Published online May 28, 2008. doi: 10.3748/wjg.14.3236
Revised: March 26, 2008
Accepted: April 2, 2008
Published online: May 28, 2008
AIM: To analyze the clinical presentation of venous diethylene glycol (DEG) poisoning in patients with preexisting severe liver disease and factors that correlate with DEG poisoning.
METHODS: Retrospective chart review was performed to analyze the epidemiology, clinical presentation, hepatorenal functions, hemodynamics and pathological characteristics of 64 patients with severe liver disease who received intravenous armillarisin-A, the solvent of which was DEG. Comparative analyses of correlating factors and causes for poisoning were based on the presence or absence of poisoning.
RESULTS: Of the 64 patients who received armillarisin-A, 15 were found to have DEG poisoning. Twelve poisoned patients died. After a mean of 5 d, the poisoned patients displayed acute renal failure. Metabolic acidosis occurred in 13 cases. BUN, Cr, and CO2 values were significantly elevated and exacerbation of digestive tract symptoms and/or symptom was noted in 11 cases. Neurological system impairment was observed in 10 cases after 2 wk. Compared to the 49 non-poisoned patients, the poisoned patients exhibited significantly lower RBC and Hb values and higher WBC count. Renal biopsy from the poisoned patients revealed acute tubular necrosis and interstitial nephritis. Significant differences in preexisting severe hepatitis, ascites, renal disease, and diuretic therapy were found between groups. Prior to diethylene glycol injections, the mean values for neutral granular cells, BUN, Cr, calcium and phosphorous ions differed significantly between groups.
CONCLUSION: Venous diethylene glycol poisoning is characterized by oliguric acute renal failure, metabolic acidosis, digestive symptoms, nervous system impairment, and a high probability of anemia and WBC proliferation. Mortality is high. Correlative factors include preexisting severe liver disease, renal disease, and infection.
- Citation: Lin BL, Zhao ZX, Chong YT, Li JG, Zuo X, Tao Y, Lou TQ, Gao ZL. Venous diethylene glycol poisoning in patients with preexisting severe liver disease in China. World J Gastroenterol 2008; 14(20): 3236-3241
- URL: https://www.wjgnet.com/1007-9327/full/v14/i20/3236.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3236
Diethylene glycol (DEG) is a chemical substance used primarily for industrial purposes. Tested in animals, DEG induces liver impairment and kidney toxicity presenting as acute renal failure (ARF)[12]. In 1937, 358 human cases of ARF resulting in 107 deaths were described following ingestion of sulfanilamide dissolved in DEG in America[3]. Similar reports of DEG poisoning appeared subsequently in the other countries[4–10], with most cases involving pediatric poisoning through oral ingestion and with fundamentally milder complications.
On April 22th 2006 and April 24th 2006, two patients in the Department of Infectious Diseases, Third Affiliated Hospital of Sun Yat-Sen University, with severe liver disease developed ARF. On April 29th 2006 and April 30th 2006, another six patients in this department with severe liver disease also developed ARF. Upon further investigation, armillarisin-A[11], a drug produced by the Qiqihar No. 2 Pharmaceutical Co. Ltd, for treatment of gall-bladder disease, was found to have been administered to all patients who developed ARF. Administration of the drug was immediately suspended. Subsequently, the situation was reported to the relevant pharmacy. All preparations of armillarisin-A were sealed and forwarded to the Guangdong Drug Examination Center for investigation. Findings revealed that DEG was present in these preparations at a concentration of 30%. Subsequent judiciary investigation disclosed that the Qiqihar No. 2 Pharmaceutical Co. Ltd, selected DEG to serve as an economic substitute for trimethylene glycol in armillarisin-A preparations.
