Published online Jun 14, 2008. doi: 10.3748/wjg.14.3569
Revised: May 4, 2008
Accepted: May 11, 2008
Published online: June 14, 2008
AIM: To study the preventive effects of Qianggan-Rongxian Decoction on liver fibrosis induced by dimethylnitrosamine (DMN) in rats.
METHODS: Male Wistar rats were randomly divided into hepatic fibrosis model group, control group and 3 treatment groups (12 rats in each group). Except for the normal control group, all the rats received 1% DMN (10 &mgr;L/kg body weight, i.p), 3 times a week for 4 wk. The rats in the 3 treatment groups including a high-dose DMN group (10 mL/kg), a medium-dose DMN group (7 mL/kg), and a low-dose DMN group (4 mL/kg) were daily gavaged with Qianggan-Rongxian Decoction, and the rats in the model and normal control groups were given saline vehicle. Enzyme-linked immunosorbent assay (ELISA) was used to determine the changes in serum hyaluronic acid (HA), laminin (LN), and type IV collagen levels. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured using routine laboratory methods. Pathologic changes, particularly fibrosis, were examined by hematoxylin and eosin (HE) and Sirius red staining. Hepatic stellate cells (HSC) were examined by transmission electron microscopy.
RESULTS: Compared with the model control group, the serum levels of HA, LN, type IV collagen, ALT and AST were decreased markedly in the other groups after treatment with Qianggan-Rongxian Decoction, especially in the medium-dose DMN group (P < 0.05). Moreover, the area-density percentage of collagen fibrosis was lower in the Qianggan-Rongxian Decoction treatment groups than in the model group, and a more significant drop was observed in the medium-dose DMN group (P < 0.05).
CONCLUSION: Qianggan-Rongxian Decoction can inhibit hepatic fibrosis due to chronic liver injury, delay the development of cirrhosis, and notably ameliorate liver function. It may be used as a safe and effective thera-peutic drug for patients with fibrosis.
-
Citation: Li CH, Pan LH, Yang ZW, Li CY, Xu WX. Preventive effect of
Qianggan-Rongxian Decoction on rat liver fibrosis. World J Gastroenterol 2008; 14(22): 3569-3573 - URL: https://www.wjgnet.com/1007-9327/full/v14/i22/3569.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3569
In China, the incidence of liver cirrhosis is still high[1]. Hepatic cirrhosis results from fibrosis[2–4]. Many factors can lead to chronic liver disease and hepatic fibrosis[5–9]. Hepatic fibrosis is associated with a number of morphological and biochemical changes leading to structural and metabolic abnormalities in the liver. Hepatic stellate cells (HSC) play a major role in various types of liver fibrosis through initial myofibroblast transformation. Although new therapeutic approaches have recently been proposed, there is no established therapy for liver fibrosis[1011]. Qianggan-Rongxian Decoction is a traditional Chinese medicine. The aim of the present study was to investigate its protective effects on rat liver fibrosis induced by dimethylnitrosamine (DMN).
The compositions of Qianggan-Rongxian Decoction mainly include 13 Chinese herbs, including 15 g Pig Bile powder, 10 g Bupleuri, 10 g Baical Skullcap Root, 10 g Pinellia Tuber, 10 g Chinese Angelica, 10 g Barbary Wolfberry Fruit, 10 g Nutgrass Galingale, 10 g Oriental Waterplantain Rhizome, 3 g Pangolin Scale, 153 g Danshen Root, 10 g White Peony Alba, 10 g Radix Glycyrrhizae ,15 g Tangshen.
