Published online Jul 21, 2008. doi: 10.3748/wjg.14.4365
Revised: May 23, 2008
Accepted: May 30, 2008
Published online: July 21, 2008
AIM: To study and determine the resting energy expenditure (REE) and oxidation rates of glucose, fat and protein in severe chronic hepatitis B patients.
METHODS: A total of 100 patients with liver diseases were categorized into three groups: 16 in the acute hepatitis group, 56 in the severe chronic hepatitis group, and 28 in the cirrhosis group. The REE and the oxidation rates of glucose, fat and protein were assessed by indirect heat measurement using the CCM-D nutritive metabolic investigation system.
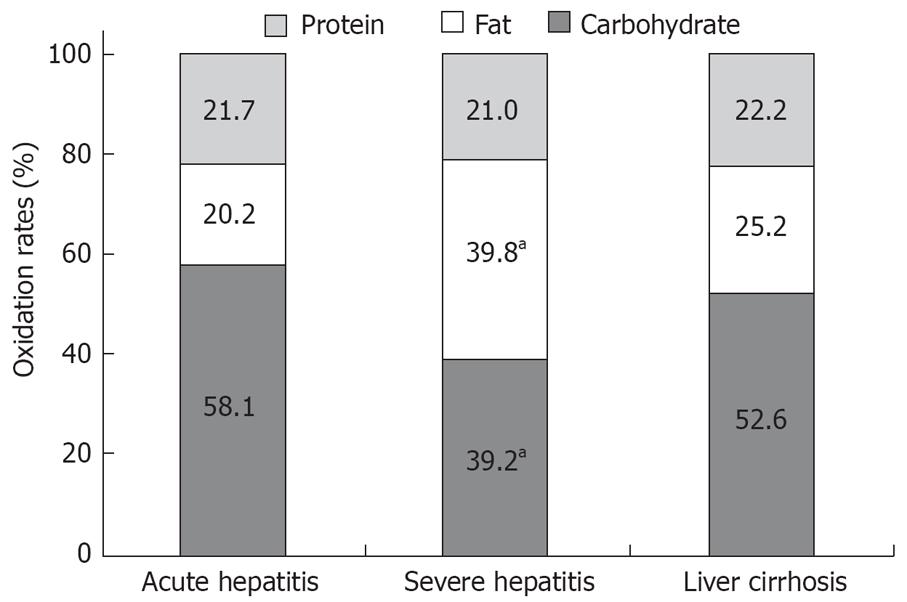
RESULTS: The REE of the severe chronic hepatitis group (20.7 ± 6.1 kcal/d per kg) was significantly lower than that of the acute hepatitis group (P = 0.014). The respiratory quotient (RQ) of the severe chronic hepatitis group (0.84 ± 0.06) was significantly lower than that of the acute hepatitis and cirrhosis groups (P = 0.001). The glucose oxidation rate of the severe hepatitis group (39.2%) was significantly lower than that of the acute hepatitis group and the cirrhosis group (P < 0.05), while the fat oxidation rate (39.8%) in the severe hepatitis group was markedly higher than that of the other two groups (P < 0.05). With improvement of liver function, the glucose oxidation rate increased from 41.7% to 60.1%, while the fat oxidation rate decreased from 26.3% to 7.6%.
CONCLUSION: The glucose oxidation rate is significantly decreased, and a high proportion of energy is provided by fat in severe chronic hepatitis. These results warrant a large clinical trail to assess the optimal nutritive support therapy for patients with severe liver disease.