Review of 64 patients who received armillarisin-A in the hospital during the relevant time period was therefore undertaken, and findings were described in the present report. Of the 15 patients subsequently died, 12 were diagnosed with DEG poisoning. No other patients with similar complications have been reported since May 2nd 2006. The investigation described in the present report has the following features: (1) all subjects were adults who received armillarisin-A with DEG intravenously; (2) clinical presentation was recorded before and after DEG poisoning, and the exact injection volumes and DEG concentrations in the preparations were recorded; (3) the majority of patients presented with concurrent severe liver disease. In the present report, the clinical presentation of venous diethylene glycol poisoning and the pathological characteristics of renal tissue of poisoned patients were described and factors that correlate with this form of poisoning were identified.
The 64 patients enrolled in the present study were treated with armillarisin-A in the Third Affiliated Hospital of Sun Yat-Sen University in Guangzhou between April 19th 2006 and May 1st 2006. All the patients, including 49 (76.6%) males, were diagnosed with severe liver diseases. Of these 64 patients, 14 had severe hepatitis, 16 had liver cirrhosis caused by hepatitis B virus, 21 had chronic active hepatitis, 6 had primary hepatocellular carcinoma, 2 underwent liver transplantation, 2 had biliary cirrhosis, and 1 had hepatolenticular degeneration and liver impairment due to malignant lymphoma and cholangiocarcinoma.
Based on the published findings long before and the consensus of experts, the Department of Health of Guangdong Province established the criteria for clinical diagnosis of DEG poisoning. The following three criteria are considered essential: (1) a history of DEG prescription (oral/venous injection), (2) acute renal impairment or renal failure characterized by oliguria or anuria occurring within 2 wk of the last ingestion/injection), and (3) elimination of all other causes of acute renal impairment or renal failure.
Diagnosis of viral hepatitis was based on the standardized “viral hepatitis prevention study” performed in 2000 by the Society of Infections Diseases and Society of Liver Diseases of the Chinese Medical Association[12].
Retrospective chart review was applied to all 64 patients who received DEG intravenously. These patients were assigned to either the poisoned group or the non-poisoned group. For each poisoned patient, analyses of epidemiology, clinical symptoms, prognosis, hepatorenal functions, hemodynamics and pathology of renal tissue were performed before and after poisoning for comparison purposes. Analyses were also performed for the poisoned group as compared to the non-poisoned group prior to receipt of DEG to identify factors predisposing to DEG poisoning.
Renal tissues from poisoned patients were examined with several methods. Ten or more renal corpuscles were extracted and subjected to HE and PASH staining followed by microscopic observation. The nature and degree of corpuscular and tubular-interstitial pathologies were evaluated. Immunofluorescence staining of frozen sections was performed to observe the deposition sites and degree of deposition of immune-complex compounds. Electron microscopy was performed to identify the ultrastructural changes in renal tissue.
Liver function and biochemical parameters were detected using an automatic chemistry analyzer. The concentration of DEG in armillarisin-A was determined by spectrophotometry.
Normality distribution was analyzed for the continuous variables. The t-test was performed to detect significant differences between groups with normality. The data are presented as mean ± SD. Group comparison for data without a normal distribution involved evaluation by independent nonparametric testing. Findings were pre-sented as the medians. The Chi-square test was performed to examine numerical data. P < 0.05 was considered statistically significant. SPSS13.0 for windows was used for all statistical analyses.
Sixty-four patients who received intravenous injections of armillarisin-A were observed. On June 30th 2006, DEG poisoning was present in 15 patients and absent in 49 patients. Comparative statistics was performed based on the presence or absence of DEG poisoning, and findings are listed in Table 1. The DEG concentration in the patients ranged from 1.2% to 6%, with a cumulative dosage volume of 2.4-114 mL, but no statistical differences in these values were observed between the poisoned and non-poisoned groups. Liver impairment was more severe in the DEG-poisoned group than in the non-DEG-poisoned group. Of the 15 poisoned patients, 12 had terminal liver disease. Data analyses revealed significant differences between the poisoned and non-poisoned groups with respect to the severity of pre-injection liver conditions, presence of ascites and renal diseases, use of diuretics, pre-injection neutral granular cell count, serum BUN, serum Cr, calcium and phosphate ion (IP) concentrations. Death occurred in 12 patients of the poisoned group and 8 patients of the non-poisoned group. Hepatic failure and multiple organ dysfunction syndromes (MODS) were identified as the main causes of death.
