Published online Jul 7, 2008. doi: 10.3748/wjg.14.4028
Revised: May 30, 2008
Accepted: June 6, 2008
Published online: July 7, 2008
AIM: To develop a novel model of colitis in rats, using a combination of iodoacetamide and enteropathogenic E. coli (EPEC), and to elucidate the pathophysiologic processes implicated in the development of ulcerative colitis (UC).
METHODS: Male Sprague-Dawley rats (n = 158) were inoculated intrarectally on a weekly basis with 4 different combinations: (a) 1% methylcellulose (MC), (b) 100 &mgr;L of 6% iodoacetamide (IA) in 1% MC, (c) 200 &mgr;L containing 4 × 108 colony factor units (CFU) of EPEC, and (d) combined treatment of (IA) followed by bacteria (B) after 2 d. Thirty days post treatment, each of the four groups was divided into two subgroups; the inoculation was stopped for one subgroup and the other subgroup continued with biweekly inoculation until the end of the experiment. Colitis was evaluated by the clinical course of the disease, the macroscopic and microscopic alterations, activity of myeloperoxidase (MPO), and by TNF-α gene expression.
RESULTS: Findings indicative of UC were seen in the combined treatment (IA + B) as well as the IA continued treatment groups: the animals showed slow rate of increase in body weight, diarrhea, bloody stools, high colonic ulcer score, as well as histological alterations characteristic of UC, with an extensive inflammatory reaction. During the course of the experiment, the MPO activity was consistently elevated and the TNF-α gene expression was upregulated compared to the control animals.
CONCLUSION: The experimental ulcerative colitis model used in the present study resembles, to a great extent, the human disease. It is reproducible with characteristics indicative of chronicity.
- Citation: Hussein IAH, Tohme R, Barada K, Mostafa MH, Freund JN, Jurjus RA, Karam W, Jurjus A. Inflammatory bowel disease in rats: Bacterial and chemical interaction. World J Gastroenterol 2008; 14(25): 4028-4039
- URL: https://www.wjgnet.com/1007-9327/full/v14/i25/4028.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4028
It is well established that ulcerative colitis (UC) is an inflammatory condition of the gastrointestinal tract (GIT), resulting from interrelated genetic and environmental factors, especially bacteria which cause disruption of the mucosal barrier thus exposing the mucosal immune system to luminal bacteria and bacterial products[1].
For several years, researchers have been addressing the question as to whether a specific pathogen could cause inflammatory bowel disease (IBD)[2]. Attempts made, so far, to find a causative bacterial strain for IBD, and particularly for UC, have been unsuccessful. Over the years, evidence has accumulated implicating endogenous luminal bacteria in the pathogenesis of UC, especially since the highest bacterial concentration and diversity are found in the colon. Much attention has been given to the role of E. coli in the onset of UC, since this organism is the predominant facultative anaerobic gram negative species of the normal intestinal flora. E. coli play an important role in promoting the stability of the intestinal microbial flora and in maintaining the normal intestinal physiology[3]. Besides commensal strains, certain clones of E. coli, possess virulent properties that cause disease in humans. More recently, it has been suggested that a particular subtype of E. coli may play a pathogenic role in UC[45]. Studies on mucosal adhesion of pathogenic bacteria in UC revealed an enhanced adhesion of E. coli isolates (obtained from stool specimens and rectal biopsies of UC patients) to buccal epithelial cells causing mucosal damage similar to that seen with enteropathogenic E. coli (EPEC)[4–7]. Adherence of EPEC strains on the intestinal mucosa is a complicated process and produces dramatic effects on the ultrastructure of the cells resulting in reduced tight junction density and leaky epithelial barrier[7–9].
More than 60 different experimental models of IBD have been developed in the past two decades[1011]. These studies confirmed the need for the presence of normal enteric flora for the development of experimental colitis[12–15]. However, none of the models mimic precisely the human disease. Therefore, more studies are required to further develop this area of research. The iodoacetamide-induced acute UC model in rat developed by Satoh et al[16] has been extensively studied[1718]. In this model, an array of morphological and functional alterations have been described, however, the model lacked features of chronicity[17–20].
The present study reports the successful development of a chronic UC model, using the combined effects of iodoacetamide, a sulfhydryl group blocker, and enteropathogenic E. coli (EPEC), a strain with adhesion properties, instilled repetitively into the descending colon of rats.
A total of 158 adult male Sprague-Dawley rats, weight range 200 ± 25 g, were used in this experiment in accordance with the criteria of the Institutional Animal Care and Use Committee for the care and use of animals. The study was conducted at the American University of Beirut. Animals were housed in rack mounted cages, with a maximum of 10 rats per cage, and kept on a 12 h light/dark cycle in a controlled temperature and humidity room. Standard laboratory pelleted formula and tap water were provided ad libitum.
The rats were randomized into 4 groups: (1) the methylcellulose treated control group (n = 37), the animals were inoculated intrarectally on a weekly basis with 100 &mgr;L of 1% methylcellulose (MC), the vehicle (Sigma, M-0512, USA); (2) the iodoacetamide-treated group (n = 42), in this group the rats were inoculated with 100 &mgr;L of 6% iodoacetamide (IA) (Sigma, I-6125, USA) dissolved in methylcellulose according to the previously described study by Satoh et al[16]; (3) The bacteria-treated (B) group (n = 37), this group was inoculated with 200 &mgr;L suspension containing 4 × 108 colony factor units of EPEC; (4) The combined treatment group (n = 42), received a combination of iodoacetamide and bacteria (IA + B); this group was inoculated intrarectally on a weekly basis with the same doses of IA followed by bacteria after 48 h. Experimental colitis was induced by regular weekly intracolonic inoculation, 7 cm proximal to the anal verge using a 2mm diameter polyethylene tube. On day 30, each group was split into 2 subgroups. In one subgroup, the inoculation of different treatments continued bi-weekly while in the second subgroup the treatment was discontinued. Three rats from each group and later subgroup (after 30 d), were anesthetized by intraperitoneal injection of sodium pentobarbital (75 mg/kg body weight) and sacrificed on day 3, 7, 14, 21, 42, 56, and 70. A portion of the colon was fixed in 10% formalin while the remaining part was stored at -80°C.
The animals were observed on a daily basis and checked for diarrhea, loose stools, gross rectal bloody stools, or any other gross abnormalities. The weight of each animal was obtained on a weekly basis to check for weight loss after induction of colitis. These observations were reported as a numerical score (Table 1).
| Macroscopic grading | ||||
| Feature | 0 | 1 | 2 | 3 |
| Stool | Normal | Loose stool | Diarrhea | Diarrhea with blood |
| Hyperemia | None | Focal | Focal and thickening of bowel wall | Extensive thickening of bowel wall |
| Adhesions | None | Mild | Moderate | Extensive |
| Megacolon | None | Mild | Moderate | Toxic megacolon |
| Ulcerations | None | Mild ulceration on one side < 1 cm | Moderate ulceration > 1 cm | Severe damage extending > 2 cm |
The evaluation of inflammation was performed according to the modified criteria for colonic changes (Table 1)[1721]. Parameters like diarrhea, hyperemia, adhesions, ulceration and megacolon were assessed to describe the inflammatory status. Each colon was assigned, in a double blind way, a score on a scale ranging from 0 (normal) to 15 (maximal activity of colitis) indicating ulcerations and severe inflammation of the colon.
