was 70.4% (19/27) in normal colon and rectum mucosa tissue samples and 36.1% (26/72) in colorectal cancer tissue samples. Endocan was also differently expressed in normal and colorectal cancer tissue samples (P = 0.005, Table 2).
| Type | n | Expression of endocan mRNA | Positive (%) | P | |
| + | - | ||||
| Normal mucous membrane | 27 | 25 | 2 | 92.6 | 0.001 |
| Colon carcinoma tissue | 72 | 24 | 48 | 33.3 | (χ2 = 25.266) |
| Type | n | Expression of endocan protein | Positive (%) | P | |
| + | - | ||||
| Normal mucous membrane | 27 | 19 | 8 | 70.4 | 0.005 |
| Colon carcinoma tissue | 72 | 26 | 46 | 36.1 | (χ2 = 7.965) |
The expression of mRNA and protein in colorectal cancer tissue samples was not correlated with age, gender, clinical stage, tumor size or lymph node metastasis, but positively correlated with the differentiation of tumors (Table 3).
| Group | n | Endocan mRNA | Endocan protein | |||||||
| + | - | Positive (%) | P | + | - | Positive (%) | P | |||
| Age | ≤ 54 | 34 | 10 | 24 | 29.4 | 0.676 (χ2 = 0.174) | 11 | 23 | 32.4 | 0.702 (χ2 = 0.146) |
| > 54 | 38 | 14 | 24 | 36.8 | 15 | 23 | 39.5 | |||
| Sex | Male | 23 | 9 | 14 | 39.1 | 0.919 (χ2 = 0.01) | 10 | 13 | 43.5 | 0.530 (χ2 = 0.395) |
| Female | 49 | 17 | 32 | 34.7 | 16 | 33 | 32.7 | |||
| Size | ≤ 3 | 11 | 3 | 8 | 27.3 | 0.643a | 5 | 6 | 45.5 | 0.483a |
| > 3 | 61 | 21 | 40 | 34.4 | 21 | 40 | 34.4 | |||
| Infiltration | Full-thickness | 64 | 21 | 43 | 32.8 | 0.791a | 21 | 43 | 32.8 | 0.099a |
| Non-full-thickness | 8 | 3 | 5 | 37.5 | 5 | 3 | 62.5 | |||
| Metastasis | Nonmetastatic tumor | 27 | 8 | 19 | 29.6 | 0.796 (χ2 = 0.067) | 9 | 18 | 33.3 | 0.899a |
| Metastatic tumor | 45 | 16 | 29 | 35.6 | 17 | 28 | 37.8 | |||
| Grade | Differentiated (well + moderately) | 57 | 23 | 34 | 40.4 | 0.031 (χ2 = 4.642) | 26 | 31 | 45.6 | 0.003 (χ2 = 8.824) |
| Poorly differentiated | 15 | 1 | 14 | 6.7 | 1 | 14 | 6.7 | |||
| TNM stage | I and II | 43 | 18 | 25 | 41.9 | 0.324 (χ2 = 0.973) | 18 | 25 | 41.9 | 0.324 (χ2 = 0.973) |
| III and IV | 29 | 8 | 21 | 27.6 | 8 | 21 | 27.6 | |||
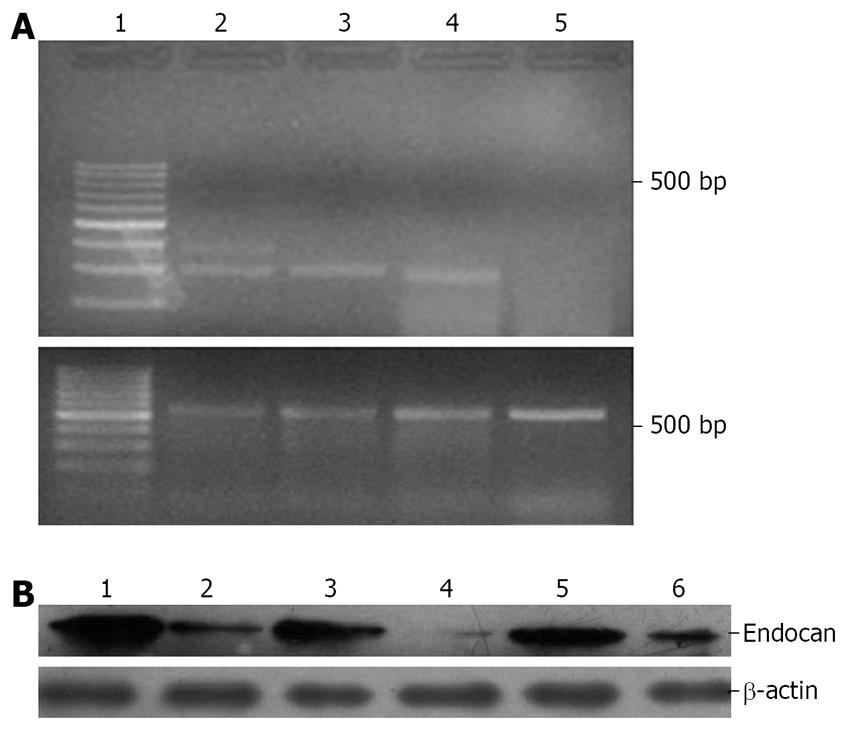
RT-PCR and Western blot were performed to further observe the relationship between the expression levels of endocan and differentiation of colorectal cancer (Figure 3). Both endocan transcript and translation were detected in colon mucous membrane and in well- and moderately-differentiated colon carcinoma, but scarely detected in poorly-differentiated carcinoma.
Endocan was originally cloned from a human endothelial cell cDNA library by Lassalle and collaborators in 1996[3]. This molecule is the product of a single gene, localized on human chromosome 5 at the position 5q11.2, that is organized into 3 exons separated by 2 introns. It encodes for a soluble proteoglycan of 50 kDa containing a mature polypeptide of 165 amino acids and a single dermatan sulphate chain, covalently linked to the serine residue at position 137[9].