| Item | DEG-poisoned group (n = 15) | Non-DEG-poisoned group (n = 49) | Statistical value | P value |
| Male sex (%) | 14 (93.3) | 35 (71.4) | 3.071 | 0.080 |
| Age (yr) | ||||
| Median | 50 | 48 | 1.11 | 0.267 |
| Range | 33-76 | 5-72 | ||
| DEG intake-ml | ||||
| Median | 24 | 36 | 0.27 | 0.787 |
| Range | 9-72 | 2.4-114 | ||
| DEG concentration (%) | ||||
| Median | 6 | 6 | 0.713 | 0.476 |
| Range | 3-6 | 1.2-6 | ||
| Alcoholics (%) | 7 (46.7) | 19 (38.8) | 0.296 | 0.586 |
| Diagnosis | 11.691 | 0.039 | ||
| TLD (%) | 12 (80.0) | 21 (42.9) | 6.344 | 0.012 |
| CH (%) | 2 (13.3) | 19 (38.8) | 0.1122 | |
| Other (%) | 1 (6.7) | 9 (18.4) | 0.2582 | |
| Diuretics (%) | 12 (80.0) | 16 (32.7) | 0.0202 | |
| Complication | ||||
| Ascites (%) | 10 (66.7) | 9 (18.4) | 0.000 | |
| Renal disease2 (%) | 5 (33.3) | 3 (6.1) | 0.0141 | |
| Serum checking | ||||
| ALT (U/L) | 180.9 ± 269.9 | 201.4 ± 284.3 | 0.251 | 0.804 |
| TB (&mgr;mol/L) | 359.2 ± 245 | 239.3 ± 221.5 | 1.767 | 0.082 |
| BUN (mmol/L) | 7.9 ± 3.8 | 4.3 ± 2.9 | 3.372 | 0.003 |
| Creatinine (&mgr;mol/L) | 94.2 ± 24.6 | 58.7 ± 22.6 | 5.141 | 0.000 |
| Ca2+ (mmol/L) | 2.37 ± 0.17 | 2.25 ± 0.21 | 2.157 | 0.035 |
| Phosphonium (mmol/L) | 0.72 ± 0.43 | 1.00 ± 0.33 | 2.574 | 0.013 |
| WBC (109/L) | 6.47 ± 2.08 | 6.00 ± 5.34 | 0.326 | 0.746 |
| NEUT | 0.716 ± 0.114 | 0.587 ± 0.153 | 3.003 | 0.004 |
| RBC (1012/L) | 3.03 ± 0.92 | 3.33 ± 0.79 | 1.208 | 0.232 |
| Hemoglobin (g/L) | 99.9 ± 25.6 | 106.6 ± 18.8 | 1.035 | 0.305 |
| Platelet count (109/L) | 106.9 ± 50.6 | 125.9 ± 73.3 | 0.293 | 0.354 |
Oliguric ARF was present for a mean of 5 d in 15 patients with intravenous DEG poisoning. The clinical characteristics of these 15 patients are presented in Table 2. The urine volume decreased rapidly. The majority of poisoned patients developed digestive tract symptoms, such as nausea, vomiting and bloating, or exhibited an increase in the severity of these symptoms. Half of the patients exhibited concomitant mild pyrexia. Ten patients displayed nervous system impairment involving the cranial nerves, including the facial, optic, oculomotor and glossopharyngeal nerves, at an average of 14 d after the initial injection. A few patients exhibited peripheral nerve involvement presenting as limb tremor and paralysis. Respiratory muscle paralysis might have been present in some patients, leading inevitably to respiratory failure. DEG poisoning was also associated with an increase in the severity of hepatic encephalopathy among patients previously exhibiting this complication. Retrospective analyses of 13 patients before and after DEG poisoning revealed that all patients experienced metabolic acidosis at an average of 9 d after injection and 4 d following development of ARF. The most severe manifestations of metabolic acidosis occurred on d 10 after initial ingestion of DEG. Twelve of the 15 patients diagnosed with DEG poisoning died. Death generally occurred 1 wk following the initial signs of renal failure. Among the 3 patients who survived the poisoning, however, urine volume was observed to recover 3 wk after poisoning and urine volume was normal 1 mo after poisoning. One of the three patients who survived underwent combined liver-kidney transplantation 16 d after exhibiting DEG poisoning.