Male Wistar rats weighing 175-200 g were obtained from the Experimental Animal Center of Chengde Medical College. The rats were randomly divided into control group, model group, and 3 treatment groups (12 rats in each group). Except for the normal control group, all the rats were abdominally injected with 1% DMN (10 &mgr;L/kg body weight, i.p.), 3 times a week for 4 wk, while the rats in the control group were abdominally injected with an equivalent amount of saline. The rats in the 3 treatment groups, including a high-dose DMN group (10 mL/kg), a medium-dose DMN group (7 mL/kg), and a low-dose DMN group (4 mL/kg), were given Qianggan-Rongxian Decoction daily via a gastric tube, once a day for 4 wk. After 4 wk, except for the dead, all the rats were anesthetized with 200 g/L urethane (5 mL/kg, abdominal injection). Blood was taken from the abdominal aorta, centrifuged at 4°C, and plasma was kept at -20°C for assay.
Quantitative enzyme-linked immunoabsorbent assay (ELISA) was used to determine serum levels of hyaluronic acid (HA), type IV collagen, and laminin (LN).
Plasma levels of alanine aminotransferase (ALT) and aspartate aminotrans-ferase (AST) were measured using routine laboratory methods.
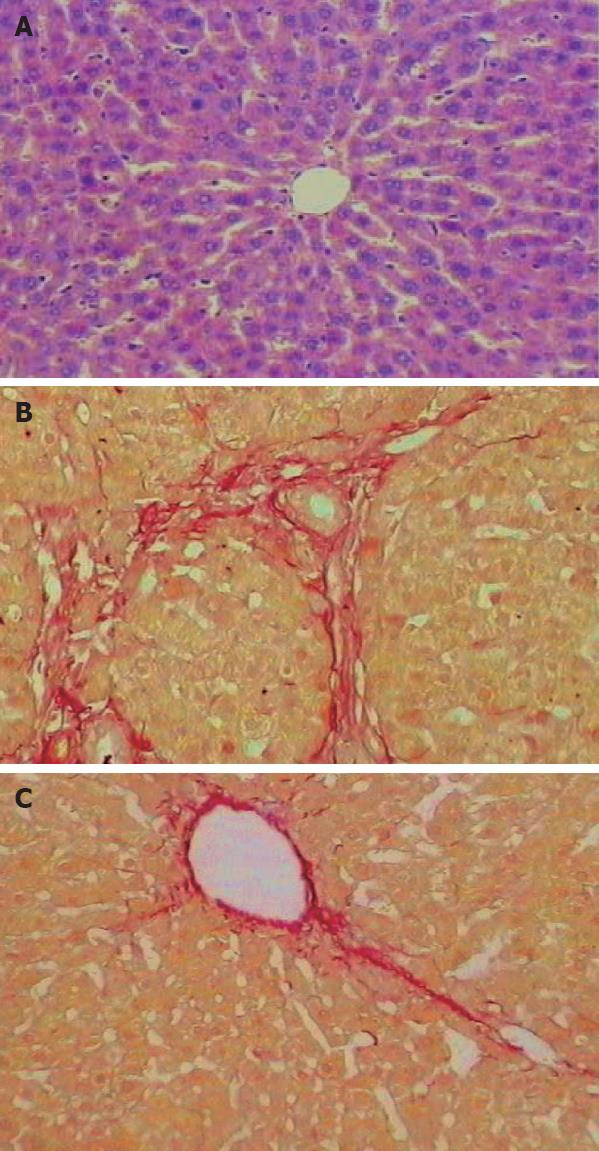
Formalin-fixed and paraffin-embedded liver tissues were cut into 4-&mgr;m thick sections which were stained with hematoxylin and eosin (HE) and Siriusred. HE staining was used to observe liver pathologic structures, Siriusred staining and CMIAS image analysis system (Beihang, China) were used to determine the area-density percentage of collagen fibrosis in hepatic tissue. At least five high-power (× 400) fields were chosen and positive collagen fibrosis (red staining) was determined. The area-density percentage of collagen fibrosis was calculated by dividing the number of positive collagen fibroses (positive optical density) over the total number of collagen fibroses (integrated optical density).