Endocan, as a proteoglycan, plays an important role in several pathophysiological processes including inflammatory disorders[10–15] and tumor progression, and in the control of fundamental cellular processes, such as adhesion[16], migration and angiogenesis. Inflammatory cytokines, such as TNF-α and LPS[17], and pro-angiogenic growth factors, such as VEGF[18], HGF/SF[19–22] and FGF-2[2324], strongly stimulate the expression and secretion of endocan in human endothelial cells.
Endocan has been identified as a potential novel endothelial cell marker and a new target for cancer therapy. It was reported that high endocan mRNA levels correlate with a poor prognosis and metastasis of several types of cancer, including breast, renal and lung cancer[12526]. A study of 78 breast cancer patients, with the aim to define the optimal prognosis classifier, was performed on 70 genes according to standard prognostic criteria, showing that endocan over expression in breast cancer is associated with a higher risk of metastasis and death within 5 years[27]. Furthermore, 1234 genes that have been identified are differentially expressed in renal cell carcinoma, and endocan mRNA level is 3-fold higher in renal cell carcinoma samples than in normal tissue samples[28]. This up-regulation of endocan expression also correlates with increased tumor vasculature and inflammation in renal cancer, which is actually the ninth most common malignancy in Western countries, and no effective treatment is available for it. Similarly, a recent extensive hybridization study showed that the endocan gene is one of the most highlighted genes, with at least a 2-fold increase in all the 8 renal cell carcinoma samples analyzed, compared to normal tissue samples[29]. Interestingly, a parallel up-regulation was also revealed for VEGF and c-Met proto-oncogene receptor for HGF/SF, both of which are heavily implicated in angiogenesis. A comparable study, by dot blotting and hybridization showed that endocan is dramatically up-regulated in several (5/14) renal cell carcinoma biopsies, and is correlated with both VEGF and VEGF receptor gene expressions[30]. A gene profiling study of tissues from 23 lung cancer patients demonstrated that endocan is one of the significant poor prognosis classifiers among the 42 genes associated with a high risk of cancer-related death.
Endocan was less reported in colon and rectum tissues. Moreover, little is known about its molecular mechanism. We mapped the regulation of endocan expression in normal membrane mucosa and colorectal cancer tissue samples. Our results reveal that endocan was significantly expressed at transcriptional and translational level in normal colorectal mucous membrane and in well- and moderately-differentiated colorectal cancer, but weakly expressed in poorly-differentiated colorectal cancer. Meanwhile, RT-PCR and Western blot also showed that the expression of endocan was upregulated in normal colon and rectum tissue samples, and down-regulated in poorly- differentiated colorectal cancer tissue samples.