The descending colon was removed and immersed in cold phosphate buffer (PBS) at pH 7.4. A 1.0 cm piece of the colon was removed proximal to the site of inoculation. It was immediately immersed in 10% buffered formalin and was processed for routine light microscopy according to standard procedures. Serial 5 &mgr;m sections were cut and stained with hematoxylin and eosin (HE) and Periodic Acid Schiff (PAS) using standard methods. The microscopic alterations were assessed according to the criteria shown in Table 2[17–22], and a numerical score of the colonic abnormalities was obtained. The histologic grades ranged from 0 (normal) to 18 (intense inflammatory reaction). The scoring was based on the findings of 2 independent observers obtained by examining six sections from each colon. Thus, the scores represented the average of 36 readings (2 observers, 6 sections per animal and 3 animals per time point). The histological abnormalities were photographed and the findings were reported as numerical scores.
| Histologic grading | ||||
| Feature | 0 | 1 | 2 | 3 |
| Abnormalities of mucosal architecture | None (Normal) | Mild or focal, not exceeding lamina propria | Moderate, not exceeding the submucosa | Severe & diffuse, exceeding the submucosa |
| Crypt abnormalities | None | Mild atrophy | Moderate atrophy, Branched crypts | Severe atrophy, branched crypts, cryptitis, crypt abscess |
| Inflammatory cell infiltration | Normal | Scattered cells | Moderate or confluent cells | Massive infiltration of cells |
| Vascular dilatation | Normal blood vessels | Mild dilatation (localized) | Moderate dilatation of several blood vessels | Severe generalized dilatation of blood vessels |
| Edema | None | Low level limited to villi | In the submucosa | All over the section |
| Mast cells | Normal scattered cells | Three cells clustered in submucosa | Clusters of > 3 cells in the submucosa | Clusters in submucosa and serosa |
The assessment of MPO activity in the mucosal scrapings was carried out as a quantitative marker for granulocytic infiltration in the colonic tissue. Protein concentration and quantification were determined in the mucosal scrapings of the descending colon using the protein assay reagent kit (Bio-Rad). One unit of MPO activity was defined as the quantity of enzyme able to convert 1 &mgr;mol/min of hydrogen peroxide at 25°C as described by Krawisz et al[23]. The results were expressed as MPO units per gram of wet tissue.
Total RNA was extracted from colonic tissues by TRIR (Invitrogen) reagent. RNA was resuspended in RNase free water, quantified and subjected to RT-PCR reaction using RT-PCR kit; Ready Mix Version (Abgene, Promega). RT-PCR was performed on the final volume of 25 &mgr;L containing 12.5 &mgr;L ready mix (optimize reaction buffer, dNTP mix, MgCl2 and DNA polymerase), 0.5 &mgr;L of 25 pmol specific primers as described for TNF-α, β-actin, 0.5 &mgr;L of reverse transcriptase and 1 &mgr;g of RNA. Reverse transcription was performed at 48°C for 30 minutes, followed by heating for 2 min at 94°C. Then 45 cycles of PCR for TNF-α and 25 cycles for β-actin was performed using the following conditions: denaturation at 94°C for 30 s, annealing temp of 55°C for 60 s and extension temperature of 68°C for 2 min, followed by a final extension step at 68°C for 7 min. The primers were TNF-α sense, 5’-AGAACT CCAGGCGGTGTCC-3’. TNF-α antisense, 3’-GATTCCTGTGGGGACTCCC T-5’ (484 bp). β-actin sense, 5’- AAC CCT AAG GCC AACCGTGAAAAG-3’; β-actin antisense, 3’-ATA CAACGGGATCTGAAGCTCG-5’ (540 bp). The PCR products were separated in 1.5 % agarose gel electrophoresis and visualized by ethiduim bromide staining (1 &mgr;g/mL). The DNA product sizes were estimated relative to 100 bp DNA ladder. A control reaction were run to rule out contamination of RNA with genomic DNA, in which reverse transcriptase was omitted from the reaction mixtures. Transcripts were normalized to the corresponding β-actin band and expressed as arbitrary density units.
Protein isolation was carried out by lysing colonic mucosal scrapings in 1.5 mL homogenization buffer (NaCl 11.7 g/L, MgCl26H2O 1 g/L, EDTA 0.76 g/L KCl 0.37 g/L, Tris 24.2 g/L, pH 7.4) using polytron homogenizer on ice. The homogenates were subjected to 30 s sonication and centrifugation at 12 000 r/min for 10 min at 4°C. The protein content of the supernatant was quantified by Bio-Rad Reagents. The protein samples diluted in sample buffer 2X (10% glycerol, 5% betamercaptoethanol, 4% SDS, 125 mmol/L Tris-HCL 1 mol/L pH 6.8 and traces of bromophenol blue) were loaded as 40 &mgr;g/lane in 12% SDS-acrylamide gels, separated by electrophoresis, and electrotransfered to nitrocellulose membranes (Bio-Rad). To detect the specific protein, the TNF-α rabbit polyclonal anti-rat (Chemicon) antibody was used at the concentrations recommended by the manufacturers. Equal loading of the protein samples was confirmed by parallel western blots for GAPDH. The intensities of the protein bands were quantitated by image scanning X-ray films and each band was measured by Image J software (NIH imaging software). Correction was performed by subtracting for level background and normalizing against GAPDH protein level.
Statistical significance of differences between treatment and control groups was determined by the Student’s t test. Where applicable, P values were reported for the 3 independent comparisons. Differences were considered statistically significant for P < 0.05. Values are presented as the mean ± SD.
The present study shows that the synergistic effect of repetitive intracolonic instillation of iodoacetamide and EPEC, resulted in chronic colitis, and the inflammatory process was sustained for the duration of the experiment: 100 d.
