Published online Jun 28, 2008. doi: 10.3748/wjg.14.3781
Revised: May 9, 2008
Accepted: May 16, 2008
Published online: June 28, 2008
The aetiology of primary sclerosing cholangitis (PSC) is not known and controversy exists as to whether PSC should be denominated an autoimmune disease. A large number of autoantibodies have been detected in PSC patients, but the specificity of these antibodies is generally low, and the frequencies vary largely between different studies. The presence of autoantibodies in PSC may be the result of a nonspecific dysregulation of the immune system, but the literature in PSC points to the possible presence of specific antibody targets in the biliary epithelium and in neutrophil granulocytes. The present review aims to give an overview of the studies of autoantibodies in PSC, with a particular emphasis on the prevalence, clinical relevance and possible pathogenetic importance of each individual marker.
- Citation: Hov JR, Boberg KM, Karlsen TH. Autoantibodies in primary sclerosing cholangitis. World J Gastroenterol 2008; 14(24): 3781-3791
- URL: https://www.wjgnet.com/1007-9327/full/v14/i24/3781.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3781
Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease of the intra- and extrahepatic biliary tree leading to progressive bile duct strictures and liver cirrhosis[1]. No effective medical treatment is currently available[2] and PSC is a major indication for liver transplantation[3]. The PSC population is heterogeneous, comprising subgroups of regular “large-duct” PSC, patients with “small-duct” affection only[4] and an “overlap-syndrome” between PSC and autoimmune hepatitis (AIH)[5]. Up to 80% of the PSC patients have concurrent inflammatory bowel disease (IBD)[6]. According to standard endoscopic and histological criteria, the IBD is most often classified as ulcerative colitis (UC), but there is also an association with colonic Crohn’s disease (CD)[78].
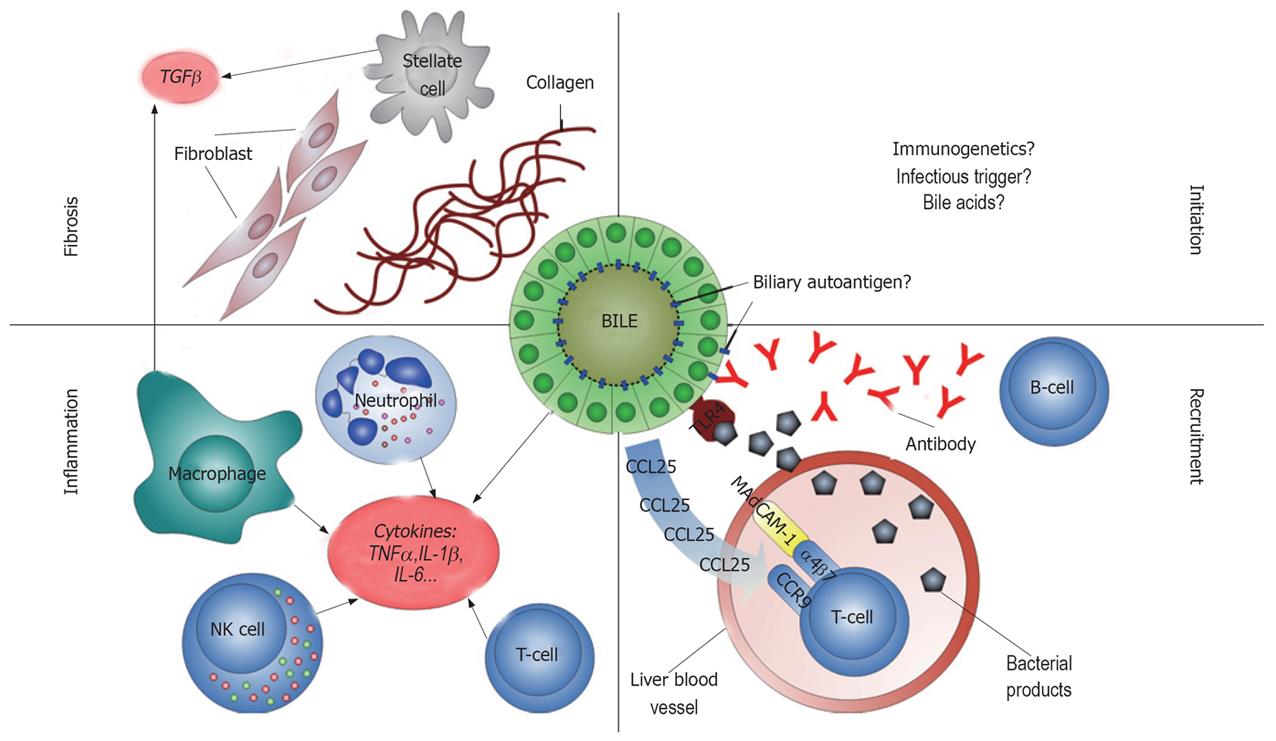
The aetiology of PSC is unknown (Figure 1). Immune responses against self antigens in the bile ducts have been proposed to play an important role in the pathogenesis, although controversy exists as to whether PSC should be denominated an autoimmune or merely immune mediated disease[9]. On one side, there are several lines of evidence supporting classification of PSC as an autoimmune disease[10]. This evidence includes (1) association with other autoimmune diseases in the same individual[11] and first degree relatives[12], (2) infiltration of T-lymphocytes in the portal tracts[13] with restriction in T cell receptor V gene usage[14], (3) a statistical association with particular human leukocyte antigen (HLA) haplotypes[15] and (4) the presence of autoantibodies[16]. On the other side, there is no documented effect of immunosuppressants in PSC[2], and in contrast to the female predominance of many diseases regarded as autoimmune, approximately 2/3 of PSC patients are male[17]. These notions suggest that additional pathogenetic factors may exist (e.g. bile acid toxicity[18]), and to what extent and at what disease stage autoimmune mechanisms contribute to the bile duct damage observed in PSC is not known.
In many autoimmune diseases, autoantibodies serve as markers of disease activity, may aid in the diagnosis of patients, and provide important insight into the pathogenesis. In clinical practice, a good marker is sensitive and specific and yields prognostic information [e.g. anti-cyclic citrullinated proteins (anti-CCP) antibodies in rheumatoid arthritis (RA)]. In studies of pathogenetic mechanisms, a good marker is tissue specific and closely linked to other observations regarding the pathogenesis (e.g. TSH receptor antibodies in Graves’ disease). In PSC patients, a large number of different autoantibodies have been reported (Table 1). Some of these autoantibodies react with biliary or colonic epithelial antigens, others with constituents of neutrophil granulocytes, and some even with various ubiquitously expressed self antigens.