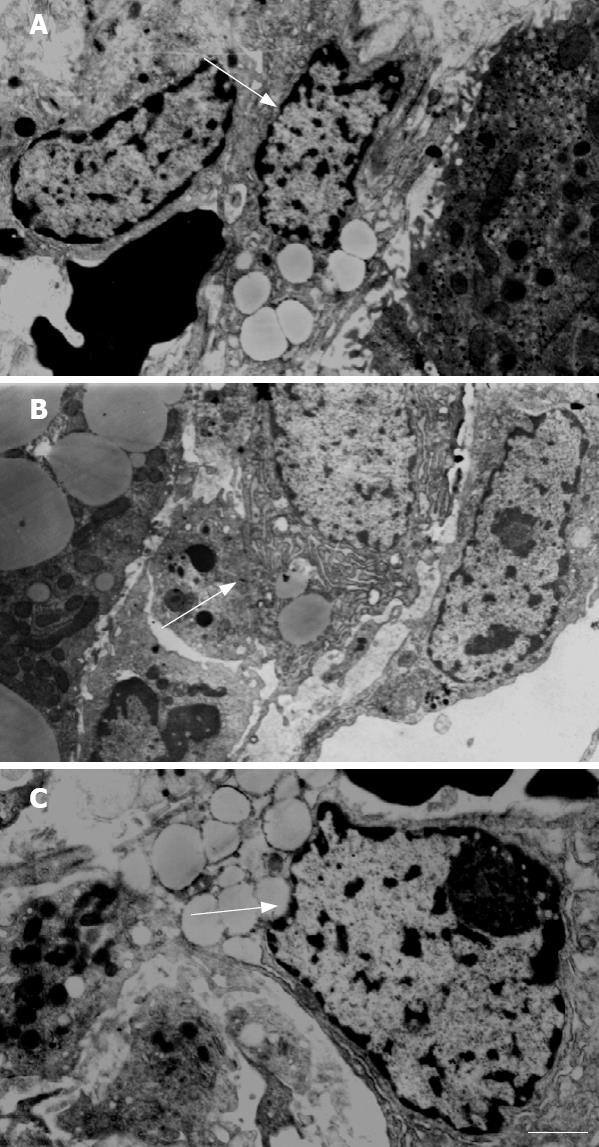
Fresh liver tissue sections (1 mm × 1 mm × 1 mm) were fixed in 10% paraform fixative, dehydrated and embedded in Epon-812 resin, and then stained with uranyl acetate and lead citrate for 15 min, respectively. Liver “transitional” HSC were observed under JEM-1200EX, 80 kV electron microscope (JEOL, Japan).
Results were expressed as mean ± SD. Quantitative data were analyzed using ANOVA with statistical software SPSS 11.0. P < 0.05 was considered statistically significant. Ridit test was used for statistical analysis of the qualita-tive data.
The serum levels of HA, LN, and type IV collagen were markedly increased in the model group compared with the control group (P < 0.05). Compared with the model group, the serum levels of HA, LN, and type IV collagen were significantly decreased in the 3 treatment groups (P < 0.05) (Table 1).
| Groups | n | HA (ng/mL) | LN (ng/mL) | Type IV collagen (ng/mL) |
| Control | 12 | 19.81 ± 2.86 | 11.02 ± 1.70 | 13.49 ± 2.49 |
| Model | 10 | 44.64 ± 3.09c | 33.27 ± 5.81c | 62.71 ± 19.16c |
| High-dose DMN group | 10 | 23.14 ± 4.58ac | 14.02 ± 2.63ac | 22.10 ± 2.44ac |
| Medium-dose DMN group | 12 | 22.58 ± 3.60a | 13.87 ± 1.45a | 25.64 ± 4.68ac |
| Low-dose DMN group | 10 | 26.08 ± 5.62ac | 19.12 ± 5.02ac | 27.64 ± 4.68ac |
At the end of the study, the liver of control rats had no appreciable alterations in the model group (Figure 1A), more fibrous tissues formed and extended into the hepatic lobules to separate them incompletely and thick intralobular septa were evident (Figure 1B). While in the 3 treatment groups, especially in the medium-dose DMN group, the pathological changes in liver were rather milder, showing less fibrous tissue proliferation (Figure 1C). The rat liver was stained with Siriusred. The area occupied by the fibrotic septa was markedly increased in the model group compared with the control group (P < 0.05). Compared with the model group, the area occupied by the fibrotic septa was significantly decreased in the 3 treatment groups (P < 0.05) (Table 2).