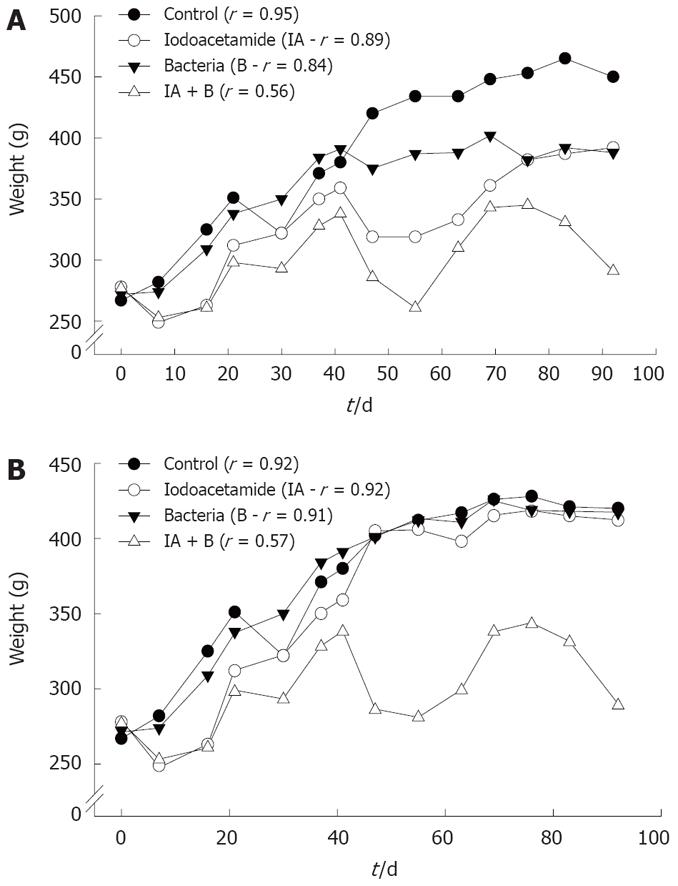
Changes in animals’ weight: As shown in Figure 1, rats in the control, bacteria or iodoacetamide-treated groups had a trend towards increase in weight, which was significantly higher compared to rats treated with the combined treatment (iodoacetamide and bacteria), in both the continued and discontinued injection subgroups. Rats in the combined treatment group (IA + B) showed the lowest rate of increase in weight gain (P < 0.005), 0.15 g/d compared to 1.83 g/d for control, 1.14 g/d for iodoacetamide, and 1.48 g/d for the bacteria treated groups. Similar results were obtained in the discontinued injection subgroups: 0.13 g/d in the combined treatment (IA + B) compared to 1.5 g/d in the normal, 1.37 g/d in the IA and 1.45 g/d in the bacteria treated groups. The decrease in weight gain supports the diagnosis of chronic UC, a phenomenon not seen in the iodoacetomide-treated animals once the inoculations were discontinued after 30 d. Furthermore, two way ANOVA analysis for two experimental parameters (i.e. treatments and duration in days) detected the presence of significant differences among the different experimental groups (P < 0.005) as well as among the various treatment intervals (P < 0.005), with respect to the effect on body weight.
Inspection of stools: Throughout the duration of the study, the control animals passed normal beaded compact stools. Similarly, none of the animals injected with the bacterial suspension of EPEC alone demonstrated abnormal stools except in a few instances of loose stools, 1 to 2 d post inoculation. By contrast, the iodoacetamide-treated group developed diarrhea consistently for 2 to 3 d post instillation that changed later to loose stools. However, after 30 d when the animals were divided into the continued and discontinued subgroups: the iodoacetamide-treated discontinued injection subgroup exhibited a pattern similar to the control group, starting 1 wk after the last IA injection and continuing for the duration of the experiment. The iodoacetamide-treated continued injection subgroup developed diarrhea for the first 2 to 3 d after the injection in nearly all animals, followed by loose stools and occasionally beaded compact stools for the remainder of the week until the next injection.
On the other hand, all animals in the combined treatment group (IA + B) both continued and discontinued subgroups, appeared sick and had severe diarrhea and bloody stools at some stage of the experiment, a feature rarely seen in the iodoacetamide-treated discontinued subgroup. These findings correlated with the lowest rate of weight increase observed in the combined treatment group (IA + B).
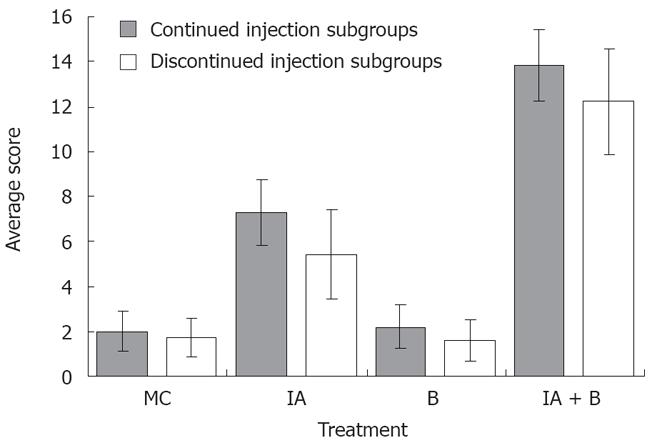
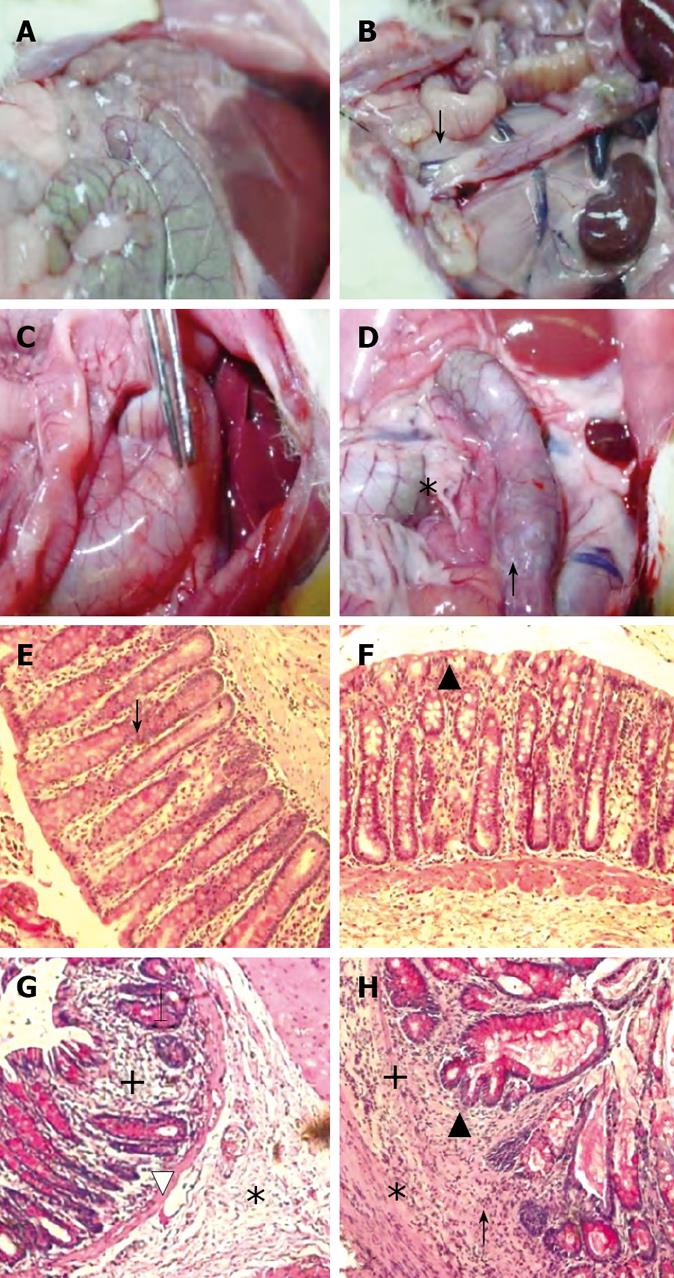
MC-treated group (Control group): The findings were considered as normal baseline. An average of three observations were made at each time point. Rats in the control group did not show any inflammation in the descending colon, however, a whitish discoloration at the site of the injection, ≤ 0.5 cm² in diameter, was easily identified. The average score was 2 ± 0.9 out of 15 indicating the absence of ulcer formation (Figure 2). These findings were observed although the experimental duration in the continued and discontinued subgroups was different (Figure 3A).