| Antibody | Prevalence (%) | (Median) | No. of patients | (Median) | No. of articles |
| Anti-BEC | 63 | (63) | 30 | (30) | 1[36] |
| pANCA | 26-94 | (68) | 13-86 | (30) | 19 (Table 2) |
| AMA | 0-9 | (0) | 15-73 | (37) | 10[44617889102112137–140] |
| Anti-LKM | 0 | (0) | 10-80 | (37) | 7[4489112137140–142] |
| Anti-SLA/LP | 0 | (0) | 10-37 | (25) | 4[4489140142] |
| ANA1 | 8-77 | (30) | 13-73 | (35) | 13[4461788999101102111112137–140] |
| SMA1 | 0-83 | (17) | 10-73 | (36) | 10[44617889111112137–140] |
| ASCA | 44 | (44) | 25 | (25) | 1[115] |
| Anti-cardiolipin | 4-63 | (27) | 23-73 | (41) | 3[617887] |
| Rheumatoid factor | 15 | (15) | 71 | (71) | 1[78] |
| AECA | 35 | (35) | 20 | (20) | 1[87] |
| Anti-TPO | 16 | (16) | 73 | (73) | 1[78] |
| Anti-GBM | 17 | (17) | 24 | (24) | 1[87] |
| Anti-sulfite oxidase | 33 | (33) | 39 | (39) | 1[130] |
| Anti-GSTT1 | 5 | (5) | 58 | (58) | 1[133] |
| Authors | PSC | PSC -IBD | PSC+IBD | UC -PSC | CD-PSC | AIH | PBC | HC | MT |
| Terjung et al[44] | 943 | 814 (142/175) | 31 | 0 | IIF | ||||
| (33/35) | (14/45) | (0/19) | 1:10 | ||||||
| Klein et al[54] | 87 | 78 | 27 | 0 | IIF | ||||
| (26/30) | (18/23) | (16/60) | (0/20) | 1:10 | |||||
| Mulder et al[102] | 79 | 775 | 825 | 886 | 28 | 5 | IIF | ||
| (19/24) | (10/13) | (9/11) | (21/24) | (7/25) | (12/252) | 1:32 | |||
| Lo et al[56] | 777 | 33 | 0 | 336 | 0 | 0 | AP | ||
| (23/30) | (15/45) | (0/32) | (1/33) | (0/14) | (0/50) | 1:10 | |||
| Seibold et al[98] | 775 | 408 | 88 | 83 | 25 | 336 | 28 | 0 | IIF |
| (17/22) | (2/5) | (15/17) | (38/46) | (20/80) | (5/15) | (7/28) | (0/30) | 1:10 | |
| Gur et al[87] | 75 | 75 | 755 | IIF | |||||
| (15/20) | (3/4) | (12/16) | 1:20 | ||||||
| Muratori et al[144] | 75 | 314 | 2 | 0 | IIF | ||||
| (18/24) | (12/39) | (1/51) | (0/18) | 1:20 | |||||
| Seibold et al[84] | 72 | 50 | 765 | 62 | 4 | 354 | 28 | 0 | IIF |
| (18/25) | (2/4) | (16/21) | (30/48) | (2/48) | (8/23) | (6/21) | (0/40) | 1:10 | |
| Zauli et al[137] | 72 | IIF | |||||||
| (33/46) | -9 | ||||||||
| Hardarson et al [145] | 69 | 75 | 675 | 76 | 8 | 506 | 0 | IIF | |
| (20/29) | (6/8) | (14/21) | (16/21) | (2/25) | (10/20) | (0/33) | 1:40 | ||
| Roozendaal et al[99] | 67 | IIF | |||||||
| (46/69) | 1:40 | ||||||||
| Bansi et al[146] | 6610 | 656 | 13 | 0 | AP | ||||
| (57/86) | (11/17) | (7/55) | (0/36) | 1:5 | |||||
| 5110 | 656 | 11 | 0 | IIF | |||||
| (44/86) | (11/17) | (6/55) | (0/36) | 1:5 | |||||
| Bansi et al[101] | 65 | 29 | 705 | 45 | 0 | AP | |||
| (41/63) | (2/7) | (39/56) | (38/85) | (0/36) | 1:05 | ||||
| Tervaert et al[88] | 62 | 716 | 33 | 0 | IIF | ||||
| (8/13) | (5/7) | (5/15) | (0/24) | -9 | |||||
| Roozendal et al[57] | 49 | 7011 | 15 | 0 | IIF | ||||
| (27/55) | (62/88) | (8/53) | (0/78) | 1:40 | |||||
| Claise et al[53] | 44 | 25 | 60 | 37 | 15 | 2411 | 0 | 0 | IIF |
| (12/27) | (3/12) | (9/15) | (18/49) | (11/75) | ( 25/105) | (0/30) | (0/50) | 1:20 | |
| Vermeulen et al[147] | 44 | 56 | 15 | 466 | 5 | IIF | |||
| (16/36) | (56/100) | (15/100) | (17/37) | (5/105) | 1:40 | ||||
| Wilschanski et al[60] | 29 | IIF | |||||||
| (7/24) | 1:20 | ||||||||
| Pokorny et al[100] | 26 | 29 | 23 | 226 | 0 | IIF | |||
| (10/39) | (5/17) | (5/22) | (2/9) | (0/7) | 1:20 |
One of the most consistent findings regarding the aetiology of PSC is the disease association with genetic variants within the HLA-complex on chromosome 6[15]. HLA class I and II genes encode molecules which present antigens to CD8+ and CD4+ T-lymphocytes, respectively, resulting in an immune response against the antigen when appropriate co-stimulation is present[19]. A relationship between particular autoantibodies and disease associated HLA variants has been detected in other autoimmune diseases[20], but in PSC the pathogenetic importance of most of the identified autoantibodies is poorly defined. The present editorial aims to give an overview of the studies of autoantibodies in PSC, with a particular emphasis on the prevalence, clinical relevance and possible pathogenetic importance of each individual marker.
The identification of antibodies against well defined biliary antigens in PSC would strongly support the hypothesis of an autoimmune aetiology. Given the high frequency of colitis among the patients, such antigens could potentially also be expressed in the colonic mucosa.
One autoantibody of this type was proposed by Das et al[21], who identified an antigenic protein expressed in both colonic and biliary epithelium, in addition to eye, skin and cartilage[22]. This 40 kDa protein was identified as human tropomyosin isoform 5 (hTM5)[23–25]. A monoclonal antibody (Das-1) was developed, and serum from UC and PSC patients inhibited the binding of Das-1 to the epithelium, indicating antibodies against hTM5 related epitope(-s) in the sera[2226]. In the cell membrane hTM5 is found complexed with a 200 kDa colonic epithelial protein (CEP), and this complex is speculated to serve as the true target for the Das-1 antibody[27]. Antibodies against hTM5 have been detected in UC patients without PSC[28], and anti-hTM5 in UC sera has recently been shown to induce cytotoxicity against colonic epithelial cells in vitro[29]. In PSC patients without concomitant UC, a single study identified antibodies against a 9-amino acid sequence from hTM (not isoform specific) in 100% (8/8) of patients as compared with 69% (33/48) of UC patients and 0/6 PBC patients[30]. The findings of Das et al have been partly reproduced by others[31], but given a number of critical concerns[32–35], further studies are required to conclusively confirm and elaborate the importance of the hTM5-CEP antigen in the pathogenesis of PSC.
A Swedish group has reported on the presence of antibodies against isolated biliary epithelial cells (BEC) at high frequencies in sera from PSC (63%) and PBC (37%) patients, versus 8% of controls (1/12)[36]. A 40 kDa antigenic protein was identified, but this protein did not react with tropomyosin antibodies, which implies that either the 40 kDa protein in this study is not a tropomyosin isoform or the antibody used reacts with other tropomyosin isoforms. Anti-BEC from PSC sera (and to a lesser extent PBC sera) induced isolated BEC to produce IL-6 and the adhesion molecule CD44, strongly suggesting pathogenetic importance. Recently the group also showed that sera from PSC patients with anti-BEC stimulated BEC to express toll-like receptors (TLR), leading to BEC cytokine production upon exposure to lipopolysaccharide (LPS, endotoxin) from gram negative bacteria[37]. This means that both LPS and antibodies against BEC are necessary to activate BEC and generate cytokine release. An association between the presence of the anti-BEC and PSC associated HLA haplotypes (DR2 and DR3) was also suggested. The relevance of the Swedish findings are further strengthened by a higher frequency of acute liver transplant rejection in patients with anti-BEC prior to transplantation (all liver diseases) than in patients with no anti-BEC[38]. However, it needs to be noted that in this study there was a high prevalence of anti-BEC in all end stage liver patients (HCV 32%, PSC 56%, PBC 75%, HBV 57%, AIH 57%, and alcoholic cirrhosis 71%). This raises concerns as to the PSC specificity of the antibody, which clearly needs to be characterised prior to further studies.
Taken together, the findings of Das et al and the Swedish group suggest that antigens expressed in the biliary epithelium may induce self-reactive immune responses under certain conditions. Whether the antigenic epitope(s) lie within the hTM5-CEP complex or elsewhere remains to be elucidated, and the clinical significance of the corresponding autoantibodies must be established.
Antibodies against cytoplasmic constituents of neutrophils (ANCAs) were initially described in patients with glomerulonephritis and systemic vasculitis[3940]. In UC patients, antibodies against nuclear antigens were reported by Calabresi et al in 1961[41] and Nielsen et al in 1983 (granulocyte specific-ANA)[42]. In PSC such antibodies were reported by Snook et al in 1989[43]. These antibodies are also present in a large proportion of patients with AIH[44] and the name ANCA was applied due to the close resemblance to ANCAs found in several of the vasculitides[4546]. ANCA is analyzed by incubating fixated human neutrophil slides with patient serum, and subsequently with secondary antibodies conjugated to a fluorophore. The indirect immunofluorescence (IIF) pattern is classified as cytoplasmic (cANCA) or perinuclear (pANCA)[4748]. Billing et al[49] and Terjung et al[50–52] have made an additional contribution to this nomenclature, documenting that the main ANCA pattern in PSC, AIH and UC is “atypical”. This means that the likely antigen is located in the nucleus rather than in the cytoplasm. The names anti-neutrophil nuclear antibodies (ANNAs)[51] and nuclear anti-neutrophil antibodies (NANAs) have thus been proposed[49].
The prevalence of ANCA (subtype not specified) in PSC patients ranges from 42% to 93%[4553–61], and that of the pANCA subtype from 26% to 94% (Table 2). Comparable prevalences of ANCA are reported in AIH and UC (Table 2). No definite evidence links ANCA to the genetic susceptibility of PSC in terms of particular HLA haplotypes[62]. One study has reported on an increased prevalence of ANCA in PSC relatives as compared with healthy controls[63] while another study could not confirm this[64].