- Citation: Fan CL, Wu YJ, Duan ZP, Zhang B, Dong PL, Ding HG. Resting energy expenditure and glucose, protein and fat oxidation in severe chronic virus hepatitis B patients. World J Gastroenterol 2008; 14(27): 4365-4369
- URL: https://www.wjgnet.com/1007-9327/full/v14/i27/4365.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4365
The liver plays a pivotal role in controlling carbohydrate metabolism by maintaining glucose concentrations in the normal range, as well as in protein and fat metabolism. This is achieved by a tightly regulated system of enzymes and kinases that regulate nutrient breakdown and synthesis in hepatocytes. Many studies have shown that cirrhotic patients have nutrient and energy metabolism imbalance, which lead to malnutrition and seriously affect their prognosis[12]. On multivariate regression analysis, the Child-Pugh’s score is a good independent predictor of malnutrition. With respect to energy metabolism, there is no consensus on energy expenditure, but there is a consensus that respiratory quotients are lower in liver cirrhosis patients than in healthy subjects. Therefore, resting energy expenditure (REE), calorie intake, and energy balance should be routinely assessed in cirrhotic patients in order to identify hypermetabolic and hypometabolic patients. The nutritional and metabolic parameters in these patients are indispensable for designing and prescribing personalized nutritional strategies for the treatment of muscle malnutrition, which can thus improve their morbidity and mortality rates[3]. Protein-energy malnutrition is frequently observed in liver cirrhosis patients; disorders of protein metabolism and energy metabolism are closely correlated with protein-energy malnutrition. It has been shown that, in protein metabolism disorders, the synthesis and degradation rates of albumin decreased and the serum half-life of albumin became longer[4]. These changes are closely correlated with the prognosis of cirrhosis patients. Therefore, it is important for clinicians to identify and treat metabolic disorders in liver cirrhosis patients. Currently, it is thought that supplementation with branched-chain amino acids (BCAAs) is useful for improving protein metabolism disorders, and that a late evening snack (LES) improves the catabolic state of advanced liver cirrhosis patients. Long-term oral supplementation with a BCAA mixture was found to be better than an ordinary diet[56]. Recently, it was shown that the ingestion of a 200 kcal rice LES can improve nutritional metabolism in cirrhosis patients. Furthermore, a short course of recombinant human growth hormone (rhGH) and insulin-like growth factor-I(IGF-I) raised albumin levels and tended to improve energy metabolism in liver cirrhosis patients. The exogenous administration of IGF-Ihas hepatoprotective and antifibrogenic actions in experimental liver cirrhosis[7–11]. Therefore, proper nutritional support is very important in promoting the recovery of liver disease patients. However, the characteristics of the energy metabolism of severe chronic hepatitis patients are not clear. Thus, the aim of the present study was to explore the characteristics of the energy metabolism of severe chronic hepatitis patients so as to provide data that could be used for optimal nutritional support.
One hundred patients with liver diseases were enrolled from July 2006 to September 2007. They were categorized into 3 groups according to their disease recovery stage: acute hepatitis group (n = 16; hepatitis A, n = 10; hepatitis E, n = 6), severe chronic hepatitis B-related hepatitis group (n = 56), and hepatitis B-related cirrhosis group (n = 28, Table 1). Of the severe chronic hepatitis B-related hepatitis patients, 14 patients (10 males, 4 females), whose status changed from the acute severe stage to the recovery stage within 8 wk, were randomly selected for assessment twice. The patients’ average age was 42.5 years (range, 36-62 years). The patients all met the diagnostic criteria of the “Symposium on Viral Hepatitis and Liver Diseases in 2000”[12]. Briefly, the severe chronic hepatitis was defined based on the following inclusion criteria: (1) a history of chronic hepatitis or liver cirrhosis; (2) severe asthenia and a serum total bilirubin more than 171 &mgr;mol/L; and (3) prothrombin time activity (PTA) less than 60%. All enrolled patients had not received nutritional support and rational regime treatment, anti-viral agents and steroid. The study was explained to the patients and/or their relatives, and written informed consent was obtained. The study was approved by the Ethics Committee of the Beijing You’an Hospital of Capital Medical University.