| Characteristics | Data |
| Age (yr) | 50 (33-76) |
| Male sex (%) | 14 (93.6) |
| Injected DEG volume (mL) | 24 (9-72) |
| ARF (%) | 15 (100) |
| Incubation period of ARF (d) | 5 (2-12) |
| Incubation periods of anuria (d) | 6 (3-13) |
| Fever (%) | 7 (46.7) |
| Incubation periods (d) | 6 (1-13) |
| Dig. tract symptoms (%) | 11 (73.3) |
| Incubation period (d) | 9 (3-19) |
| Nerv. syst. impair (%) | 10 (66.7) |
| Incubation periods (d) | 14 (7-24) |
| Cranial nerves (%) | 10 (64.7) |
| Peripheral nerves (%) | 5 (33.3) |
| Central nerv. syst. (%) | 6 (40.0) |
| Metab. acidosis (n = 13) (%) | 13 (100) |
| Incubation periods of abnormal Cr and/or BUN (d) | 5 (2-12) |
| Time of peak Cr (d) | 11 (6-19) |
| Time of peak BUN (d) | 14 (6-23) |
| Incubation periods of abnormal CO2 (d) | 9 (2-14) |
| Time of peak CO2 (d) | 10 (5-16) |
| Death (%) | 12 (80.0) |
| Death time after injection (d) | 12.5 (8-65) |
| Causes of death (n = 12) | |
| MODS (%) | 7 (58.3) |
| Infection (%) | 4 (33.3) |
| Dig. tract bleed (%) | 1 (8.3) |
When the liver function, renal function and peripheral blood cell counts before DEG poisoning were compared with the peak value after DEG poisoning: (1) the patients’ blood urea nitrogen (BUN), creatinine (Cr), and phosphate (P) concentrations increased significantly after DEG poisoning, while serum CO2 concentration dropped significantly, but serum calcium had no remarkable change; (2) DEG did not cause aggravation of liver function, while serum total bilirubin level, GGT, ALP and prothrombin time did not change significantly; (3) the peripheral blood cell counts increased significantly after DEG poisoning, while the red blood cell counts and hemoglobin value dropped significantly, but platelet counts did not change obviously (Table 3).