Multiple neutrophil antigens contribute to different ANCA IIF patterns (Table 3). A study published in abstract form by Terjung et al[65] in 2005 proposed that the main antigen of atypical pANCA in AIH, UC and PSC patients is tubulin beta 5 chain (TBB5), a nuclear membrane-associated protein present in myeloid cell lines. Further studies of anti-TBB5 are necessary to characterise the clinical and pathogenetic relevance of these findings. Other nuclear antigens have also been proposed as nuclear targets of pANCA in AIH and UC, notably the high mobility group (HMG) non-histone chromosomal proteins HMG1 and HMG2[66–68] and Histone H1[69]. These have not been studied in patients with PSC.
| Antibody | Frequency range % (median) | No. of patients range (median) | Number of studies |
| Anti-lactoferrin | 4-50 (29) | 12-76 (24) | 10[55578485878899102137144] |
| Anti-myeloperoxidase | 0-33 (2) | 12-73 (40) | 7[577884858799102] |
| Anti-BPI | 5-46 (29) | 36-76 (69) | 5[5557597899] |
| Anti-cathepsin G | 0-35 (21) | 14-76 (55) | 5[5557848799] |
| Anti-proteinase 3 | 0-44 (4) | 25-73 (62) | 5[57788799102] |
| Anti-elastase | 0-35 (9) | 23-76 (69) | 4[558799102] |
| Anti-α-enolase | 11-33 (27) | 15-55 (36) | 3[5789147] |
| Anti-catalase | 16-60 (38) | 15-55 (35) | 2[5789] |
| Anti-α-antigen | 33 (33) | 12 (12) | 1[85] |
| Anti-h-lamp-2 | 71 (71) | 73 (73) | 1[78] |
| Anti-TBB51 |
A variety of cytoplasmic proteins have also been proposed to be targets for ANCAs in PSC. In ANCA-associated small vessel vasculitis (Wegener’s disease, microscopic polyangiitis and Churg-Strauss syndrome) the main proportion of specific ANCAs are directed against proteinase 3 (PR3, mainly cytoplasmic IIF pattern) and myeloperoxidase (MPO, mainly perinuclear IIF pattern)[70]. In these diseases, increased ANCA levels may predict clinical relapse, but there is limited correlation between titres and disease activity. The prevalence of anti-PR3 and anti-MPO in PSC patients is low (Table 3).
Bactericidal/permeability increasing protein (BPI) has functional domains which bind the inner core region of LPS[71]. This binding triggers anti-bacterial activity, neutralization of endotoxin and delivery of endotoxin rich particles to host cells[72]. Anti-BPI is detected in many clinical settings. In PSC, anti-BPI has been found in 5% to 46% of the patients (Table 3), which is similar to UC (3%-39%)[5973–77], compared with 0% to 5% of healthy controls[5778]. Anti-BPI is also reported in RA, systemic lupus erythematosus (SLE) and systemic sclerosis[79], and interestingly there is a high prevalence of anti-BPI in cystic fibrosis patients colonized with gram negative bacteria[80].
Another LPS-binding ANCA target is lactoferrin, which is released from neutrophils during inflammation and has bactericidal and immune modulating effects[81]. Antibodies against lactoferrin have been detected in several autoimmune diseases including RA[82], SLE[83], reactive arthritis[82] and ankylosing spondylitis. The reported prevalence of anti-lactoferrin in PSC (4%-54%, Table 3) is similar to that in UC (4%-50%), and considerably higher than in CD (0%-9%)[73757684–86] and healthy controls (0%)[8788].
Antibodies against the proteases elastase and cathepsin G are found in up to 35% of patients with PSC (Table 3). Catalase prevents cell damage from reactive oxygen-derived free radicals, and antibodies against catalase have been detected in up to 60% of PSC patients, compared with up to 10% of healthy controls[5789]. Finally, human lysosomal-associated membrane protein 2 (h-lamp-2) is a target of ANCA in vasculitides[90]. In a single study, anti-h-lamp-2 was detected in a large proportion of PSC patients (71%) versus only 15% of healthy controls[78]. No disease controls were investigated. This finding has not yet been reproduced.
The large range of different ANCAs in PSC (Table 3) has been critically interpreted as the ANCAs serving as nonspecific epiphenomena of an immune response against dying neutrophils at an inflammatory site[9192]. ANCAs (i.e. anti-MPO and anti-PR3) may, however, activate neutrophils[70], and anti-BPI may inhibit clearance of LPS[93]. Also, widely and even ubiquitously expressed antigens sometimes serve as antigens in tissue specific autoimmunity [e.g. anti-mitochondrial antibodies (AMAs) in PBC].
Another possibility is related to the predominant theory on UC and CD, which involves an aberrant response to gut luminal antigens in genetically susceptible hosts[94]. A series of antibodies against bacterial antigens have been detected in IBD patients, and ANCAs may represent such antibodies[94]. One study from 1995 indicated that colonic lamina propria B-cells in UC produce pANCA[95]. In another study, absorption of human pANCA-positive sera with enteric bacterial antigens reduced or abolished the specific perinuclear staining[96]. The targets of these pANCAs are not known, but a study published in abstract form in 2006 indicates that antibodies giving rise to the atypical pANCA pattern have dual reactivity against both TBB5 and the microbial tubulin FtsZ[97]. How these cross-reacting antibodies may lead to hepatobiliary pathology can only be speculated upon.
The sensitivity of ANCA in PSC is high in some studies, whereas specificity is low. In one study of the diagnostic precision of autoantibodies in liver diseases, atypical pANCA with cut-off titre 1:40 had a specificity of 78% and sensitivity of 61% for PSC (AUC, 0.69; 95%CI, 0.61-0.77)[44]. Identification of the principal antigenic target of ANCAs in PSC would allow prospective studies to define this diagnostic role further. Currently, ANCA does not contribute diagnostically or during the clinical follow-up of PSC patients.
In terms of correlation between ANCA and particular clinical characteristics of PSC, no clear interpretation can be made from available data. If ANCAs were to represent markers for intestinal affection in PSC, a higher ANCA prevalence should be detected in PSC patients with IBD than in patients without IBD. This has only been shown in one small study by Seibold et al[98]. In another study, anti-lactoferrin was more prevalent in PSC with UC than without[99]. A few papers relate ANCA positivity to biliary tract complications like biliary calculi or cholangiocarcinoma[100], or more extensive involvement of the biliary tree (both intra- and extrahepatic as compared with intrahepatic only)[101]. The presence of pANCA has also been found to correlate with disease stage (cirrhosis or liver transplantation)[100102], and in one study anti-BPI and anti-cathepsin G were more prevalent in PSC patients with cirrhosis[99]. Most other papers reported no difference in ANCA positivity between early and advanced PSC, and found no correlation between titres and disease activity[455799103]. ANCAs seem to persist after liver transplantation[98104], even though the titres may vary during follow-up[103].
AMA may be considered one of the most useful autoantibodies in the diagnosis of cholestatic liver disease, since AMAs are virtually absent in PSC patients (Table 1) compared with a 90%-95% prevalence in PBC[105]. The AMA antigens are different epitopes of the pyruvate dehydrogenase complex (PDC), especially the PDC-E2[106107]. Mitochondrial antigens are expressed in all nucleated cells, and AMAs are classically detected by IIF. The presence of AMAs in PBC is an example of how autoimmunity against a ubiquitous antigen may be involved in the pathogenesis of a highly tissue specific disease. One of several proposed theories in PBC hypothesizes that in biliary epithelial cells the main AMA-antigen (PDC-E2) is not glutathiolated (as opposed to in other cells), causing persisting antigenicity of PDC-E2 when biliary epithelial cells undergo apoptosis[108]. Modification of AMA antigens in the liver by xenobiotics may also contribute[108]. A similar post-translational modification of proteins is known to contribute to antigenicity in several autoimmune diseases (e.g. antibodies against citrullinated proteins in RA)[109].
Anti-liver kidney microsomes type 1 (anti-LKM1), anti-soluble liver antigen/liver pancreas antigen (anti-SLA/LP) and anti-liver cytosolic protein type 1 (anti-LC1) are autoantibodies used in diagnosis of AIH[105]. These have not been detected in PSC patients (Table 1).
ANA and SMA are directed against ubiquitous antigens. ANA is the hallmark of SLE and other connective tissue diseases, but are also among the most prevalent autoantibodies in AIH[110]. ANAs may represent a large number of nuclear targets while SMAs are similarly undefined and directed against actin and other cytoplasmic filamentous proteins. ANA is reported in 8%-77% of the PSC patients (Table 1). No particular ANA subspecificities seem to predominate; anti-dsDNA has been reported in 3%-29%[617887111], anti-ENA in 4%-12%[78111112], anti SSA/B in 1%-28%[7887] and anti-RNP, anti-SCL70, anti-Sm and anti-ssDNA in a minority of patients[87]. SMAs have been reported in 0%-83% of PSC patients (Table 1) but the prevalence is also high in AIH, various malignancies and infections[107]. ANA and SMA often co-exist, they lack organ and disease specificity, and should probably be concluded as irrelevant for the diagnostic process and pathogenesis in PSC.