B-treated group: The findings in this group were similar to the control group except for the occasional findings of vasodilatation, and the presence of a white granulomatous area at the site of inoculation, with an overall size ≤ 0.5 cm² (Figure 3B). The overall average score for the group was 2.0 ± 0.96 out of 15, as illustrated in Figure 2.
IA-treated group: Perianal redness was sometimes noticed prior to sacrifice. The ascending and transverse colon were invariably normal. At most time points, inflammation was restricted to the descending colon including the occasional presence of an area of redness of the jejunum around the site of injection. There was dilatation of blood vessels and hyperemia around the site of the inoculation, along with the presence of adhesions (Figure 3C). The size of the inoculation site was 1 to 1.5 cm². The overall average score for the mucosal damage at the various time points in the continuous injection subgroup was 7 ± 0.9 out of 15, while the average score in the discontinued injection subgroup was 5.2 ± 1.6 out of 15. These findings correlated with the findings of less severe symptoms in treatment-discontinued subgroup compared to the treatment-continued subgroup (Figure 2).
IA + B-treated group: Perianal redness was consistently observed, accompanied by staining with stools and blood. Protrusion of structures through the abdominal wall of the rat prior to surgery was clearly noticeable. The dilated descending colon was severely inflamed. The animals exhibited multiple abdominal adhesions over the descending colon with extensive generalized dilatations of the abdominal vessels (Figure 3D). Occasionally, megacolon was observed in this group and at times toxic megacolon was seen particularly in the continuous injection subgroup. It is important to note that the descending colon was hyperemic with vasodilatation starting from day 3 and persisting throughout the duration of the experiment (Figure 3D). The mucosa was always thickened, with a larger ulcer (2-3 cm2). The overall average score was 13.5 ± 1.4 for the continued and 12 ± 1.8 for the discontinued injection subgroups (Figure 2).
In brief, the ulcer score in the control group was significantly lower than that in the iodoacetamide-treated group (P < 0.005) and the combined treatment group (P < 0.005) but not significantly different from the bacteria-treated group. Moreover, there was a significant difference in the ulcer score between the iodoacetamide and the bacteria-treated groups (P < 0.005) as well as between the combined treatment and the iodoacetamide groups (P < 0.05). Furthermore, the score of the combined treatment group was significantly higher than that in the bacteria-treated animals (P < 0.005).
The microscopic alterations focused mainly on the descending colon proximal to the site of the inoculation and its surroundings. The findings were compared with commonly reported colonic alterations seen in chronic ulcerative colitis. All layers of the colon were thoroughly studied and reference was made to the normal intestinal mucosa obtained from control animals.
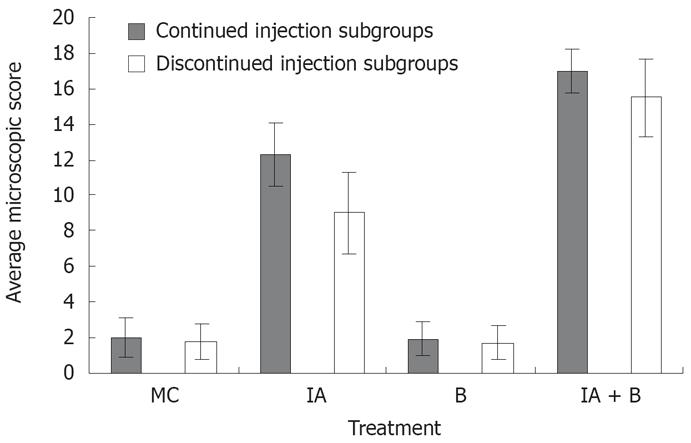
MC and B-treated groups: The mucosal architecture of the MC group was considered as normal. There was no ulceration of the epithelial lining. The crypts, and the lamina propria inflammatory infiltrate were normal (Figure 3E). The overall average histologic score was 2 ± 1.2 (Figure 4), with no significant difference between the continued and discontinued injection subgroups. Similar findings were observed in the B-treated group, with an average score of 1.89 ± 1.1 (Figures 3F and 4).
IA-treated group: The iodoacetamide-treated continued injection subgroup showed focal mucosal ulceration and depletion of the epithelial lining; the inflammation involved the entire descending colon, sigmoid colon, rectum and anus (Figures 3G and 4). In addition, there was massive infiltration of inflammatory cells and an increase in the gut-associated lymphoid tissues. The crypts were partially destroyed, hypertrophied, and surrounded by inflammatory cells and edema (Figure 3G). The mucosa and submucosa contained several cell types including neutrophils, lymphocytes, macrophages, eosinophils, and clusters of mast cells. The overall average histologic score for IA-treated animals was 12 ± 1.35. However, when the treatment with IA was stopped, the tissues regained to a great extent their normal appearance, starting 1 wk after discontinuing the injections. The epithelial lining was almost normal, with little inflammatory infiltrate, scattered mast cells and no vasodilatation. The overall histologic score for IA-treated discontinued injection subgroup animals was 9 ± 1.
Combined (IA + B)-treated group: Severe inflam-mation was noted proximal to the site of inoculation with frequent involvement of all four layers of the colon. Histological examination revealed diffuse distortion of the mucosal architecture, with marked crypt atrophy and extensive infiltration by mononuclear cells (Figure 3H). The mucosal surface was irregular, villiform, and heavily infiltrated by various types of inflammatory cells exceeding that in the submucosa. The crypts showed loss of parallelism, with irregularity in crypt size, spacing and shape. In addition, cryptitis and crypt abscesses were present (Figure 3H). Mast cells were clustered in the submucosa. The sites affected by inflammation were edematous, ulcerated, and devoid of any glands. Furthermore, there was thinning or complete absence of the muscularis mucosa. Mucosal ulcers were covered with a large cellular debris (necrotized tissue and products of inflammation). These findings were observed throughout the duration of the experiment in both subgroups. The highest overall histologic score was seen in the continued injection subgroup, with an average score of 16.2 ± 0.9, compared to 15.4 ± 1.7 for the discontinued injection subgroup (Figure 4).
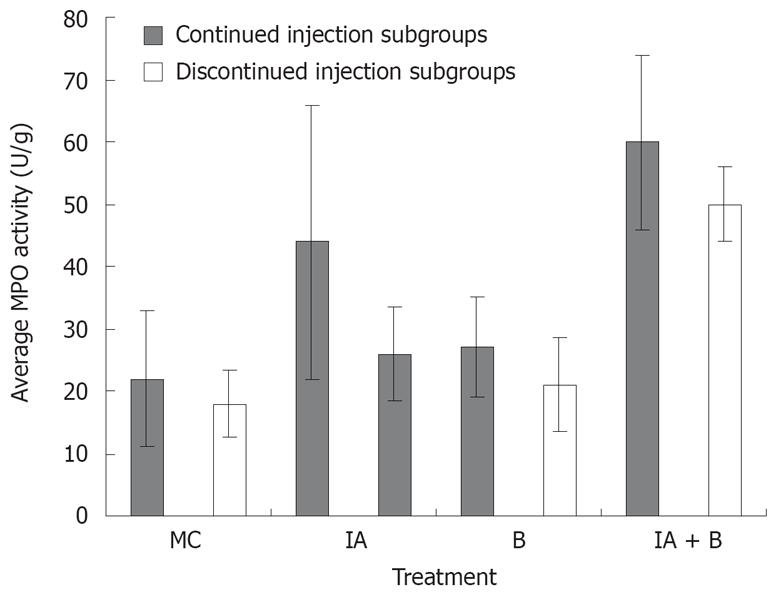
Assessment of MPO activity is considered a good estimate of the intestinal inflammatory status. MPO activity was consistently the highest in the combined (IA + B) treatment group receiving continuous inoculations, at all time points from day 7 to day 30. This high activity was maintained until the last day of the experiment (Figure 5).