Plasma levels of ALT and AST were higher in the model group than in the control group (P < 0.05), while ALT and AST levels were significantly lower in the Qianggan-Rongxian Decoction treatment groups than in the model group. No difference was found in the serum levels of ALT and AST between the medium-dose DMN group and normal group (Table 3).
HSC were normal in the control group and typical myofibroblasts were observed in the fibrous septum of the model group (Figure 2A). The elongated cell body contained indented nuclei and numerous microfilaments outlined by a lamina-like structure. Collagen fibers of variable size were seen all around the myofibroblasts (Figure 2B). “Transitional” HSC could be observed under the electron microscope (Figure 2C).
Hepatic fibrosis at the intermediate and crucial stage is characterized by reversibility. If treated properly at this stage, cirrhosis could be successfully prevented. However, it remains a problem to prevent cirrhosis or to control its progression. Great efforts have been made to find safe and effective drugs. Recent clinical and experimental observations demonstrated that Chinese medicines have some preventive and therapeutic values against fibrosis[12–14]. Astragalus, one of the compositions of Qianggan-Rongxian Decoction, can relieve stasis by activating blood circulation, and eliminate fullness by strengthening the “spleen”, supplementing and smoothening “Qi”, reinforcing the body’s immunological function. It also could preserve the integrity of hepatocytes, eliminate toxic free radicals, inhibit lipid peroxidation of cytomembrane, relieve necrosis of hepatocytes, and reduce fibrosis[15–19]. Thoroughfare is mainly used to activate blood circulation, remove stasis, and dredge the liver[20]. Qianggan-Rongxian Decoction has been used for 20 years to prevent liver fibrosis in clinical practice. However, its effect and associated mechanisms need further study. DMN-induced experimental model may be helpful in understanding the relationship between liver injury and development of hepatic fibrosis[21]. As estimated by histological analysis of liver tissue stained with Sirius red[22], it can be used to detect different degrees of hepatic fibrosis, and examination of the liver can reveal a progressive increase in fibrosis scores and expansion of fibrous septa[23]. Serum HA, LN and collagen type IV levels were significantly increased in the rats as detected by ELISA, showing that simultaneous determination of HA, LN and collagen type IV levels is an optimal choice[24]. We treated the rat liver fibrosis induced by injection of DMN with Qianggan-Rongxian Decoction. After 4 wk, no appreciable alterations were found in the control group. However, the rats in the model group had an almost integrity fibrosis septum, and pseudolobules could be seen in nearly all sections. While the rats that received Qianggan-Rongxian Decoction had less fibrosis, reticular fibrosis in the interlobular septum was limited and no pseudo-lobules could be seen. HSC play a central role in the pathogenesis of liver fibrosis and are able to regulate matrix degradation in the liver. Following liver injury, HSC become activated and express extra-cellular matrix. Qianggan-Rongxian Decoction can inhibit transition from HSC to myofibroblasts and fibroblasts. In addition, Qianggan-Rongxian Decoction could decrease the area-density percentage of collagen fibrosis. HA, LN, and type IV collagen are good serum markers of hepatic fibrosis. In this study, the serum levels of these 3 markers in the model group were much higher than those in the control group (P < 0.05). The serum levels of HA, LN and type IV collagen were significantly lower in the Qianggan-Rongxian Decoction treatment groups than in the control group. ALT and AST are the indexes of liver functions. Since ALT in cytoplasm of liver cells is discharged into blood when degeneration, hyper permeability and necrosis of liver cells occur, increased serum ALT levels reflect the degree of liver cell injury. Our study showed that Qianggan-Rongxian Decoction could decrease the serum levels of ALT and AST in rats with hepatic injury induced by DMN, indicating that Qianggan-Rongxian Decoction may work by protecting liver against fibrosis. The mechanism underlying rat liver fibrosis induced by DMN is associated with immune function, which is similar to the mechanism underlying human liver fibrosis[25]. Thus, DMN-induced rat liver fibrosis may be a useful model for determination of liver fibrosis during drug screening. The mechanism of Qianggan-Rongxian Decoction may need further study.