| Group | n | Age (yr) | Male/Female | ALT (U/L) | AST (U/L) | T-Bil (mg/dL) | ALB (g/L) | PA (g/L) | CHE (g/L) | CH (g/L) |
| 1 | 16 | 34 ± 16 | 10/6 | 154.3 ± 184.8 | 68.3 ± 58.4 | 4.4 ± 6.4 | 36.6 ± 4.4 | 142.0 ± 66.0 | 6050.9 ± 2243.6 | 164.9 ± 49.4 |
| 2 | 56 | 46 ± 13 | 49/9 | 189.2 ± 374.9 | 156.8 ± 159.4 | 20.4 ± 9.8 | 29.8 ± 4.6 | 52.5 ± 27.5 | 2723.6 ± 1422.2 | 52.3 ± 36.2 |
| 3 | 28 | 53 ± 10 | 24/4 | 68.3 ± 118.9 | 80.2 ± 80.6 | 5.8 ± 7.2 | 29.5 ± 7.2 | 62.2 ± 34 | 2628.6 ± 1416.3 | 114.9 ± 42.7 |
The REE and the carbohydrate, protein and fat oxidation rates were determined by indirect heat measurement. The REE was determined using the CCM-D nutrition metabolism investigation system (Medgraphics Company, United States). The average O2 amount consumed and the CO2 amount produced per minute by the subjects were used to calculate the actual REE using the Weir formula; subsequently, the respiratory quotient, the ratio of the average O2 amount consumed to the CO2 amount produced per minute by the subjects, was calculated. Twenty-four-hour urine samples were collected from all subjects for the determination of the urea nitrogen level using the HITACHI7170 automatic biochemistry analyzer (Japan). The protein oxidation rate, non-protein-respiratory quotient (npRQ), the carbohydrate (CHO) and the fat oxidation rates were calculated from the collected data using a computer program. The predicted REE value was calculated using the Harris-Benedict formula based on the subject’s height, weight and age.
The subjects were required to fast for at least 8 h prior to testing. In order to avoid muscle activation; they stayed in bed in the morning for at least 30 min. The room temperature was kept between 24°C and 26°C, with a humidity of 45%-60%. The volume and gas were calibrated for the CCM-D nutrition metabolism investigation system; and the subject’s data on height, weight, gender and age were put into the system. The value of energy metabolism may be related to body weight. Therefore, the REE/kg of all patients were also evaluated.
Serum samples were collected from all subjects, and the following liver function indices were determined by the HITACHI7170 automatic biochemistry analyzer (Japan): alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBIL), albumin (ALB), proalbumin (PA), cholinesterase (CHE), and cholesterol (CH).
The data is presented as mean ± SD. The means were compared between groups using variance analysis. Data from severe virus hepatitis patients in the severe stage and the recovery stage were compared using the paired t test. SPSS 10.0 statistical software was used for all analyses. P < 0.05 was considered statistically significant.
The REE of the severe chronic hepatitis group and the acute hepatitis group was not significantly different from that of the cirrhosis group. However, the REE per kg (REE/kg) of the acute hepatitis group was significantly higher than that of the chronic severe hepatitis group and the cirrhosis group (P = 0.014), but the difference in the REE/kg between the chronic severe hepatitis group and the cirrhosis group was not significant. The respiratory quotient of the severe chronic hepatitis group (0.84 ± 0.06) was significantly lower than that of the acute hepatitis group and the cirrhosis group (P = 0.001). The respiratory quotients of the chronic hepatitis group (0.90 ± 0.05) and the cirrhosis group (0.88 ± 0.08) were not significantly different (Table 2).