| Item | n | BP | AP | t-value | P | CI |
| TB (&mgr;mol/L) | 15 | 376.7 ± 244.6 | 354.7 ± 257.1 | 0.945 | 0.362 | -28.36-72.44 |
| PT (s) | 15 | 24.4 ± 13.1 | 22.4 ± 8.8 | 1.33 | 0.210 | -1.34-5.41 |
| GGT (U/L) | 14 | 163.2 ± 225.5 | 109.4 ± 115.8 | 1.451 | 0.170 | -26.3-133.8 |
| ALP (U/L) | 14 | 217.0 ± 265.4 | 146.7 ± 148.8 | 1.888 | 0.082 | 10.2-150.7 |
| BUN (mmol/L) | 15 | 7.4 ± 3.9 | 31.2 ± 9.68 | 8.373 | 0.000 | 17.61-30.00 |
| Cr (&mgr;mol/L) | 15 | 94.2 ± 24.1 | 691.6 ± 197.8 | 10.659 | 0.000 | 475.28-719.51 |
| CO2 (mmol/L) | 13 | 24.4 ± 3.9 | 13.1 ± 2.6 | 11.75 | 0.000 | 9.20-13.39 |
| Ca2+ (mmol/L) | 14 | 2.38 ± 0.18 | 2.41 ± 0.22 | -0.737 | 0.474 | -0.13-0.07 |
| Phosphonium (mmol/L) | 14 | 0.73 ± 0.45 | 1.31 ± 0.50 | -4.088 | 0.001 | -0.90-(-0.28) |
| WBC (109/L) | 15 | 6.59 ± 2.33 | 9.78 ± 3.75 | 3.325 | 0.008 | 1.05-5.33 |
| RBC (1012/L) | 15 | 2.99 ± 0.94 | 2.32 ± 0.76 | 2.968 | 0.014 | 0.16-1.17 |
| Hb (g/L) | 15 | 99.6 ± 25.1 | 79.5 ± 23.6 | 2.823 | 0.018 | 4.25-36.11 |
| PLT (109/L) | 15 | 119.6 ± 50.1 | 94.6 ± 72.6 | 1.336 | 0.211 | -16.75-66.93 |
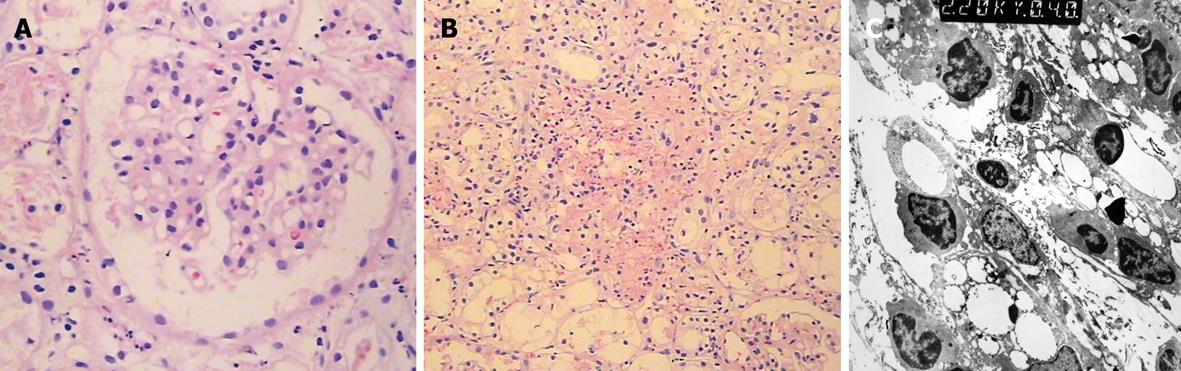
Renal tissues were taken from two patients with DEG poisoning on the third and forth days after ARF, respectively. Biopsies of renal tissue indicated significant tubular pathological changes, partial dissolution and necrosis of epithelial cells, and interstitial inflammatory cell infiltration (Figure 1). No pathological changes in the glomerular basement membrane of these patients were observed.
In 1937, the Massengill Company (USA) developed an “elixir of sulfanilamide”, a preparation of 9-10 g of sulfanilamide dissolved in 100 mL of DEG. Other cases of DEG poisoning have been largely associated with foul play or deliberate consumption of alcoholic mixtures containing DEG[3–1013]. In this study, injection of armillarisin-A produced by the Qiqihar No. 2 Pharmaceutical Co. Ltd. resulted in events similar to those described previously in response to DEG poisoning. Sixty-four patients with severe liver disease received venous armillarisin-A injections containing high concentrations of DEG (325.9 mg/mL and 30% concentration as reported by the Heilongjiang Province Drug Inspection Center and the Guangdong Province Drug Inspection Center, respectively). Fifteen patients were diagnosed with DEG poisoning. The rate of poisoning was 23.4%.