In summary, Qianggan-Rongxian Decoction may play a role in anti-fibrotic therapy by protecting liver cells and inhibiting the deposition of collagen fibers in liver, thus providing a safe and effective strategy for inhibition of cirrhosis in clinic practice.
In China, the incidence of liver cirrhosis is still high, liver fibrosis and cirrhosis are due to chronic liver injury. Although new therapeutic approaches have recently been proposed, there is no established therapy for liver fibrosis. The authors investigated the preventive effect of Qianggan-Rongxian Decoction on rat liver fibrosis induced by dimethylnitrosamine (DMN).
Qianggan-Rongxian Decoction can protect the liver against fibrosis induced by DMN in rats.
Enzyme-linked immunosorbent assay (ELISA) and hematoxylin and eosin as well as Sirius red staining and transmission electron microscopy demonstrated that Qianggan-Rongxian Decoction could prevent liver against fibrosis.
ELISA can determine the changes in serum levels of hyaluronic acid (HA), laminin (LA), and type IV collagen. Pathologic changes, particularly fibrosis can be examined by light microscopy. Hepatic stellate cells (HSC) can be examined by transmission electron microscopy
Qianggan-Rongxian Decoction is a kind of Chinese medicine, which may protect the liver against fibrosis.
This is an interesting article. Qianggan-Rongxian Decoction can protect the liver against fibrosis and inhibit the deposition of collagen fibers in liver, thus providing a safe and effective strategy for inhibition of cirrhosis in clinical practice. The paper is well organized and the results are clearly described and commented.
| 1. | Du WD, Zhang YE, Zhai WR, Zhou XM. Dynamic changes of type I, III and IV collagen synthesis and distribution of collagen-producing cells in carbon tetrachloride-induced rat liver fibrosis. World J Gastroenterol. 1999;5:397-403. |
| 2. | Canturk NZ, Canturk Z, Ozden M, Dalcik H, Yardimoglu M, Tulubas F. Protective effect of IGF-1 on experimental liver cirrhosis-induced common bile duct ligation. Hepatogastroenterology. 2003;50:2061-2066. |
| 3. | López-Lirola A, González-Reimers E, Martín Olivera R, Santolaria-Fernández F, Galindo-Martín L, Abreu-González P, González-Hernández T, Valladares-Parrilla F. Protein deficiency and muscle damage in carbon tetrachloride induced liver cirrhosis. Food Chem Toxicol. 2003;41:1789-1797. |
| 4. | Pan NS, Li ST, Wang Y, Li MF, Han Z. Therapeutic effect of "anti-hepatic-fibrosis 268" on hepatic fibrosis in rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35:528-531. |
| 5. | Floreani A, Guido M, Bortolami M, Della Zentil G, Venturi C, Pennelli N, Naccarato R. Relationship between apoptosis, tumour necrosis factor, and cell proliferation in chronic cholestasis. Dig Liver Dis. 2001;33:570-575. |
| 6. | Jia JD. [Further systematize and standardize the diagnosis and treatment of liver cirrhosis]. Zhonghua Ganzangbing Zazhi. 2005;13:401-402. |
| 7. | Zalatnai A. Molecular aspects of stromal-parenchymal interactions in malignant neoplasms. Curr Mol Med. 2006;6:685-693. |
| 8. | Breitkopf K, Sawitza I, Gressner AM. Characterization of intracellular pathways leading to coinduction of thrombospondin-1 and TGF-beta1 expression in rat hepatic stellate cells. Growth Factors. 2005;23:77-85. |