All these data show that endocan is differently expressed in colon and rectum tissue and other tissues. According to the previous results, endocan is almost not expressed in normal human tissue except in lung tissue. Our study showed that endocan was also expressed in normal colon and rectum tissue, but its expression was down-regulated in colorectal cancer, suggesting that regulation may be complex in colon and rectum. As we know, there are a lot of germs in human colon. Most of the outer germs are killed by gastric acid when they get into the stomach through the mouth. In the upper part of the small intestine, the number of germs is also small. However, this number increases gradually at the end of the ileum and reaches its maximum in the colon, where the contents is neutral or alkaline and movement is slower, thus making the germs propagate at a fast pace. There are 109-1011 germs per gram of colon contents. However, these germs can decompose protein, which is called degradation. In this process, the germs also produce some virulent substances, amino acids, peptide, amine, and hydrogen sulphide and proper indole, all of which can activate macrophages and monocytes to secrete a large number of cell factors, such as IL-1 and TNF-α, which can stimulate expression of endocan. That is why we can detect a high expression of endocan in normal colon and rectum tissue. However, the expression of endocan was down-regulated in poorly-differentiated colorectal cancer, suggesting that endocan may be closely related with differentiation and development of colorectal cancer.
Endocan plays a key role in the regulation of certain processes, such as cell adhesion, inflammatory disorders and tumor progression and correlates with poor prognosis and metastasis in several types of cancer, including breast, renal and lung cancer, indicating that endocan may also play a role in the pathology of cancer cells and/or may be a tumor marker. However, few studies are available on endocan expression in colorectal tissue. This study was to map endocan expression in colorectal tissue and analyze the relationship between endocan expression and tumor differentiation.
Colorectal cancer accounted for about 1 million new cases in 2002 and its incidence increases. The results of this study indicate that endocan may be used as a special molecule in the early diagnosis and treatment of colorectal cancer.
The results of this study show that endocan expression plays a role in the pathogenesis of colorectal cancer.
The expression level of endocan plays an important role in the pathogenesis of colorectal cancer. Endocan may be used in the treatment of colorectal cancer in clinical practice.
The authors showed that the expression level of endocan was lower in colon cancer tissue than in normal colon tissue. The study was well-designed and the data are original and informative.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. |
| 2. | Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4-S66. |
| 3. | Lassalle P, Molet S, Janin A, Heyden JV, Tavernier J, Fiers W, Devos R, Tonnel AB. ESM-1 is a novel human endothelial cell-specific molecule expressed in lung and regulated by cytokines. J Biol Chem. 1996;271:20458-20464. |
| 4. | Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803-1804. |
| 5. | Mostofi FK. International histologic classification of tumors. A report by the Executive Committee of the International Council of Societies of Pathology. Cancer. 1974;33:1480-1484. |
| 6. | Tang KF, Pantoja CR, Redman RM, Lightner DV. Development of in situ hybridization and RT-PCR assay for the detection of a nodavirus (PvNV) that causes muscle necrosis in Penaeus vannamei. Dis Aquat Organ. 2007;75:183-190. |
| 7. | Ikeda K, Monden T, Kanoh T, Tsujie M, Izawa H, Haba A, Ohnishi T, Sekimoto M, Tomita N, Shiozaki H. Extraction and analysis of diagnostically useful proteins from formalin-fixed, paraffin-embedded tissue sections. J Histochem Cytochem. 1998;46:397-403. |
| 8. | Chung JY, Braunschweig T, Baibakov G, Galperin M, Ramesh A, Skacel M, Gannot G, Knezevic V, Hewitt SM. Transfer and multiplex immunoblotting of a paraffin embedded tissue. Proteomics. 2006;6:767-774. |