| Group | REE (kcal/d) | REE/kg (kcal/d) | RQ | npRQ | CHO (g/d per kg) | Fat (g/d per kg) | Protein (g/d per kg) |
| 1 | 1586.7 ± 783.0 | 25.8 ± 11.3 | 0.90 ± 0.05 | 0.92 ± 0.21 | 3.8 ± 2.1 | 0.5 ± 0.5 | 1.1 ± 0.6 |
| 2 | 1388.5 ± 334.5 | 20.7 ± 6.1 | 0.84 ± 0.06a | 0.88 ± 0.18 | 1.8 ± 1.0a | 0.9 ± 0.7 | 0.9 ± 0.6 |
| 3 | 1317.9 ± 266.3 | 19.8 ± 3.6 | 0.88 ± 0.08 | 0.91 ± 0.09 | 2.7 ± 1.4 | 0.9 ± 2.0 | 1.0 ± 0.6 |
| P value | 0.126 | 0.014 | 0.001 | 0.441 | 0 | 0.721 | 0.525 |
The proportion of energy supplied by the three major substrates (carbohydrate, fat and protein) differed among the groups. Protein oxidation rates were not significantly different among the groups; they ranged from 21.0% to 22.2%. The carbohydrate oxidation rate of the severe hepatitis group (39.2%) was significantly lower than that of the acute hepatitis group and the cirrhosis group (P = 0.048). The fat oxidation rate of the severe hepatitis group (39.8%) was significantly higher than that of the acute hepatitis group and the cirrhosis group (P = 0.01). The carbohydrate and fat oxidation rates of the acute hepatitis group and the cirrhosis group were not significantly different (P = 0.472). Energy supplied by carbohydrate oxidation accounted for 50% or more of the total energy supplies (Figure 1). The actual consumptions per kg weight per day of protein and fat did not differ significantly among the three groups. However, the carbohydrate consumption per kg weight per day was highest in the acute hepatitis group and lowest in the chronic severe hepatitis group; there were significant differences among the groups in carbohydrate consumption (P < 0.0001, Table 2).
The energy metabolism of the 14 patients with severe chronic hepatitis whose disease improved from the severe stage to the recovery stage was assessed twice. Four of these patients had been given growth hormone (4.5 IU/d for 2 wk). Significant improvement in the biochemical indices was seen in the patients at the recovery stage: ALT decreased from 561.2 ± 818.5 U/L to ALT 35.7 ± 9.37 U/L, T-Bil decreased from 17.4 ± 5.5 mg/dL to 5.3 ± 2.7 mg/dL, and PTA increased from 38.2% ± 29% to 72.7% ± 35.9%. After the improvement of liver function, the REE was not significantly changed compared to that obtained prior to the improvement; there was no significant change in the REE per kg weight. However, the carbohydrate oxidation rate increased from 41.7% to 60.1%, while the fat oxidation rate decreased from 26.3% to 7.6%. There was a non-significant trend for the respiratory quotient (RQ) value to increase (P = 0.105, Table 3).
| Patient | REE (kcal/d) | REE/kg (kcal/d per kg) | RQ | CHO (%) | Fat (%) | Protein (%) |
| Severe stage | 1366.1 ± 140.7 | 19.1 ± 3.3 | 0.86 ± 0.05 | 41.7 ± 18.3 | 26.3 ± 27.2 | 32.1 ± 24.8 |
| Recovery stage | 1306.7 ± 497.8 | 17.9 ± 6.2 | 0.91 ± 0.06 | 60.1 ± 11.7 | 7.6 ± 38.6 | 32.3 ± 30.9 |
| P value | 0.761 | 0.638 | 0.105 | 0.075 | 0.232 | 0.990 |
The liver plays a unique role in carbohydrate metabolism by maintaining glucose concentration levels in the normal range. This is achieved by a tightly regulated system of enzymes and kinases that regulate glucose breakdown and synthesis in hepatocytes. This process is under the control of glucoregulatory mediators, of which insulin plays a key role. Therefore, the liver is the major organ of substrate and energy metabolism. It has recently been noted that the energy metabolism of patients with end-stage liver diseases, such as cirrhosis, was altered compared to normal controls, they showed evidence of malnutrition, a high metabolism, a lower RQ value, and a relatively higher fat oxidation rate[24611]. Long-term oral supplementation with a BCAA mixture is better than ordinary food taken as a late evening snack for improving the serum albumin level and the energy metabolism of cirrhosis patients[510].
Severe chronic hepatitis is a severe liver disease in which extended liver tissue necrosis caused by chronic viral hepatitis or hepatitis cirrhosis may lead to liver failure. Nutritional support is an important component of comprehensive therapy. However, the characteristics of the energy metabolism of severe chronic hepatitis patients have never been previously reported. The present study found that the energy metabolism of patients with severe chronic hepatitis had several unique characteristics.