| 9. | Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248-258. |
| 10. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. |
| 11. | Kitamura K, Tada S, Nakamoto N, Toda K, Horikawa H, Kurita S, Tsunematsu S, Kumagai N, Ishii H, Saito H. Rho/Rho kinase is a key enzyme system involved in the angiotensin II signaling pathway of liver fibrosis and steatosis. J Gastroenterol Hepatol. 2007;22:2022-2033. |
| 12. | Liu C, Jiang CM, Liu CH, Liu P, Hu YY. Effect of Fuzhenghuayu decoction on vascular endothelial growth factor secretion in hepatic stellate cells. Hepatobiliary Pancreat Dis Int. 2002;1:207-210. |
| 13. | Liu P, Liu CH, Wang HN, Hu YY, Liu C. Effect of salvianolic acid B on collagen production and mitogen-activated protein kinase activity in rat hepatic stellate cells. Acta Pharmacol Sin. 2002;23:733-738. |
| 14. | Kusunose M, Qiu B, Cui T, Hamada A, Yoshioka S, Ono M, Miyamura M, Kyotani S, Nishioka Y. Effect of Sho-saiko-to extract on hepatic inflammation and fibrosis in dimethylnitrosamine induced liver injury rats. Biol Pharm Bull. 2002;25:1417-1421. |
| 15. | Wang RT, Shan BE, Li QX. [Extracorporeal experimental study on immuno-modulatory activity of Astragalus memhranaceus extract]. Zhongguo Zhongxiyi Jiehe Zazhi. 2002;22:453-456. |
| 16. | Chu DT, Lin JR, Wong W. [The in vitro potentiation of LAK cell cytotoxicity in cancer and aids patients induced by F3--a fractionated extract of Astragalus membranaceus]. Zhonghua Zhongliu Zazhi. 1994;16:167-171. |
| 17. | Zhang YD, Shen JP, Zhu SH, Huang DK, Ding Y, Zhang XL. Effects of astragalus (ASI, SK) on experimental liver injury. Yao Xue Xue Bao. 1992;27:401-406. |
| 18. | Tan YW, Yin YM, Yu XJ. [Influence of Salvia miltiorrhizae and Astragalus membranaceus on hemodynamics and liver fibrosis indexes in liver cirrhotic patients with portal hypertension]. Zhongguo Zhongxiyi Jiehe Zazhi. 2001;21:351-353. |
| 19. | Liu P, Hu YY, Liu C, Xu LM, Liu CH, Sun KW, Hu DC, Yin YK, Zhou XQ, Wan MB. Multicenter clinical study on Fuzhenghuayu capsule against liver fibrosis due to chronic hepatitis B. World J Gastroenterol. 2005;11:2892-2899. |
| 20. | Chen H, Weng L. [Comparison on efficacy in treating liver fibrosis of chronic hepatitis B between Astragalus Polygonum anti-fibrosis decoction and jinshuibao capsule]. Zhongguo Zhongxiyi Jiehe Zazhi. 2000;20:255-257. |
| 21. | Mancini R, Jezequel AM, Benedetti A, Paolucci F, Trozzi L, Orlandi F. Quantitative analysis of proliferating sinusoidal cells in dimethylnitrosamine-induced cirrhosis. An immunohistochemical study. J Hepatol. 1992;15:361-366. |
| 22. | Lee MH, Yoon S, Moon JO. The flavonoid naringenin inhibits dimethylnitrosamine-induced liver damage in rats. Biol Pharm Bull. 2004;27:72-76. |
| 23. | Hsu YC, Chiu YT, Lee CY, Lin YL, Huang YT. Increases in fibrosis-related gene transcripts in livers of dimethylnitrosamine-intoxicated rats. J Biomed Sci. 2004;11:408-417. |
| 24. | Liang XH, Zheng H. [Value of simultaneous determination of serum hyaluronic acid, collagen type IV and the laminin level in diagnosing liver fibrosis]. Hunan Yike Daxue Xuebao. 2002;27:67-68. |