One week following induction of inflammation, the MPO activity in the combined (IA + B) treatment group reached an average of 41 ± 2.2 U/g tissue which was significantly higher than the respective control values (11.7 ± 2.1, P < 0.005) and almost twice the values of the bacteria-treated group (28.5 ± 2.8, P < 0.005) and almost twice those of IA group (23.2 ± 7.7, P < 0.005). The MPO activity in the IA (23.2 ± 7.7) and B (28.5 ± 2.8) treated animals were significantly higher than in the MC-treated group (Figure 5). A pattern similar to that observed on day 7 was also noted on day 14, 21, 28, 42, 56 and 70 (Figure 5).
In general, in the continued injection experiment, the MPO score in the control group was significantly lower than that in all the other groups (P < 0.005) but not significantly different from the bacteria-treated group. Furthermore, MPO level in the combined treatment group was significantly higher than that in the bacteria-treated animals (P < 0.005). Even in the discontinued injection subgroups, there was a significant difference in MPO activity score between the control group and the combined treatment group (P < 0.005) In brief, the combined treatment group had a significantly higher MPO activity score than the iodoacetamide-treated group (P < 0.05).
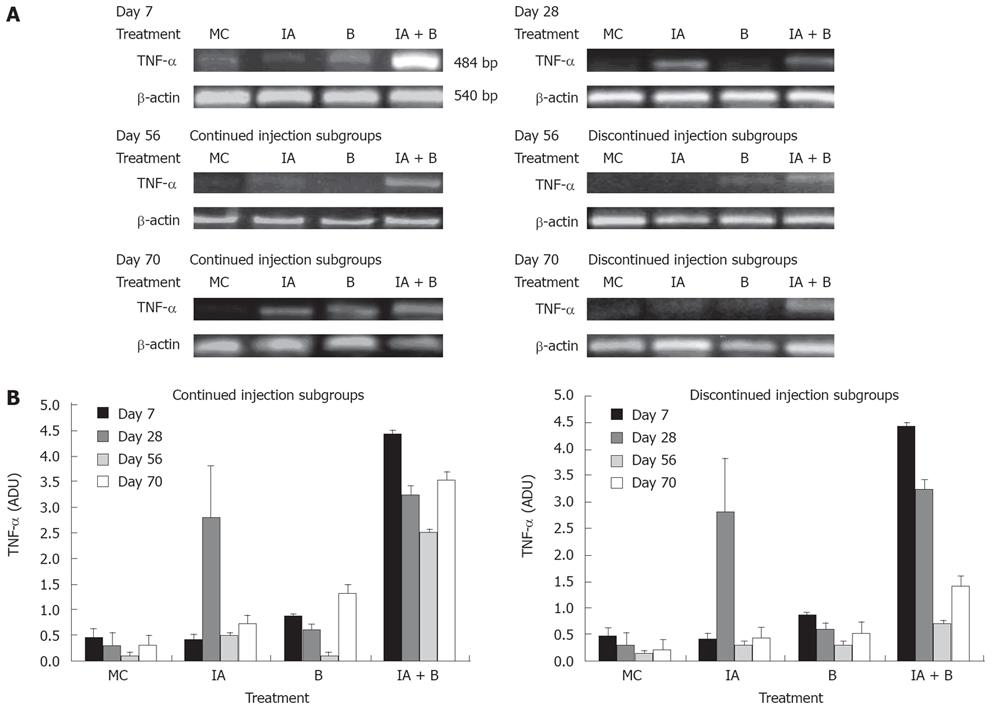
TNF-α is a prototypical proinflammatory cytokine and a key regulatory factor in various inflammatory processes involved in the pathogenesis of ulcerative colitis [24–27]. In this study, TNF-α mRNA expression levels were assessed as arbitrary density units (ADU) in relation to the endogenous level of β-actin. Our findings are similar to previous studies that reported an increase in TNF-α expression at both mRNA and protein levels in patients with IBD.
TNF-αmRNA expression: On day 7, TNF-α mRNA expression was increased 7-to-8 fold in the combined treatment (IA + B) group compared to the other groups (Figure 6A). On day 28, there was a significant rise in TNF-α expression in the IA-treated group reaching 7-fold higher than that in the B-group, slightly higher than that in the combined (IA + B) group which was slightly down regulated but still significantly higher than the other groups (Figure 6B). On day 56, there was a transient overall decline in the expression of the TNF-α mRNA in the IA-continued injection subgroup followed by an elevation in the levels on day 70. However, in the discontinued injection subgroup, there was a time-dependent decrease in the TNF-α levels following day 28 to day 70 post-treatment. On day 56, the TNF-α mRNA levels were reduced in all groups (Figure 6B). The combined treatment (IA + B) continued injection subgroup maintained a relatively higher level of the TNF-α mRNA compared to IA-treated or the B-treated and MC-treated control groups (Figure 6B). However, the discontinued injection subgroup exhibited mRNA expression below the detection limits (Figure 6B). During the course of the entire study, the increase in TNF-α mRNA reached its peak in the combined treatment (IA + B) group (both in the continued and discontinued injection subgroups) except for a transient rise in the iodoacetamide-treated group at day 28 (Figure 6B). However, it is important to note that after the inoculations were stopped, TNF-α mRNA expression decreased in the discontinued compared to the continued injection subgroup and this difference was observed at all time points, with greater expression in the combined treatment (IA + B) subgroup.