ASCA is an antibody against baker’s yeast (microbial antigens) and therefore does not represent a typical autoantibody. ASCA was first described in patients with CD in 1988[113]. The antigenic epitope of ASCA is located on the S. cerevisiae mannan, which is a polymer of mannose[114]. In a single study, 44% (11/25) of PSC patients were ASCA positive (57% with concurrent IBD and 39% without IBD) compared with 23% (28/123) of PBC and 18% (12/67) of AIH patients[115]. The presence of ASCA is interesting as a specific example of immune responses towards gut luminal antigens in IBD. As a serological marker in PSC, however, ASCA does not seem to contribute.
Anti-phospholipid antibodies are directed against phospholipids or phospholipid associated proteins, and are associated with thromboembolic disease. They are commonly detected in connective tissue disorders (e.g. SLE) but also in 1% to 5% of healthy subjects and during infections[116]. Three studies have investigated the presence of anti-cardiolipin antibodies in PSC with the prevalence ranging from 4% to 63% (Table 1). Interestingly, Angulo et al[78] found a positive correlation between anti-cardiolipin titres and Mayo risk score and histological disease stage, and there are anecdotal reports of an elevated risk of thrombosis in PSC patients[117]. An increased risk of hepatic artery thrombosis post liver transplantation has also been proposed[118]. Anti-cardiolipin antibodies have also been reported at low frequencies in UC (16%-26%)[119–121] and CD (16%-27%) patients[120121].
In addition to ANA and SMA, several other non-specific autoantibodies are detected in PSC. Rheumatoid factor is detected in connective tissue diseases, infections and lymphoproliferative diseases[122], but is only found in 15% of PSC patients[78]. Anti-endothelial cell antibodies (AECAs) are directed against antigens in endothelial cells and have been reported in 35% of PSC patients in a single small study[87] but are observed in many other clinical conditions including vasculitis, SLE, systemic sclerosis and IBD[123124]. The clinical and pathogenetic roles of AECAs are not clear[123124].
A few autoantibodies in PSC are probably related to co-morbidity. In one study, the prevalence of thyroid diseases in PSC patients was 8%[11]. This probably explains the elevated levels of anti-thyroid peroxidase (Table 3) and other thyroid related antibodies in PSC patients[547887]. An association between PSC and celiac disease has been reported[125–127]. Recently the celiac disease related anti-tissue transglutaminase was detected in 7% of PSC patients in a large pre-transplant cohort from the Mayo Clinic (11/155), versus 6% (7/112) of PBC and 35% (15/43) of AIH[128] patients. This may in part be explained by shared susceptibility HLA-alleles (DQ2 and DQ8)[129]. Finally, in a single small study, antibodies against the glomerular basement membrane (anti-GBM) were detected in 17% of patients with PSC, while all healthy controls were negative[87]. The significance of this finding is not known.
Antibodies against sulfite oxidase were detected by Preuss et al. in 33% (13/39) of PSC patients compared with 5% (5/96) of PBC and 9% (7/77) of AIH patients[130]. Sulfite oxidase is a mitochondrial enzyme previously thought to be the antigen of anti-M4 (an AMA subtype)[131] but this does not seem to be correct[132]. The authors report lower prevalence in PSC patients treated with UDCA but the role of anti-sulfite oxidase antibodies in PSC remains to be established[130].
Glutathione S-transferase theta 1 (GSTT1) was recently investigated as a candidate autoantigen in PSC by Ardesjö et al using immunoscreening[133]. This group created a cDNA library based on mRNA from human ductus choledochus[133]. The GSTT1 antigen was identified screening one single PSC patient serum for antibodies against bacteria expressing the cDNA encoded proteins. Upon testing in a larger population of PSC patients (n = 58), antibodies against GSTT1 were only found in three patients, thus concluding GSTT1 as unlikely to serve as an important autoantigen in PSC. Nevertheless, the study points to the possible need for the application of broader screening methods in the search for autoantigens in PSC. The role of autoantibodies and B-cells in other autoimmune diseases has gained renewed interest the last few years[134], not only as pathogenetic factors[135], but also as therapeutic targets (e.g. Rituximab)[136]. It is thus likely that further insight into the role of autoantibodies in PSC may be of clinical importance and further studies are warranted.
A large number of autoantibodies have been detected in PSC patients. The specificity of these antibodies is generally low and the frequencies vary largely between different studies. Interpretation of the literature is difficult because of small patient sample sizes and variable methodology for antibody detection. The presence of autoantibodies in PSC is often attributed to a nonspecific dysregulation of the immune system, but the literature in PSC points to the possible presence of specific antibody targets both in the biliary epithelium and in neutrophils. Further characterisation of such targets would probably yield important insight into the pathogenesis of PSC. The investigation of larger populations may also further define the role of autoantibodies in PSC as diagnostic tools.
| 1. | Chapman RW, Arborgh BA, Rhodes JM, Summerfield JA, Dick R, Scheuer PJ, Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980;21:870-877. [Cited in This Article: ] |
| 2. | Cullen SN, Chapman RW. The medical management of primary sclerosing cholangitis. Semin Liver Dis. 2006;26:52-61. [Cited in This Article: ] |
| 3. | Brandsaeter B, Friman S, Broome U, Isoniemi H, Olausson M, Backman L, Hansen B, Schrumpf E, Oksanen A, Ericzon BG. Outcome following liver transplantation for primary sclerosing cholangitis in the Nordic countries. Scand J Gastroenterol. 2003;38:1176-1183. [Cited in This Article: ] |
| 4. | Wee A, Ludwig J. Pericholangitis in chronic ulcerative colitis: primary sclerosing cholangitis of the small bile ducts? Ann Intern Med. 1985;102:581-587. [Cited in This Article: ] |
| 5. | Washington MK. Autoimmune liver disease: overlap and outliers. Mod Pathol. 2007;20 Suppl 1:S15-S30. [Cited in This Article: ] |
| 6. | Broome U, Bergquist A. Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. 2006;26:31-41. [Cited in This Article: ] |
| 7. | Fausa O, Schrumpf E, Elgjo K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin Liver Dis. 1991;11:31-39. [Cited in This Article: ] |
| 8. | Loftus EV Jr, Harewood GC, Loftus CG, Tremaine WJ, Harmsen WS, Zinsmeister AR, Jewell DA, Sandborn WJ. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005;54:91-96. [Cited in This Article: ] |
| 9. | Cullen S, Chapman R. Primary sclerosing cholangitis. Autoimmun Rev. 2003;2:305-312. [Cited in This Article: ] |
| 10. | Rose NR, Bona C. Defining criteria for autoimmune diseases (Witebsky's postulates revisited). Immunol Today. 1993;14:426-430. [Cited in This Article: ] |
| 11. | Saarinen S, Olerup O, Broome U. Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:3195-3199. [Cited in This Article: ] |
| 12. | Bergquist A, Lindberg G, Saarinen S, Broome U. Increased prevalence of primary sclerosing cholangitis among first-degree relatives. J Hepatol. 2005;42:252-256. [Cited in This Article: ] |
| 13. | Ponsioen CY, Kuiper H, Ten Kate FJ, van Milligen de Wit M, van Deventer SJ, Tytgat GN. Immunohistochemical analysis of inflammation in primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 1999;11:769-774. [Cited in This Article: ] |
| 14. | Broome U, Grunewald J, Scheynius A, Olerup O, Hultcrantz R. Preferential V beta3 usage by hepatic T lymphocytes in patients with primary sclerosing cholangitis. J Hepatol. 1997;26:527-534. [Cited in This Article: ] |