First, the REE of severe chronic hepatitis patients was not significantly different from that of acute hepatitis and cirrhosis patients, who did not have increased energy metabolism. The REE per kg weight was similar in the severe chronic hepatitis group to that in the cirrhosis group, and both were lower than that in the acute hepatitis group. This may be due to the fact that the REE/kg value in the severe chronic hepatitis patients was lower than that in normal controls. This is different from the high energy metabolism condition found in cirrhosis patients, which is widely acknowledged. Tajika[2] found that energy metabolism was normal in 58% patients, and only 12% of patients had low energy metabolism. This can be related to the high energy metabolism of acute hepatitis patients. Plauth[6] showed that chronic hepatitis C patients had a high energy metabolism related to their viral load; and their high energy metabolism resolved with anti-viral treatment. Therefore, it is likely that high energy metabolism is related to the presence of acute hepatitis.
Second, severe the chronic hepatitis group had a notably higher fat oxidation rate than the acute hepatitis group and the cirrhosis group, and a lower glucose oxidation rate than the acute hepatitis group and the cirrhosis group. There was no difference in the protein oxidation rate among the three groups.
Third, the REE before and after liver function improvement in the severe hepatitis patients was compared. The REE of these patients was not changed, but they had a lower fat oxidation rate and a higher carbohydrate oxidation rate; however, these differences were not statistically significant, perhaps as a result of the small sample size. Recently, Plauth[6] showed that TIPS could improve energy metabolism and malnutrition in cirrhosis patients. Growth hormone treatment can improve the liver function and energy metabolism of severe hepatitis patients. From these results, it would appear that severe chronic hepatitis patients cannot utilize carbohydrate. With a notable decrease in the glucose oxidation rate, they can only obtain energy by increased fat utilization. Glucose utilization returns to normal when the patients recover. The inability to use glucose may be due to insulin resistance in chronic liver disease patients[8]. An increase in the RQ value can be used as a marker of recovery in these patients. Tajika[2] also found that, in cirrhosis patients, the non-protein respiratory quotient (npRQ) was an independent risk factor for survival; and patients with a lower npRQ had a worse prognosis.
In the present study, substrate and energy metabolism of cirrhosis patients was not significantly different from that of acute hepatitis patients. This may be related to patient selection. The cirrhotic patients selected were in Child grade A or B, resulting in no significant difference between the cirrhosis patient group and the acute hepatitis group. Multivariate regression analysis confirmed that the Child-Pugh’s score is a better independent predictor of malnutrition than the other variables. However, the REE, TEE, calorie intake and energy balance need to be routinely assessed in cirrhotic patients in order to identify hypermetabolic and hypometabolic patients, which account for approximately 30% of patients. In these patients, the nutritional and metabolic parameters are indispensable for designing and prescribing personalized nutritional strategies for the treatment of the patients’ muscle malnutrition, thus improving their morbidity and mortality rates.
In conclusion, in the present study, severe chronic hepatitis patients had a lower resting energy metabolism per kg weight than acute hepatitis patients, but similar to cirrhosis patients. Severe chronic hepatitis patients had a significantly higher fat oxidation rate and a significantly lower glucose oxidation rate than acute hepatitis and cirrhosis patients. As the severe chronic hepatitis patients’ condition improved, the glucose oxidation rate increased. An increase in the RQ value can be used as a marker of recovery in these patients. The REE and the oxidation rates of various substrates determined by indirect heat measurement can be used to determine the optimal nutritive support therapy for severe liver disease patients.
The liver plays a pivotal role in glucose, fat, protein and energy metabolism. Many studies have shown that patients with liver cirrhosis have nutrient and energy metabolism imbalance, which lead to malnutrition and seriously affect their prognosis. However, the characteristics of the glucose, fat, protein and energy metabolism in patients with severe chronic hepatitis are not clear.
It has recently been noted that the energy metabolism of patients with end-stage liver diseases such as cirrhosis was altered compared to normal controls. They showed evidence of malnutrition, a high metabolism, a lower respiratory quotient (RQ) value, and a relatively higher fat oxidation rate. Long-term oral supplementation with a branched-chain amino acids (BCAA) mixture is better than ordinary food taken as a late evening snack for improving the serum albumin level and the energy metabolism of cirrhotic patients. Severe chronic hepatitis, which extended hepatic cell necrosis caused by chronic viral hepatitis B, leads to liver failure. Nutritional support is an important component of comprehensive therapy. However, the characteristics of the glucose, fat, protein and energy metabolism in patients with severe chronic hepatitis remain unclear.