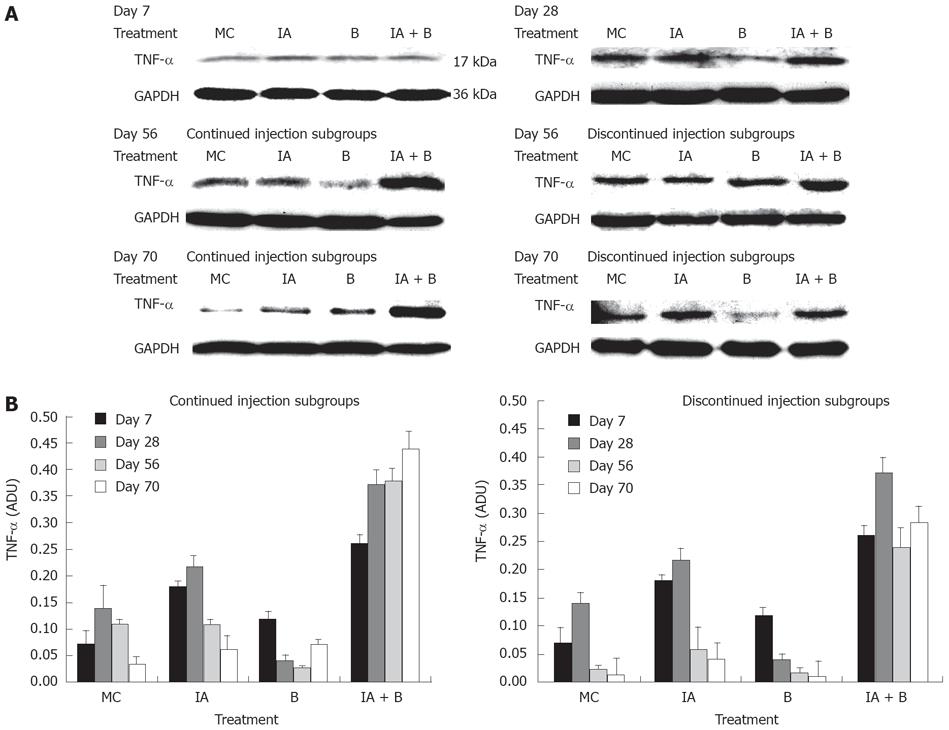
TNF-αprotein expression: Western blot analysis of TNF-α protein expression showed that at 7 d post treatment, all groups exhibited close levels of TNF-α protein. In both MC and B-treated groups the protein levels were 2-fold lower than the protein levels in the IA-treated and the combined treatment (IA + B) groups (Figure 7A). During the entire study, TNF-α protein levels were the highest in the combined treatment (IA + B) continued injection subgroup (Figure 7A), whereas in the discontinued injection subgroup all animals showed reduced expression following the cessation of the injections (Figure 7B). It is worth mentioning that the combined treatment (IA + B) discontinued injection subgroup maintained the highest values of TNF-α protein levels. In general, there was a good correlation between TNF-α protein and mRNA expression levels in all the experimental groups and subgroups, indicating the effect of treatment on both post-transcriptional and post-translational processes of TNF-α.
Over the past three decades, several models of UC have been developed, with a variable range of clinical manifestations resembling those observed in human IBD, however, none of these closely mimic the clinical entity of human UC [10111617]. Using animal models, there is much indirect evidence to suggest an interaction between luminal flora and the immune system, based mostly on the disruption of the immunoregulatory mechanisms[28–30]. The present study showed that a UC model, using an SH blocker and enteropathogenic E. coli, closely resembles the human situation and is reproducible with findings indicative of chronicity. The characteristics of the present model were colonic disease induced for nearly hundred days, the presence of clinical features such as weight loss (Figure 1), diarrhea and rectal bleeding, accompanied with macroscopic (Figure 2) and histological alterations (Figures 3 and 4) typical of chronic ulcerative colitis in humans[16172931]. Moreover, an analysis of colonic myeloperoxidase activity[32] showed a consistent elevation of activity in the combined IA + B treatment group, indicative of severe mucosal inflammation of the descending colon (Figure 5). Furthermore, upregulation of TNF-α mRNA and protein expression in the IA + B group supported the chronicity of the inflammatory process (Figures 6 and 7).
Analysis of body weight gain across the various experimental groups and subgroups revealed that rats treated with a combination of IA + B exhibited the lowest rate of weight gain in both the continued and the discontinued injected subgroups compared to MC, IA-treated discontinued injection subgroup and B-treated group. In addition, the IA continued injection subgroup behaved similar to the combined treatment continued injection subgroup, but with a higher overall growth rate. Once the injections in the IA-treated subgroup were discontinued (day 30), the growth rate increased again. Moreover, the combined treatment group showed an undulating course of increase and decrease in weight, which may indicate periods of relapse and remission of the disease. Such a decrease was observed consistently in both the continued and discontinued combined treatment subgroups as well as in the iodoacetamide-treated continued injection subgroup. On the other hand, the iodoacetamide-treated subgroup with discontinued treatment behaved like the controls after the injections were stopped (Figure 1B). The low weight gain (in the IA + B group) was due probably due to malabsorption secondary to a defective intestinal barrier. This barrier may not be restored appropriately because of persistent activation of mucosal inflammation[17], with malabsorption in addition to diarrhea, affecting the physiology of other parts of the gut[3334]. The changes in weight gain in the experimental groups (IA + B) were paralleled by the increased rates of diarrhea, loose and bloody stools, and sometimes swollen abdomen and megacolon.
Abdominal examination revealed tenderness over the descending colon as well as various degrees of megacolon in the combined treatment group. These findings provide further support to the validity of our animal model in inducing UC. Besides, the IA-treated animals in the continued injection subgroup showed similar findings but were not as consistent, particularly with regard to the development of megacolon. This feature was not encountered in the B-treated or MC control groups.
The validity of the combined treatment induced-UC was further supported by the high range of scores for macroscopic alterations. These included generalized vasodilatation, adhesions, enlargement of the descending colon and ulcer formation (Figure 2). These observations suggest that continuous stimulation by iodoacetamide is necessary for maintaining the inflammatory process. In this case, the score range in the iodoacetamide-treated and combined treatment groups showed moderate to severe inflammation in the descending colon (Figure 3C and D) and is consistent with the clinical findings seen in UC[1418]. In general, the gross abnormalities observed in the combined treatment group were more consistent and more characteristic of chronic UC. There was diffuse hyperemia, with adhesions, megacolon, ulcerations, vasodilatation, and redness in the nearby segment of the jejunum. The overall score in the combined IA + B group with continued treatment ranged between 10-15, while the score ranged from 9-14 in the discontinued injection subgroup. Therefore, despite discontinuing the injections, the inflammatory process maintained its course, a point of special interest for further investigations.
In addition, the microscopic findings in the descending colon revealed histological abnormalities, particularly in the combined IA + B treatment group, followed by the IA-treated group with continuous injection (Figure 3). Such changes were seen mostly in the mucosa. These observations are in line with the findings seen in UC. However, in a few instances, the inflammation in the combined IA + B treatment group was very severe, involving all four layers of the colon, the mucosa, submucosa, muscular layer and serosa. Histological assessment of the colonic damage showed that during the entire duration of the experiment, the combined IA + B treatment group had the highest score, in both continued and discontinued subgroups (between 8 to 16). These changes were less pronounced in the iodoacetamide groups and were minimal or absent in the B-treated and MC-treated control groups (Figure 4). Therefore, changes in the microscopic structure of the combined treatment subgroups mimicked the changes commonly encountered in severe UC in humans[303536]. These include extensive hyperemia and loss of epithelial lining, ulceration of the mucosa, severe depletion of goblet cells, loss of crypts, crypt abscesses, cryptitis, with dense inflammatory cell infiltration, severe dilatation of several blood vessels, loss or thinning of muscularis mucosa, and invasion by lymphoid cells[31719].