| 15. | Karlsen TH, Schrumpf E, Boberg KM. Genetic epidemiology of primary sclerosing cholangitis. World J Gastroenterol. 2007;13:5421-5431. [Cited in This Article: ] |
| 16. | Terjung B, Worman HJ. Anti-neutrophil antibodies in primary sclerosing cholangitis. Best Pract Res Clin Gastroenterol. 2001;15:629-642. [Cited in This Article: ] |
| 17. | Broome U, Olsson R, Loof L, Bodemar G, Hultcrantz R, Danielsson A, Prytz H, Sandberg-Gertzen H, Wallerstedt S, Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996;38:610-615. [Cited in This Article: ] |
| 18. | O'Mahony CA, Vierling JM. Etiopathogenesis of primary sclerosing cholangitis. Semin Liver Dis. 2006;26:3-21. [Cited in This Article: ] |
| 19. | Klein J, Sato A. The HLA system. First of two parts. N Engl J Med. 2000;343:702-709. [Cited in This Article: ] |
| 20. | Van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54:1117-1121. [Cited in This Article: ] |
| 21. | Das KM. Immunopathogenesis of primary sclerosing cholangitis: possible role of a shared colonic and biliary epithelial antigen. J Gastroenterol Hepatol. 2004;19:S290. [Cited in This Article: ] |
| 22. | Das KM, Sakamaki S, Vecchi M, Diamond B. The production and characterization of monoclonal antibodies to a human colonic antigen associated with ulcerative colitis: cellular localization of the antigen by using the monoclonal antibody. J Immunol. 1987;139:77-84. [Cited in This Article: ] |
| 23. | Das KM, Dasgupta A, Mandal A, Geng X. Autoimmunity to cytoskeletal protein tropomyosin. A clue to the pathogenetic mechanism for ulcerative colitis. J Immunol. 1993;150:2487-2493. [Cited in This Article: ] |
| 24. | Geng X, Biancone L, Dai HH, Lin JJ, Yoshizaki N, Dasgupta A, Pallone F, Das KM. Tropomyosin isoforms in intestinal mucosa: production of autoantibodies to tropomyosin isoforms in ulcerative colitis. Gastroenterology. 1998;114:912-922. [Cited in This Article: ] |
| 25. | Mirza ZK, Sastri B, Lin JJ, Amenta PS, Das KM. Autoimmunity against human tropomyosin isoforms in ulcerative colitis: localization of specific human tropomyosin isoforms in the intestine and extraintestinal organs. Inflamm Bowel Dis. 2006;12:1036-1043. [Cited in This Article: ] |
| 26. | Mandal A, Dasgupta A, Jeffers L, Squillante L, Hyder S, Reddy R, Schiff E, Das KM. Autoantibodies in sclerosing cholangitis against a shared peptide in biliary and colon epithelium. Gastroenterology. 1994;106:185-192. [Cited in This Article: ] |
| 27. | Kesari KV, Yoshizaki N, Geng X, Lin JJ, Das KM. Externalization of tropomyosin isoform 5 in colon epithelial cells. Clin Exp Immunol. 1999;118:219-227. [Cited in This Article: ] |
| 28. | Biancone L, Monteleone G, Marasco R, Pallone F. Autoimmunity to tropomyosin isoforms in ulcerative colitis (UC) patients and unaffected relatives. Clin Exp Immunol. 1998;113:198-205. [Cited in This Article: ] |
| 29. | Ebert EC, Geng X, Lin J, Das KM. Autoantibodies against human tropomyosin isoform 5 in ulcerative colitis destroys colonic epithelial cells through antibody and complement-mediated lysis. Cell Immunol. 2006;244:43-49. [Cited in This Article: ] |
| 30. | Sakamaki S, Takayanagi N, Yoshizaki N, Hayashi S, Takayama T, Kato J, Kogawa K, Yamauchi N, Takemoto N, Nobuoka A. Autoantibodies against the specific epitope of human tropomyosin(s) detected by a peptide based enzyme immunoassay in sera of patients with ulcerative colitis show antibody dependent cell mediated cytotoxicity against HLA-DPw9 transfected L cells. Gut. 2000;47:236-241. [Cited in This Article: ] |
| 31. | Halstensen TS, Das KM, Brandtzaeg P. Epithelial deposits of immunoglobulin G1 and activated complement colocalise with the M(r) 40 kD putative autoantigen in ulcerative colitis. Gut. 1993;34:650-657. [Cited in This Article: ] |
| 32. | Snook JA, Lowes JR, Wu KC, Priddle JD, Jewell DP. Serum and tissue autoantibodies to colonic epithelium in ulcerative colitis. Gut. 1991;32:163-166. [Cited in This Article: ] |
| 33. | Cantrell M, Prindiville T, Gershwin ME. Autoantibodies to colonic cells and subcellular fractions in inflammatory bowel disease: do they exist? J Autoimmun. 1990;3:307-320. [Cited in This Article: ] |
| 34. | Khoo UY, Bjarnason I, Donaghy A, Williams R, Macpherson A. Antibodies to colonic epithelial cells from the serum and colonic mucosal washings in ulcerative colitis. Gut. 1995;37:63-70. [Cited in This Article: ] |
| 35. | Hamilton MI, Bradley NJ, Srai SK, Thrasivoulou C, Pounder RE, Wakefield AJ. Autoimmunity in ulcerative colitis: tropomyosin is not the major antigenic determinant of the Das monoclonal antibody, 7E12H12. Clin Exp Immunol. 1995;99:404-411. [Cited in This Article: ] |
| 36. | Xu B, Broome U, Ericzon BG, Sumitran-Holgersson S. High frequency of autoantibodies in patients with primary sclerosing cholangitis that bind biliary epithelial cells and induce expression of CD44 and production of interleukin 6. Gut. 2002;51:120-127. [Cited in This Article: ] |
| 37. | Karrar A, Broome U, Sodergren T, Jaksch M, Bergquist A, Bjornstedt M, Sumitran-Holgersson S. Biliary epithelial cell antibodies link adaptive and innate immune responses in primary sclerosing cholangitis. Gastroenterology. 2007;132:1504-1514. [Cited in This Article: ] |
| 38. | Ge X, Ericzon BG, Nowak G, oHrstrom H, Broome U, Sumitran-Holgersson S. Are preformed antibodies to biliary epithelial cells of clinical importance in liver transplantation? Liver Transpl. 2003;9:1191-1198. [Cited in This Article: ] |
| 39. | Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J (Clin Res Ed). 1982;285:606. [Cited in This Article: ] |
| 40. | Van der Woude FJ, Rasmussen N, Lobatto S, Wiik A, Permin H, van Es LA, van der Giessen M, van der Hem GK, The TH. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425-429. [Cited in This Article: ] |
| 41. | Calabresi P, Thayer WR, Spiro HM. Demonstration of circulating antinuclear globulins in ulcerative culitis. J Clin Invest. 1961;40:2126-2133. [Cited in This Article: ] |
| 42. | Nielsen H, Wiik A, Elmgreen J. Granulocyte specific antinuclear antibodies in ulcerative colitis. Aid in differential diagnosis of inflammatory bowel disease. Acta Pathol Microbiol Immunol Scand C. 1983;91:23-26. [Cited in This Article: ] |
| 43. | Snook JA, Chapman RW, Fleming K, Jewell DP. Anti-neutrophil nuclear antibody in ulcerative colitis, Crohn's disease and primary sclerosing cholangitis. Clin Exp Immunol. 1989;76:30-33. [Cited in This Article: ] |
| 44. | Terjung B, Bogsch F, Klein R, Sohne J, Reichel C, Wasmuth JC, Beuers U, Sauerbruch T, Spengler U. Diagnostic accuracy of atypical p-ANCA in autoimmune hepatitis using ROC- and multivariate regression analysis. Eur J Med Res. 2004;9:439-448. [Cited in This Article: ] |
| 45. | Duerr RH, Targan SR, Landers CJ, LaRusso NF, Lindsay KL, Wiesner RH, Shanahan F. Neutrophil cytoplasmic antibodies: a link between primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1991;100:1385-1391. [Cited in This Article: ] |
| 46. | Rump JA, Scholmerich J, Gross V, Roth M, Helfesrieder R, Rautmann A, Ludemann J, Gross WL, Peter HH. A new type of perinuclear anti-neutrophil cytoplasmic antibody (p-ANCA) in active ulcerative colitis but not in Crohn's disease. Immunobiology. 1990;181:406-413. [Cited in This Article: ] |
| 47. | Savige J, Dimech W, Fritzler M, Goeken J, Hagen EC, Jennette JC, McEvoy R, Pusey C, Pollock W, Trevisin M. Addendum to the International Consensus Statement on testing and reporting of antineutrophil cytoplasmic antibodies. Quality control guidelines, comments, and recommendations for testing in other autoimmune diseases. Am J Clin Pathol. 2003;120:312-318. [Cited in This Article: ] |
| 48. | Savige J, Gillis D, Benson E, Davies D, Esnault V, Falk RJ, Hagen EC, Jayne D, Jennette JC, Paspaliaris B. International Consensus Statement on Testing and Reporting of Antineutrophil Cytoplasmic Antibodies (ANCA). Am J Clin Pathol. 1999;111:507-513. [Cited in This Article: ] |