The characteristics of the energy metabolism of severe chronic hepatitis patients have not been previously reported. The authors studied the disturbed homeostasis of energy, carbohydrate, fat and protein metabolism in severe chronic viral hepatitis B patients, and found that these patients had increased fat oxidation and reduced carbohydrate metabolism, which intended to improve when the liver function became normal. An increase in the RQ value can be used as a marker of recovery in these patients.
The measurement of resting energy expenditure (REE) and the oxidation rates of various substrates can be used to determine the optimal nutritive support therapy for severe liver disease patients. This research is expected to warrant a large clinical trail to assess the optimal nutritive support therapy for severe liver disease patients.
The authors have estimated REE and RQ of patients with severe chronic hepatitis B. And most of energy consumed by these patients is provided by fat, not carbohydrate. The study is interesting and valuable.
| 1. | Guglielmi FW, Panella C, Buda A, Budillon G, Caregaro L, Clerici C, Conte D, Federico A, Gasbarrini G, Guglielmi A. Nutritional state and energy balance in cirrhotic patients with or without hypermetabolism. Multicentre prospective study by the ‘Nutritional Problems in Gastroenterology’ Section of the Italian Society of Gastroenterology (SIGE). Dig Liver Dis. 2005;37:681-688. |
| 2. | Tajika M, Kato M, Mohri H, Miwa Y, Kato T, Ohnishi H, Moriwaki H. Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition. 2002;18:229-234. |
| 3. | Ding HG, Wang JT, Wang BE. Malnutrition, energy metabolism abnormality and nutrients support in cirrhotic patients. Zhongguo Linchuang Yingyang Zazhi. 2002;10:281-283. |
| 4. | Kato M, Moriwaki H. Metabolic disorders in patients with liver cirrhosis. Hepatol Res. 2004;30S:59-62. |
| 5. | Nakaya Y, Okita K, Suzuki K, Moriwaki H, Kato A, Miwa Y, Shiraishi K, Okuda H, Onji M, Kanazawa H. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23:113-120. |
| 6. | Plauth M, Schutz T, Buckendahl DP, Kreymann G, Pirlich M, Grungreiff S, Romaniuk P, Ertl S, Weiss ML, Lochs H. Weight gain after transjugular intrahepatic portosystemic shunt is associated with improvement in body composition in malnourished patients with cirrhosis and hypermetabolism. J Hepatol. 2004;40:228-233. |
| 7. | Holstein A, Hinze S, Thiessen E, Plaschke A, Egberts EH. Clinical implications of hepatogenous diabetes in liver cirrhosis. J Gastroenterol Hepatol. 2002;17:677-681. |
| 8. | Jessen N, Buhl ES, Schmitz O, Lund S. Impaired insulin action despite upregulation of proximal insulin signaling: novel insights into skeletal muscle insulin resistance in liver cirrhosis. J Hepatol. 2006;45:797-804. |
| 9. | Raddatz D, Ramadori G. Carbohydrate metabolism and the liver: actual aspects from physiology and disease. Z Gastroenterol. 2007;45:51-62. |
| 10. | Yamanaka-Okumura H, Nakamura T, Takeuchi H, Miyake H, Katayama T, Arai H, Taketani Y, Fujii M, Shimada M, Takeda E. Effect of late evening snack with rice ball on energy metabolism in liver cirrhosis. Eur J Clin Nutr. 2006;60:1067-1072. |
| 11. | Wallace JD, Abbott-Johnson WJ, Crawford DH, Barnard R, Potter JM, Cuneo RC. GH treatment in adults with chronic liver disease: a randomized, double-blind, placebo-controlled, cross-over study. J Clin Endocrinol Metab. 2002;87:2751-2759. |
| 12. | Chinese society of Hepatology. Program of prevention and treatment for viral hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324-329. |