Liver impairment was more severe in the DEG-poisoned group than in the non-DEG-poisoned group. Metabolism of DEG involves the actions of alcohol dehydrogenase and aldehyde dehydrogenase[1]. Alcohol dehydrogenase ordinarily converts DEG to an aldehyde and aldehyde dehydrogenase ordinarily converts this aldehyde to certain acids. As alcohol dehydrogenase and aldehyde dehydrogenase are mainly restricted to the liver, loss of these enzymes as a consequence of severe liver disease may significantly impair DEG metabolism. Furthermore, secondary infection is a common complication in patients with terminal liver disease. Peritonitis caused by Gram-negative bacilli and hepatobiliary infection are the most prevalent complications. Data also indicate that the pre-injection rate of neutral granular cells in the poisoned group was significantly higher than that in the non-poisoned group. Infection-induced endotoxemia increases alcohol dehydrogenase activity[14], and accumulation of the aldehyde intermediate can provoke DEG poisoning. Concurrently, serious liver diseases often produce massive ascites requiring diuretic therapy. Resultant renal hemodynamic changes occurring in response to such therapy may inevitably lead to exacerbation of renal damage. Therefore, lower dosages and concentrations of DEG can provoke poisoning via the intravenous route in patients with severe liver disease as compared to the oral route in patients without severe liver disease.
In the present study, the poisoned patients had a significantly higher incidence of renal disease and significantly higher serum BUN and Cr concentrations than the non-poisoned patients, suggesting that patients with renal disease are more susceptible to DEG poisoning than those without renal disease.
Currently available information about DEG indicates that this glycol induces acute poisoning, but no chronic poisoning. This apparent discrepancy can be explained by the short half-life of DEG (approximately 3 h)[15]. DEG poisoning was previously considered similar to ethylene glycol poisoning, which is associated with renal impairment attributable to renal accumulation of calcium ions and to the final product, oxalic acid, with resultant accumulation of calcium oxalates. Recent findings show that the final product of DEG metabolism is a 2-hydroxy-ethoxyacetic acid rather than an oxalic acid. DEG-induced pathological changes and necrosis of tubular epithelial cells are attributable to a metabolic intermediate that poisons tubular epithelial cells rather than to deposition of calcium oxalates[16–19]. Renal impairment is observed at early stages of poisoning and is prominent in all cases of poisoning, as was observed in the present study.
The clinical characteristics of patients poisoned by intravenous DEG were similar to those of patients poisoned following oral ingestion of DEG in the present study. It was reported that renal impairment occurs at early times following ingestion, with metabolic acidosis and delayed neurological impairment mainly involving the cranial and peripheral nerves commonly observed[20–24]. Poisoning via the intravenous route differs notably from poisoning via the oral route in that exhibition of mild fever and an increase in severity of digestive tract symptoms before occurrence of renal failure, along with a later occurrence of organ impairment, is specific for intravenous poisoning. This finding may be attributable to the age of patients in the present study and to their preexisting severe liver disease which could have limited the actions of alcohol and aldehyde dehydrogenases. Prospective research is warranted for further clarification. Due to the scarcity of DEG poisoning survivors, it is difficult to evaluate the process of systematic recovery. While previous reports indicate that recovery of the nervous system after oral DEG poisoning requires 4-6 mo[25], the present findings disclose that nervous system recovery occurs 1 mo following intravenous poisoning.
In the present study, 80% mortality was observed in the poisoned patients. Seven died of MODS, 4 died of severe infection, and 1 died of severe digestive tract bleeding. The lethal dose of DEG varies with species[26]. It was reported that DEG at a cumulative dosage of 0.22-4 mL/kg with a concentration of 17.5%-72% in humans can lead to death[67]. The DEG concentration in the present study ranged from 3% to 6%, with a cumulative dosage volume of 9-72 mL, but no statistical differences in these values were observed between the poisoned and non-poisoned groups, indicating that the severity of preexisting liver disease leading to loss of alcohol and aldehyde dehydrogenase activities constitutes a primary important predisposing factor for poisoning.