| 49. | Billing P, Tahir S, Calfin B, Gagne G, Cobb L, Targan S, Vidrich A. Nuclear localization of the antigen detected by ulcerative colitis-associated perinuclear antineutrophil cytoplasmic antibodies. Am J Pathol. 1995;147:979-987. [Cited in This Article: ] |
| 50. | Terjung B, Herzog V, Worman HJ, Gestmann I, Bauer C, Sauerbruch T, Spengler U. Atypical antineutrophil cytoplasmic antibodies with perinuclear fluorescence in chronic inflammatory bowel diseases and hepatobiliary disorders colocalize with nuclear lamina proteins. Hepatology. 1998;28:332-340. [Cited in This Article: ] |
| 51. | Terjung B, Spengler U, Sauerbruch T, Worman HJ. "Atypical p-ANCA" in IBD and hepatobiliary disorders react with a 50-kilodalton nuclear envelope protein of neutrophils and myeloid cell lines. Gastroenterology. 2000;119:310-322. [Cited in This Article: ] |
| 52. | Terjung B, Worman HJ, Herzog V, Sauerbruch T, Spengler U. Differentiation of antineutrophil nuclear antibodies in inflammatory bowel and autoimmune liver diseases from antineutrophil cytoplasmic antibodies (p-ANCA) using immunofluorescence microscopy. Clin Exp Immunol. 2001;126:37-46. [Cited in This Article: ] |
| 53. | Claise C, Johanet C, Bouhnik Y, Kapel N, Homberg JC, Poupon R. Antineutrophil cytoplasmic autoantibodies in autoimmune liver and inflammatory bowel diseases. Liver. 1996;16:28-34. [Cited in This Article: ] |
| 54. | Klein R, Eisenburg J, Weber P, Seibold F, Berg PA. Significance and specificity of antibodies to neutrophils detected by western blotting for the serological diagnosis of primary sclerosing cholangitis. Hepatology. 1991;14:1147-1152. [Cited in This Article: ] |
| 55. | Lindgren S, Nilsson S, Nassberger L, Verbaan H, Wieslander J. Anti-neutrophil cytoplasmic antibodies in patients with chronic liver diseases: prevalence, antigen specificity and predictive value for diagnosis of autoimmune liver disease. Swedish Internal Medicine Liver Club (SILK). J Gastroenterol Hepatol. 2000;15:437-442. [Cited in This Article: ] |
| 56. | Lo SK, Fleming KA, Chapman RW. Prevalence of anti-neutrophil antibody in primary sclerosing cholangitis and ulcerative colitis using an alkaline phosphatase technique. Gut. 1992;33:1370-1375. [Cited in This Article: ] |
| 57. | Roozendaal C, de Jong MA, van den Berg AP, van Wijk RT, Limburg PC, Kallenberg CG. Clinical significance of anti-neutrophil cytoplasmic antibodies (ANCA) in autoimmune liver diseases. J Hepatol. 2000;32:734-741. [Cited in This Article: ] |
| 58. | Schwarze C, Terjung B, Lilienweiss P, Beuers U, Herzog V, Sauerbruch T, Spengler U. IgA class antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis and autoimmune hepatitis. Clin Exp Immunol. 2003;133:283-289. [Cited in This Article: ] |
| 59. | Stoffel MP, Csernok E, Herzberg C, Johnson T, Carroll SF, Gross WL. Anti-neutrophil cytoplasmic antibodies (ANCA) directed against bactericidal/permeability increasing protein (BPI): a new seromarker for inflammatory bowel disease and associated disorders. Clin Exp Immunol. 1996;104:54-59. [Cited in This Article: ] |
| 60. | Wilschanski M, Chait P, Wade JA, Davis L, Corey M, St Louis P, Griffiths AM, Blendis LM, Moroz SP, Scully L. Primary sclerosing cholangitis in 32 children: clinical, laboratory, and radiographic features, with survival analysis. Hepatology. 1995;22:1415-1422. [Cited in This Article: ] |
| 61. | Zachou K, Liaskos C, Rigopoulou E, Gabeta S, Papamichalis P, Gatselis N, Georgiadou S, Dalekos GN. Presence of high avidity anticardiolipin antibodies in patients with autoimmune cholestatic liver diseases. Clin Immunol. 2006;119:203-212. [Cited in This Article: ] |
| 62. | Mehal WZ, Lo SK, Chapman RW, Fleming KA. The immunogenetic basis for anti-neutrophil cytoplasmic antibody production in primary sclerosing cholangitis and ulcerative colitis. J Hepatol. 1994;21:910-911. [Cited in This Article: ] |
| 63. | Seibold F, Slametschka D, Gregor M, Weber P. Neutrophil autoantibodies: a genetic marker in primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1994;107:532-536. [Cited in This Article: ] |
| 64. | Bansi DS, Lo S, Chapman RW, Fleming KA. Absence of antineutrophil cytoplasmic antibodies in relatives of UK patients with primary sclerosing cholangitis and ulcerative colitis. Eur J Gastroenterol Hepatol. 1996;8:111-116. [Cited in This Article: ] |
| 65. | Terjung B, Muennich M, Gottwein J. Identification of myleloid-specific tubulin-beta isotype 5 as target antigen of antineutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 2005;42:288A. [Cited in This Article: ] |
| 66. | Sobajima J, Ozaki S, Osakada F, Uesugi H, Shirakawa H, Yoshida M, Nakao K. Novel autoantigens of perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA) in ulcerative colitis: non-histone chromosomal proteins, HMG1 and HMG2. Clin Exp Immunol. 1997;107:135-140. [Cited in This Article: ] |
| 67. | Sobajima J, Ozaki S, Uesugi H, Osakada F, Inoue M, Fukuda Y, Shirakawa H, Yoshida M, Rokuhara A, Imai H. High mobility group (HMG) non-histone chromosomal proteins HMG1 and HMG2 are significant target antigens of perinuclear anti-neutrophil cytoplasmic antibodies in autoimmune hepatitis. Gut. 1999;44:867-873. [Cited in This Article: ] |
| 68. | Sobajima J, Ozaki S, Uesugi H, Osakada F, Shirakawa H, Yoshida M, Nakao K. Prevalence and characterization of perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA) directed against HMG1 and HMG2 in ulcerative colitis (UC). Clin Exp Immunol. 1998;111:402-407. [Cited in This Article: ] |
| 69. | Eggena M, Cohavy O, Parseghian MH, Hamkalo BA, Clemens D, Targan SR, Gordon LK, Braun J. Identification of histone H1 as a cognate antigen of the ulcerative colitis-associated marker antibody pANCA. J Autoimmun. 2000;14:83-97. [Cited in This Article: ] |
| 70. | Kallenberg CG, Heeringa P, Stegeman CA. Mechanisms of Disease: pathogenesis and treatment of ANCA-associated vasculitides. Nat Clin Pract Rheumatol. 2006;2:661-670. [Cited in This Article: ] |
| 71. | Gazzano-Santoro H, Parent JB, Grinna L, Horwitz A, Parsons T, Theofan G, Elsbach P, Weiss J, Conlon PJ. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754-4761. [Cited in This Article: ] |
| 72. | Schultz H, Csernok E, Schuster A, Schmitz TS, Ernst M, Gross WL. Anti-neutrophil cytoplasmic antibodies directed against the bactericidal/permeability-increasing protein (BPI) in pediatric cystic fibrosis patients do not recognize N-terminal regions important for the anti-microbial and lipopolysaccharide-binding activity of BPI. Pediatr Allergy Immunol. 2000;11:64-70. [Cited in This Article: ] |
| 73. | Roozendaal C, Pogany K, Horst G, Jagt TG, Kleibeuker JH, Nelis GF, Limburg PC, Kallenberg CG. Does analysis of the antigenic specificities of anti-neutrophil cytoplasmic antibodies contribute to their clinical significance in the inflammatory bowel diseases? Scand J Gastroenterol. 1999;34:1123-1131. [Cited in This Article: ] |
| 74. | Cooper T, Savige J, Nassis L, Paspaliaris B, Neeson P, Neil J, Knight KR, Daskalakis M, Doery JC. Clinical associations and characterisation of antineutrophil cytoplasmic antibodies directed against bactericidal/permeability-increasing protein and azurocidin. Rheumatol Int. 2000;19:129-136. [Cited in This Article: ] |
| 75. | Brimnes J, Nielsen OH, Wiik A, Heegaard NH. Autoantibodies to molecular targets in neutrophils in patients with ulcerative colitis. Dig Dis Sci. 1999;44:415-423. [Cited in This Article: ] |
| 76. | Walmsley RS, Zhao MH, Hamilton MI, Brownlee A, Chapman P, Pounder RE, Wakefield AJ, Lockwood CM. Antineutrophil cytoplasm autoantibodies against bactericidal/permeability-increasing protein in inflammatory bowel disease. Gut. 1997;40:105-109. [Cited in This Article: ] |