Assessment of MPO activity provides a reproducible and qualitative estimate of mucosal inflammation[23] and may serve as a quantitative index of disease severity. At the site of the mucosal injury, MPO content is a marker of the magnitude of neutrophil infiltration. The combined treatment group exhibited the highest MPO activity in the descending colon (Figure 5). It is therefore, clear that the degree of inflammation was the highest in the combined IA + B treatment group; in particular the subgroup with continued injection. The inflammatory activity was maintained at a much higher rate than in the control, B-treated or the IA-treated groups. However, it is important to note that once the injections were discontinued, the combined IA + B treatment subgroup maintained its highest activity compared to the other groups. This observation provides further support to the notion that the inflammation persisted in the combined treatment group regardless of continued or discontinued injections. Once again, these findings are in line with the findings of chronic UC in humans[3536].
Further characterization of our animal model was carried out at the molecular level in order to evaluate the expression of inflammatory signaling. We analyzed the mRNA and protein expressions of TNF-α, a proinflammatory cytokine which can induce COX-2 and COX-2 protein expression. It should be noted that increased local expression of TNF-α is of prime importance in driving the chronic inflammatory reaction and the development of tissue injury[2526]. There is significant correlation between the production of TNF-α and the severity of UC. High levels of proinflammatory cytokines in the mucosa lead to excessive production of matrix degrading enzymes by the gut fibroblasts, loss of mucosal integrity and ulceration. In UC, high levels of TNF-α have been documented in the lamina propria as well as increased TNF-α mRNA and protein expression in the mucosa associated with tissue injury[3437]. TNF-α is the most important mediator of response to Gram-negative bacteria and also plays a critical role in the immune response to other micro-organisms[36]. There is much evidence to suggest that either enteric bacteria or immune dysfunction play a pivotal pathogenic role in IBD[12]. Endotoxins or lipopolysaccharides (LPS), derived from the outer membrane of Gram negative enteropathogenic E. coli interact with CD14 on the surface membrane of mononuclear cells, thus triggering a signal cascade that leads to the production and release of TNF-α, which is strongly involved in the pathogenesis of ulcerative colitis[38]. In this respect, both TNF-α and LPS may represent putative therapeutic targets for the treatment of UC.
The present study has demonstrated that mucosal scrapings of the colon contained significantly elevated levels of TNF-α mRNA and protein in both the combined treatment (IA + B) subgroups and at all time points compared to control animals (Figures 6 and 7). However, an occasional increase in TNF-α mRNA expression was noted in the IA-treated group on day 28 and 70.
The abnormalities resulting in chronic mucosal inflammation can be divided into two types; defects in immune regulation pathways and defects involved in the barrier function of the epithelium[589]. Models of disrupted barriers function have resulted in mucosal inflammation[5]. Chemical agents such as acetic acid, dextran sodium sulfate, and iodoacetamide, can injure the epithelium. In a normal animal, all means of chemical injuries result in a transient inflammatory process. Repair mechanisms step in and a stable mucosal barrier is reestablished. Indeed, a leading concept in the pathogenesis of chronic intestinal inflammation is the break in mucosal tolerance, an active process by which an injurious immune response is prevented, suppressed or shifted to a non-injuring class of immune reaction[3940]. The intestine is in permanent contact with billions of bacteria belonging to the normal gut flora, food protein, and potentially pathogenic bacteria and has to discriminate and define selective action towards pathogenic and non-pathogenic components. Mucosal tolerance exists in order to prevent an immune response against the body’s “own” bacteria that would otherwise give rise to chronic intestinal inflammation. EPEC colonize the intestinal mucosa and, by subverting intestinal epithelial cell cytoskeleton function, produce a characteristic histopathological response. EPEC with invasive properties cause gross destruction of the epithelial architecture and tight junction proteins. Thus the presence of a chemical agent in addition to EPEC can create an optimal environment for the development and maintenance of chronic UC.
Experimental studies indicate that defects in intestinal permeability induced by iodoacetamide may facilitate the passage of LPS, derived from the Gram-negative enteropathogenic E. coli or other enteric bacterial flora, into the circulation. It is speculated that enhanced intestinal permeability, probably caused by the inflammation induced by iodoacetamide, could precede and prepare the ground for the development of UC in rats.
In conclusion, the development of animal models is critical in elucidating the molecular pathways by which human ulcerative colitis develops and progresses to an advanced stage. Indeed, identifying the molecular pathways involved in initiating this disorder may allow us to gain an insight into the strategy(ies) that can lead to IBD prevention. Furthermore, the present study may permit the characterization of molecular and morphologic effects of the disease that may enhance our ability to develop therapeutic agents with higher efficacy or with fewer side effects.
Furthermore, such a study may result in the development of agents that target molecular pathways known to play a role in the initiation of IBD, specifically in the subset of asymptomatic individuals at high-risk for the development of UC.
We employed the two-hit hypothesis, both chemical and bacterial, for our chronic ulcerative colitis (UC) model, based on several lines of indirect evidence implicating gut flora in the pathogenesis of inflammatory bowel disease in general, and ulcerative colitis in particular. Bacterial flora appears to be always involved in the development of ulcerative colitis, and a sulfhydryl group blocker such as iodoacetamide may further increase the metabolic stress on the epithelial cells especially when it is instilled into the colon followed by enteropathogenic E. coli. The resident cells of the lamina propria, under such conditions, constitute an important target for endotoxins. The resulting secretion of cytokines causes the induction of a chronic colitis. This model requires a regular inoculation schedule to maintain the inflammatory process.
A chronic ulcerative colitis model which mimics to a great extent the human disease was developed. Our model is characterized by clinical, macroscopic, microscopic and molecular parameters that are similar to human UC.
The present study confirmed one of the possible mechanisms of ulcerative colitis whereby a chemical reagent introduced into the colon induces colonic inflammation with morphological and clinical features suggestive of ulcerative colitis. The inflammatory condition was further provoked by inoculating E. coli bacteria at the same site allowing for an interaction between the bacteria, the mucosal barrier and the intestinal tissues, in particular the mucosal layer. Such interactions aggravated the inflammatory reaction as evidenced by the upregulation of peroxidase, indicating an increase in the inflammatory cells, and the upregulation of TNF alpha, a prototypical proinflammatory cytokine secreted by the inflammatory cells and a key regulatory factor involved in ulcerative colitis. Such a phenomenon was maintained for the entire duration of the experiment.
The present study provides a well characterized experimental model for ulcerative colitis essential for elucidating the mechanisms and molecular pathways involved. It also sheds light on the effect of the colonic microecology and the potential of manipulating the microbial environment in the intestines through the use of probiotics. This model may allow insight into strategies that can lead to the prevention of inflammatory bowel disease (IBD) and for the assessment of agents that can be used in the management of UC.
Inflammatory bowel disease (IBD): IBD is characterized by chronic, recurrent inflammation of the GI tract of unknown etiology. The disease varies in the extent and severity of the symptoms. IBD is known to develop between the ages of 15 to 35 years. Ulcerative colitis (UC): UC is an IBD characterized by passage of bloody diarrhea, which usually constitutes the earliest sign of the disease. Progression of UC may be associated with fever, abdominal pain and weight loss. UC is often termed left-sided colitis for it affects mainly the mucosa of the descending colon. The disease may extend to the proximal part of the large bowel; when the entire colon is affected, the disease is called pancolitis. Crohn’s disease (CD): CD is an IBD that can involve any part of the digestive tract, from the oral cavity to the anus. It causes deep chronic ulcerations involving the mucosa and the deeper layers.