| 9. | Bechard D, Gentina T, Delehedde M, Scherpereel A, Lyon M, Aumercier M, Vazeux R, Richet C, Degand P, Jude B. Endocan is a novel chondroitin sulfate/dermatan sulfate proteoglycan that promotes hepatocyte growth factor/scatter factor mitogenic activity. J Biol Chem. 2001;276:48341-48349. |
| 10. | Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349-355. |
| 11. | Timar J, Lapis K, Dudas J, Sebestyen A, Kopper L, Kovalszky I. Proteoglycans and tumor progression: Janus-faced molecules with contradictory functions in cancer. Semin Cancer Biol. 2002;12:173-186. |
| 12. | Sanderson RD, Yang Y, Suva LJ, Kelly T. Heparan sulfate proteoglycans and heparanase--partners in osteolytic tumor growth and metastasis. Matrix Biol. 2004;23:341-352. |
| 13. | Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104-1110. |
| 14. | Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765-4777. |
| 15. | Xiong JX, Wu ZS, Wu Q, Zhou Q, Gui SY and Wang Y. Expression of endothelial cell specific molecular-1 in renal cell carcinoma. Bulletin of Chinese Cancer. 2004;13:309-312. |
| 16. | Scherpereel A, Depontieu F, Grigoriu B, Cavestri B, Tsicopoulos A, Gentina T, Jourdain M, Pugin J, Tonnel AB, Lassalle P. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34:532-537. |
| 17. | Bu LJ, Zuo L, Ren CL, Zhang SM, Zhou Q, Gui SY, Wang Y. LPS effects on the growth of human umbilical vein endothelial cells and expression of ESM-1. Acta Universitatis Medicinalis Anhui. 2005;40:7-9. |
| 18. | Abid MR, Yi X, Yano K, Shih SC, Aird WC. Vascular endocan is preferentially expressed in tumor endothelium. Microvasc Res. 2006;72:136-145. |
| 19. | Sarrazin S, Adam E, Lyon M, Depontieu F, Motte V, Landolfi C, Lortat-Jacob H, Bechard D, Lassalle P, Delehedde M. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim Biophys Acta. 2006;1765:25-37. |
| 20. | Seidel C, Borset M, Hjertner O, Cao D, Abildgaard N, Hjorth-Hansen H, Sanderson RD, Waage A, Sundan A. High levels of soluble syndecan-1 in myeloma-derived bone marrow: modulation of hepatocyte growth factor activity. Blood. 2000;96:3139-3146. |
| 21. | Delehedde M, Sergeant N, Lyon M, Rudland PS, Fernig DG. Hepatocyte growth factor/scatter factor stimulates migration of rat mammary fibroblasts through both mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt pathways. Eur J Biochem. 2001;268:4423-4429. |
| 22. | Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332-337. |
| 23. | Aitkenhead M, Wang SJ, Nakatsu MN, Mestas J, Heard C, Hughes CC. Identification of endothelial cell genes expressed in an in vitro model of angiogenesis: induction of ESM-1, (beta)ig-h3, and NrCAM. Microvasc Res. 2002;63:159-171. |
| 24. | Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, Herndon M, Gallo RL. Dermatan sulfate released after injury is a potent promoter of fibroblast growth factor-2 function. J Biol Chem. 1998;273:28116-28121. |
| 25. | Xiong JX, Wu Q, Zhou Q, Wu ZS, Gui SY, Wang Y. Expression and relation between endothelial cell specific molecule-1 mRNA and protein in renal cell carcinoma. J Clin Exp Pathol. 2004;20:187-189. |
| 26. | Perey L, Benhattar J, Peters R, Jaunin P, Leyvraz S. High tumour contamination of leukaphereses in patients with small cell carcinoma of the lung: a comparison of immunocytochemistry and RT-PCR. Br J Cancer. 2001;85:1713-1721. |
| 27. | van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530-536. |
| 28. | Lenburg ME, Liou LS, Gerry NP, Frampton GM, Cohen HT, Christman MF. Previously unidentified changes in renal cell carcinoma gene expression identified by parametric analysis of microarray data. BMC Cancer. 2003;3:31. |
| 29. | Amatschek S, Koenig U, Auer H, Steinlein P, Pacher M, Gruenfelder A, Dekan G, Vogl S, Kubista E, Heider KH. Tissue-wide expression profiling using cDNA subtraction and microarrays to identify tumor-specific genes. Cancer Res. 2004;64:844-856. |
| 30. | Borczuk AC, Shah L, Pearson GD, Walter KL, Wang L, Austin JH, Friedman RA, Powell CA. Molecular signatures in biopsy specimens of lung cancer. Am J Respir Crit Care Med. 2004;170:167-174. |
| 31. | Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 2004;5:292-302. |