| 77. | Vecchi M, Sinico A, Bianchi MB, Radice A, Gionchetti P, Campieri M, de Franchis R. Recognition of bactericidal/permeability-increasing protein by perinuclear anti-neutrophil cytoplasmic antibody-positive sera from ulcerative colitis patients: prevalence and clinical significance. Scand J Gastroenterol. 1998;33:1284-1288. [Cited in This Article: ] |
| 78. | Angulo P, Peter JB, Gershwin ME, DeSotel CK, Shoenfeld Y, Ahmed AE, Lindor KD. Serum autoantibodies in patients with primary sclerosing cholangitis. J Hepatol. 2000;32:182-187. [Cited in This Article: ] |
| 79. | Khanna D, Aggarwal A, Bhakuni DS, Dayal R, Misra R. Bactericidal/permeability-increasing protein and cathepsin G are the major antigenic targets of antineutrophil cytoplasmic autoantibodies in systemic sclerosis. J Rheumatol. 2003;30:1248-1252. [Cited in This Article: ] |
| 80. | Carlsson M, Eriksson L, Pressler T, Kornfalt R, Mared L, Meyer P, Wiik A, Wieslander J, Segelmark M. Autoantibody response to BPI predict disease severity and outcome in cystic fibrosis. J Cyst Fibros. 2007;6:228-233. [Cited in This Article: ] |
| 81. | Baveye S, Elass E, Mazurier J, Spik G, Legrand D. Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clin Chem Lab Med. 1999;37:281-286. [Cited in This Article: ] |
| 82. | Locht H, Skogh T, Wiik A. Characterisation of autoantibodies to neutrophil granule constituents among patients with reactive arthritis, rheumatoid arthritis, and ulcerative colitis. Ann Rheum Dis. 2000;59:898-903. [Cited in This Article: ] |
| 83. | Chen M, Zhao MH, Zhang YK, Wang HY. Antineutrophil cytoplasmic autoantibodies in patients with systemic lupus erythematosus recognize a novel 69 kDa target antigen of neutrophil granules. Nephrology (Carlton). 2005;10:491-495. [Cited in This Article: ] |
| 84. | Seibold F, Weber P, Schoning A, Mork H, Goppel S, Scheurlen M. Neutrophil antibodies (pANCA) in chronic liver disease and inflammatory bowel disease: do they react with different antigens? Eur J Gastroenterol Hepatol. 1996;8:1095-1100. [Cited in This Article: ] |
| 85. | Peen E, Almer S, Bodemar G, Ryden BO, Sjolin C, Tejle K, Skogh T. Anti-lactoferrin antibodies and other types of ANCA in ulcerative colitis, primary sclerosing cholangitis, and Crohn's disease. Gut. 1993;34:56-62. [Cited in This Article: ] |
| 86. | Kossa K, Coulthart A, Ives CT, Pusey CD, Hodgson HJ. Antigen specificity of circulating anti-neutrophil cytoplasmic antibodies in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995;7:783-789. [Cited in This Article: ] |
| 87. | Gur H, Shen G, Sutjita M, Terrberry J, Alosachie I, Barka N, Lin HC, Peter JB, Meroni PL, Kaplan M. Autoantibody profile of primary sclerosing cholangitis. Pathobiology. 1995;63:76-82. [Cited in This Article: ] |
| 88. | Tervaert JW, Mulder AH, Horst G, Haagsma EB, Kleibeuker JH, Kallenberg CG. Antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis, ulcerative colitis, and autoimmune diseases. Gastroenterology. 1992;102:1090-1091. [Cited in This Article: ] |
| 89. | Orth T, Kellner R, Diekmann O, Faust J, Meyer zum Buschenfelde KH, Mayet WJ. Identification and characterization of autoantibodies against catalase and alpha-enolase in patients with primary sclerosing cholangitis. Clin Exp Immunol. 1998;112:507-515. [Cited in This Article: ] |
| 90. | Kain R, Matsui K, Exner M, Binder S, Schaffner G, Sommer EM, Kerjaschki D. A novel class of autoantigens of anti-neutrophil cytoplasmic antibodies in necrotizing and crescentic glomerulonephritis: the lysosomal membrane glycoprotein h-lamp-2 in neutrophil granulocytes and a related membrane protein in glomerular endothelial cells. J Exp Med. 1995;181:585-597. [Cited in This Article: ] |
| 91. | Weismuller TJ, Wedemeyer J, Kubicka S, Strassburg CP, Manns MP. The challenges in primary sclerosing cholangitis--aetiopathogenesis, autoimmunity, management and malignancy. J Hepatol. 2008;48 Suppl 1:S38-S57. [Cited in This Article: ] |
| 92. | Wiik A. Neutrophil-specific autoantibodies in chronic inflammatory bowel diseases. Autoimmun Rev. 2002;1:67-72. [Cited in This Article: ] |
| 93. | Schultz H. From infection to autoimmunity: a new model for induction of ANCA against the bactericidal/permeability increasing protein (BPI). Autoimmun Rev. 2007;6:223-227. [Cited in This Article: ] |
| 94. | Shih DQ, Targan SR. Immunopathogenesis of inflammatory bowel disease. World J Gastroenterol. 2008;14:390-400. [Cited in This Article: ] |
| 95. | Targan SR, Landers CJ, Cobb L, MacDermott RP, Vidrich A. Perinuclear anti-neutrophil cytoplasmic antibodies are spontaneously produced by mucosal B cells of ulcerative colitis patients. J Immunol. 1995;155:3262-3267. [Cited in This Article: ] |
| 96. | Seibold F, Brandwein S, Simpson S, Terhorst C, Elson CO. pANCA represents a cross-reactivity to enteric bacterial antigens. J Clin Immunol. 1998;18:153-160. [Cited in This Article: ] |
| 97. | Terjung B, Soehne J, Worman HJ, Sauerbruch T, Spengler U. Molecular mimicry between target antigen of ANCA and microbial protein FtsZ in autoimmune liver disorders. Hepatology. 2006;44:229A. [Cited in This Article: ] |
| 98. | Seibold F, Weber P, Klein R, Berg PA, Wiedmann KH. Clinical significance of antibodies against neutrophils in patients with inflammatory bowel disease and primary sclerosing cholangitis. Gut. 1992;33:657-662. [Cited in This Article: ] |
| 99. | Roozendaal C, Van Milligen de Wit AW, Haagsma EB, Horst G, Schwarze C, Peter HH, Kleibeuker JH, Tervaert JW, Limburg PC, Kallenberg CG. Antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis: defined specificities may be associated with distinct clinical features. Am J Med. 1998;105:393-399. [Cited in This Article: ] |
| 100. | Pokorny CS, Norton ID, McCaughan GW, Selby WS. Anti-neutrophil cytoplasmic antibody: a prognostic indicator in primary sclerosing cholangitis. J Gastroenterol Hepatol. 1994;9:40-44. [Cited in This Article: ] |
| 101. | Bansi DS, Fleming KA, Chapman RW. Importance of antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis and ulcerative colitis: prevalence, titre, and IgG subclass. Gut. 1996;38:384-389. [Cited in This Article: ] |
| 102. | Mulder AH, Horst G, Haagsma EB, Limburg PC, Kleibeuker JH, Kallenberg CG. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993;17:411-417. [Cited in This Article: ] |
| 103. | Lo SK, Fleming KA, Chapman RW. A 2-year follow-up study of anti-neutrophil antibody in primary sclerosing cholangitis: relationship to clinical activity, liver biochemistry and ursodeoxycholic acid treatment. J Hepatol. 1994;21:974-978. [Cited in This Article: ] |
| 104. | Haagsma EB, Mulder AH, Gouw AS, Horst G, Meerman L, Slooff MJ, Kallenberg CG. Neutrophil cytoplasmic autoantibodies after liver transplantation in patients with primary sclerosing cholangitis. J Hepatol. 1993;19:8-14. [Cited in This Article: ] |
| 105. | Invernizzi P, Lleo A, Podda M. Interpreting serological tests in diagnosing autoimmune liver diseases. Semin Liver Dis. 2007;27:161-172. [Cited in This Article: ] |
| 106. | Howard MJ, Fuller C, Broadhurst RW, Perham RN, Tang JG, Quinn J, Diamond AG, Yeaman SJ. Three-dimensional structure of the major autoantigen in primary biliary cirrhosis. Gastroenterology. 1998;115:139-146. [Cited in This Article: ] |
| 107. | Czaja AJ. Autoantibodies in autoimmune liver disease. Adv Clin Chem. 2005;40:127-164. [Cited in This Article: ] |
| 108. | Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737-745. [Cited in This Article: ] |
| 109. | Eggleton P, Haigh R, Winyard PG. Consequence of neo-antigenicity of the 'altered self'. Rheumatology (Oxford). 2008;47:567-571. [Cited in This Article: ] |
| 110. | Terjung B, Spengler U. Role of auto-antibodies for the diagnosis of chronic cholestatic liver diseases. Clin Rev Allergy Immunol. 2005;28:115-133. [Cited in This Article: ] |
| 111. | Zauli D, Schrumpf E, Crespi C, Cassani F, Fausa O, Aadland E. An autoantibody profile in primary sclerosing cholangitis. J Hepatol. 1987;5:14-18. [Cited in This Article: ] |