This is a solid and valuable piece of work. It is an extension of the authors’ previous work on experimental colitis. It is definitely an original work of great scientific value. The experimental design is sound, results are well presented, and conclusions are documented by the findings of the study.
| 1. | Scaldaferri F, Fiocchi C. Inflammatory bowel disease: progress and current concepts of etiopathogenesis. J Dig Dis. 2007;8:171-178. |
| 3. | Rath HC. The role of endogenous bacterial flora: bystander or the necessary prerequisite? Eur J Gastroenterol Hepatol. 2003;15:615-620. |
| 4. | Rath HC. Role of commensal bacteria in chronic experimental colitis: lessons from the HLA-B27 transgenic rat. Pathobiology. 2002;70:131-138. |
| 5. | Nazli A, Yang PC, Jury J, Howe K, Watson JL, Soderholm JD, Sherman PM, Perdue MH, McKay DM. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am J Pathol. 2004;164:947-957. |
| 6. | Schuppler M, Lotzsch K, Waidmann M, Autenrieth IB. An abundance of Escherichia coli is harbored by the mucosa-associated bacterial flora of interleukin-2-deficient mice. Infect Immun. 2004;72:1983-1990. |
| 7. | Yu J, Kaper JB. Cloning and characterization of the eae gene of enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1992;6:411-417. |
| 8. | Nougayrede JP, Fernandes PJ, Donnenberg MS. Adhesion of enteropathogenic Escherichia coli to host cells. Cell Microbiol. 2003;5:359-372. |
| 9. | Muza-Moons MM, Schneeberger EE, Hecht GA. Enteropathogenic Escherichia coli infection leads to appearance of aberrant tight junctions strands in the lateral membrane of intestinal epithelial cells. Cell Microbiol. 2004;6:783-793. |
| 10. | Hoffmann JC, Pawlowski NN, Kuhl AA, Hohne W, Zeitz M. Animal models of inflammatory bowel disease: an overview. Pathobiology. 2002;70:121-130. |
| 11. | Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. J Pharmacol Toxicol Methods. 2004;50:81-92. |
| 12. | Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478-485. |
| 13. | Cash HL, Hooper LV. Commensal bacteria shape intestinal immune system development. ASM News. 2005;71:77-83. |
| 14. | Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495-549. |
| 15. | Mayer L. The development of animal models of inflammatory bowel disease. Int Rev Immunol. 2000;19:77-90. |
| 16. | Satoh H, Sato F, Takami K, Szabo S. New ulcerative colitis model induced by sulfhydryl blockers in rats and the effects of antiinflammatory drugs on the colitis. Jpn J Pharmacol. 1997;73:299-309. |
| 17. | Jurjus A, Barada K, Khoury N, Assef MD, Foltzer CJ, Reimund JM, Kedinger M. Morphological and biochemical alterations in the jejunum following iodoacetamide-induced colitis in rats. Can J Physiol Pharmacol. 2006;84:1191-1203. |
| 18. | Szabo S, Trier JS, Frankel PW. Sulfhydryl compounds may mediate gastric cytoprotection. Science. 1981;214:200-202. |
| 19. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. |
| 20. | Dotan I, Mayer L. Immunopathogenesis of inflammatory bowel disease. Curr Opin Gastroenterol. 2002;18:421-427. |
| 21. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. |
| 22. | Fabia R, Ar’Rajab A, Johansson ML, Willen R, Andersson R, Molin G, Bengmark S. The effect of exogenous administration of Lactobacillus reuteri R2LC and oat fiber on acetic acid-induced colitis in the rat. Scand J Gastroenterol. 1993;28:155-162. |
| 23. | Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344-1350. |
| 24. | Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999;34:66-74. |
| 25. | Rogler G, Andus T. Cytokines in inflammatory bowel disease. World J Surg. 1998;22:382-389. |
| 26. | Xia B, Crusius J, Meuwissen S, Pena A. Inflammatory bowel disease: definition, epidemiology, etiologic aspects, and immunogenetic studies. World J Gastroenterol. 1998;4:446-458. |
| 27. | Kusugami K, Fukatsu A, Tanimoto M, Shinoda M, Haruta J, Kuroiwa A, Ina K, Kanayama K, Ando T, Matsuura T. Elevation of interleukin-6 in inflammatory bowel disease is macrophage- and epithelial cell-dependent. Dig Dis Sci. 1995;40:949-959. |
| 29. | Terashima S, Hoshino Y, Kanzaki N, Kogure M, Gotoh M. Ulcerative duodenitis accompanying ulcerative colitis. J Clin Gastroenterol. 2001;32:172-175. |
| 30. | Van Damme N, De Keyser F, Demetter P, Baeten D, Mielants H, Verbruggen G, Cuvelier C, Veys EM, De Vos M. The proportion of Th1 cells, which prevail in gut mucosa, is decreased in inflammatory bowel syndrome. Clin Exp Immunol. 2001;125:383-390. |
| 31. | Jankey N, Price LA. Small intestinal histochemical and histological changes in ulcerative colitis. Gut. 1969;10:267-269. |
| 32. | Perez-Navarro R, Ballester I, Zarzuelo A, Sanchez de Medina F. Disturbances in epithelial ionic secretion in different experimental models of colitis. Life Sci. 2005;76:1489-1501. |
| 33. | Fries W, Mazzon E, Squarzoni S, Martin A, Martines D, Micali A, Sturniolo GC, Citi S, Longo G. Experimental colitis increases small intestine permeability in the rat. Lab Invest. 1999;79:49-57. |
| 34. | Goncalves NS, Ghaem-Maghami M, Monteleone G, Frankel G, Dougan G, Lewis DJ, Simmons CP, MacDonald TT. Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infect Immun. 2001;69:6651-6659. |
| 35. | Amati L, Caradonna L, Leandro G, Magrone T, Minenna M, Faleo G, Pellegrino NM, Jirillo E, Caccavo D. Immune abnormalities and endotoxemia in patients with ulcerative colitis and in their first degree relatives: attempts at neutralizing endotoxin-mediated effects. Curr Pharm Des. 2003;9:1937-1945. |
| 36. | Lu J, Wang A, Ansari S, Hershberg RM, McKay DM. Colonic bacterial superantigens evoke an inflammatory response and exaggerate disease in mice recovering from colitis. Gastroenterology. 2003;125:1785-1795. |
| 37. | Porras M, Martin MT, Soler M, Vergara P. Intestinal motor disorders associated with cyclical bacterial overgrowth in a rat model of enteritis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G58-G64. |
| 38. | Sasaki M, Sitaraman SV, Babbin BA, Gerner-Smidt P, Ribot EM, Garrett N, Alpern JA, Akyildiz A, Theiss AL, Nusrat A. Invasive Escherichia coli are a feature of Crohn's disease. Lab Invest. 2007;87:1042-1054. |
| 39. | Schulzke JD, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann N Y Acad Sci. 2006;1072:288-299. |
| 40. | Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182-205. |