| 112. | Granito A, Muratori P, Muratori L, Pappas G, Cassani F, Worthington J, Ferri S, Quarneti C, Cipriano V, de Molo C. Antibodies to SS-A/Ro-52kD and centromere in autoimmune liver disease: a clue to diagnosis and prognosis of primary biliary cirrhosis. Aliment Pharmacol Ther. 2007;26:831-838. [Cited in This Article: ] |
| 113. | Main J, McKenzie H, Yeaman GR, Kerr MA, Robson D, Pennington CR, Parratt D. Antibody to Saccharomyces cerevisiae (bakers' yeast) in Crohn's disease. BMJ. 1988;297:1105-1106. [Cited in This Article: ] |
| 114. | Sendid B, Colombel JF, Jacquinot PM, Faille C, Fruit J, Cortot A, Lucidarme D, Camus D, Poulain D. Specific antibody response to oligomannosidic epitopes in Crohn's disease. Clin Diagn Lab Immunol. 1996;3:219-226. [Cited in This Article: ] |
| 115. | Muratori P, Muratori L, Guidi M, Maccariello S, Pappas G, Ferrari R, Gionchetti P, Campieri M, Bianchi FB. Anti-Saccharomyces cerevisiae antibodies (ASCA) and autoimmune liver diseases. Clin Exp Immunol. 2003;132:473-476. [Cited in This Article: ] |
| 116. | Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752-763. [Cited in This Article: ] |
| 117. | Kirby DF, Blei AT, Rosen ST, Vogelzang RL, Neiman HL. Primary sclerosing cholangitis in the presence of a lupus anticoagulant. Am J Med. 1986;81:1077-1080. [Cited in This Article: ] |
| 118. | Bjoro K, Brandsaeter B, Foss A, Schrumpf E. Liver transplantation in primary sclerosing cholangitis. Semin Liver Dis. 2006;26:69-79. [Cited in This Article: ] |
| 119. | Dalekos GN, Manoussakis MN, Goussia AC, Tsianos EV, Moutsopoulos HM. Soluble interleukin-2 receptors, antineutrophil cytoplasmic antibodies, and other autoantibodies in patients with ulcerative colitis. Gut. 1993;34:658-664. [Cited in This Article: ] |
| 120. | Aichbichler BW, Petritsch W, Reicht GA, Wenzl HH, Eherer AJ, Hinterleitner TA, Auer-Grumbach P, Krejs GJ. Anti-cardiolipin antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1999;44:852-856. [Cited in This Article: ] |
| 121. | Koutroubakis IE, Petinaki E, Anagnostopoulou E, Kritikos H, Mouzas IA, Kouroumalis EA, Manousos ON. Anti-cardiolipin and anti-beta2-glycoprotein I antibodies in patients with inflammatory bowel disease. Dig Dis Sci. 1998;43:2507-2512. [Cited in This Article: ] |
| 122. | Tuomi T. Which antigen to use in the detection of rheumatoid factors? Comparison of patients with rheumatoid arthritis and subjects with 'false positive' rheumatoid factor reactions. Clin Exp Immunol. 1989;77:349-355. [Cited in This Article: ] |
| 123. | Alessandri C, Bombardieri M, Valesini G. Pathogenic mechanisms of anti-endothelial cell antibodies (AECA): their prevalence and clinical relevance. Adv Clin Chem. 2006;42:297-326. [Cited in This Article: ] |
| 124. | Youinou P. New target antigens for anti-endothelial cell antibodies. Immunobiology. 2005;210:789-797. [Cited in This Article: ] |
| 125. | Hay JE, Wiesner RH, Shorter RG, LaRusso NF, Baldus WP. Primary sclerosing cholangitis and celiac disease. A novel association. Ann Intern Med. 1988;109:713-717. [Cited in This Article: ] |
| 126. | Volta U, Rodrigo L, Granito A, Petrolini N, Muratori P, Muratori L, Linares A, Veronesi L, Fuentes D, Zauli D. Celiac disease in autoimmune cholestatic liver disorders. Am J Gastroenterol. 2002;97:2609-2613. [Cited in This Article: ] |
| 127. | Ludvigsson JF, Elfstrom P, Broome U, Ekbom A, Montgomery SM. Celiac disease and risk of liver disease: a general population-based study. Clin Gastroenterol Hepatol. 2007;5:63-69. [Cited in This Article: ] |
| 128. | Rubio-Tapia A, Abdulkarim AS, Wiesner RH, Moore SB, Krause PK, Murray JA. Celiac disease autoantibodies in severe autoimmune liver disease and the effect of liver transplantation. Liver Int. 2008;28:467-476. [Cited in This Article: ] |
| 129. | Rubio-Tapia A, Murray JA. The liver in celiac disease. Hepatology. 2007;46:1650-1658. [Cited in This Article: ] |
| 130. | Preuss B, Berg C, Altenberend F, Gregor M, Stevanovic S, Klein R. Demonstration of autoantibodies to recombinant human sulphite oxidase in patients with chronic liver disorders and analysis of their clinical relevance. Clin Exp Immunol. 2007;150:312-321. [Cited in This Article: ] |
| 131. | Klein R, Berg PA. Anti-M4 antibodies in primary biliary cirrhosis react with sulphite oxidase, an enzyme of the mitochondrial inter-membrane space. Clin Exp Immunol. 1991;84:445-448. [Cited in This Article: ] |
| 132. | Palmer JM, Yeaman SJ, Bassendine MF, James OF. M4 and M9 autoantigens in primary biliary cirrhosis--a negative study. J Hepatol. 1993;18:251-254. [Cited in This Article: ] |
| 133. | Ardesjo B, Hansson CM, Bruder CE, Rorsman F, Betterle C, Dumanski JP, Kampe O, Ekwall O. Autoantibodies to glutathione S-transferase theta 1 in patients with primary sclerosing cholangitis and other autoimmune diseases. J Autoimmun. 2008;30:273-282. [Cited in This Article: ] |
| 134. | Foreman AL, Van de Water J, Gougeon ML, Gershwin ME. B cells in autoimmune diseases: insights from analyses of immunoglobulin variable (Ig V) gene usage. Autoimmun Rev. 2007;6:387-401. [Cited in This Article: ] |
| 135. | Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008;26:651-675. [Cited in This Article: ] |
| 136. | Levesque MC, St Clair EW. B cell-directed therapies for autoimmune disease and correlates of disease response and relapse. J Allergy Clin Immunol. 2008;121:13-21; quiz 22-23. [Cited in This Article: ] |
| 137. | Zauli D, Grassi A, Cassani F, Ballardini G, Bortolotti R, Muratori L, Fusconi M, Bianchi FB. Autoimmune serology of primary sclerosing cholangitis. Dig Liver Dis. 2001;33:391-392. [Cited in This Article: ] |
| 138. | Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980;79:200-206. [Cited in This Article: ] |
| 139. | Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99-103. [Cited in This Article: ] |
| 140. | Ballot E, Homberg JC, Johanet C. Antibodies to soluble liver antigen: an additional marker in type 1 auto-immune hepatitis. J Hepatol. 2000;33:208-215. [Cited in This Article: ] |
| 141. | Lindgren S, Braun HB, Michel G, Nemeth A, Nilsson S, Thome-Kromer B, Eriksson S. Absence of LKM-1 antibody reactivity in autoimmune and hepatitis-C-related chronic liver disease in Sweden. Swedish Internal Medicine Liver club. Scand J Gastroenterol. 1997;32:175-178. [Cited in This Article: ] |
| 142. | Miyakawa H, Kawashima Y, Kitazawa E, Kawaguchi N, Kato T, Kikuchi K, Imai E, Fujikawa H, Hashimoto E, Schlumberger W. Low frequency of anti-SLA/LP autoantibody in Japanese adult patients with autoimmune liver diseases: analysis with recombinant antigen assay. J Autoimmun. 2003;21:77-82. [Cited in This Article: ] |
| 143. | Boberg KM, Fausa O, Haaland T, Holter E, Mellbye OJ, Spurkland A, Schrumpf E. Features of autoimmune hepatitis in primary sclerosing cholangitis: an evaluation of 114 primary sclerosing cholangitis patients according to a scoring system for the diagnosis of autoimmune hepatitis. Hepatology. 1996;23:1369-1376. [Cited in This Article: ] |
| 144. | Muratori L, Muratori P, Zauli D, Grassi A, Pappas G, Rodrigo L, Cassani F, Lenzi M, Bianchi FB. Antilactoferrin antibodies in autoimmune liver disease. Clin Exp Immunol. 2001;124:470-473. [Cited in This Article: ] |
| 145. | Hardarson S, Labrecque DR, Mitros FA, Neil GA, Goeken JA. Antineutrophil cytoplasmic antibody in inflammatory bowel and hepatobiliary diseases. High prevalence in ulcerative colitis, primary sclerosing cholangitis, and autoimmune hepatitis. Am J Clin Pathol. 1993;99:277-281. [Cited in This Article: ] |
| 146. | Bansi DS, Bauducci M, Bergqvist A, Boberg K, Broome U, Chapman R, Fleming K, Jorgensen R, Lindor K, Rosina F. Detection of antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis: a comparison of the alkaline phosphatase and immunofluorescent techniques. Eur J Gastroenterol Hepatol. 1997;9:575-580. [Cited in This Article: ] |