In the present study, the patients with DEG poisoning had higher serum calcium values and lower serum IP values than the non-poisoned patients, the serum IP concentrations were significantly increased after intravenous DEG poisoning. The importance of calcium and phosphates in DEG poisoning remains to be determined.
Although the DEG-poisoned patients described in the present study presented with concomitant severe liver diseases, no exacerbating degenerative features of general liver function (no changes in bilirubin, aldehyde dehydrogenase, albumin, and hemostatic function) were found. Furthermore, no significant increase in gamma-glutamyl transpeptidase or alkaline phosphatase was noted, indicating that venous injections of DEG do not directly affect the hepatobiliary system and drug-induced liver damage is absent. These observations may be attributable to the fact that, in contrast to oral ingestion of DEG[27], venous injection of this glycol does not participate in liver metabolism.
Additional analyses indicated that DEG-poisoned patients might present with anemia characterized by decreased red blood cells and hemoglobin. A similar form of anemia was observed in ethylene glycol poisoning. The DEG-poisoned patients described in the present study also presented with an increase in white blood cell count, with a significant increase in neutral granular cells but no remarkable changes in eosinophils. This phenomenon can be attributed to an increase in the severity of infection in patients with severe liver disease and is indicative of acute renal failure as a result of DEG poisoning rather than allergen induction.
Renal biopsy findings revealed that DEG induced tubular epithelial cell dissolution and necrosis and renal interstitial inflammatory cell infiltration, but no pathological changes in the glomerulus. These alterations differ from those associated with the hepatorenal complications and glomerulonephritis induced by severe liver disease and are therefore important in differential diagnosis.
In conclusion, venous diethylene glycol poisoning is characterized by oliguric acute renal failure, metabolic acidosis, digestive symptoms, nervous system impairment, and a high probability of anemia and WBC proliferation. Mortality is high, and correlative factors include preexist-ing severe liver disease, renal disease, and infection.
Diethylene glycol (DEG) is a chemical substance used primarily for industrial purposes. DEG induces kidney toxicity presenting as acute renal failure (ARF) and has been used as a chemical substance for industrial purposes in many countries since 1937. In 2006, 64 patients with severe liver disease received venous armillarisin-A injections containing high concentrations of DEG, and 15 were diagnosed with DEG poisoning in Guangzhou, China. In the present report, the clinical presentation of venous diethylene glycol poisoning and the pathological characteristics of renal tissue from poisoned patients were described and factors that correlate with this form of poisoning were identified.
All the clinical researches about DEG poisoning based on events of herbal toxicity, have been limited in oral DEG intake and normal persons.
The investigation described in the present report was characterized by the following features: (1) all subjects were adults who received armillarisin-A with DEG intravenously, (2) clinical presentation was recorded before and after DEG poisoning and the exact injection volumes and DEG concentrations in the preparations were recorded, (3) the majority of patients presented with concurrent severe liver diseases.
This work may help to know the clinical presentation of venous diethylene glycol (DEG) poisoning in patients with preexisting severe liver disease and factors that correlate with this form of poisoning.
This is a nice report on an outbreak of IV DEG poisoning. Authors analyzed the features of venous DEG poisoning and serious consequences to remind government of paying attentions to drug safety and supervising.
| 1. | Mathews JM, Parker MK, Matthews HB. Metabolism and disposition of diethylene glycol in rat and dog. Drug Metab Dispos. 1991;19:1066-1070. |
| 2. | Kraul H, Jahn F, Braunlich H. Nephrotoxic effects of diethylene glycol (DEG) in rats. Exp Pathol. 1991;42:27-32. |
| 3. | Geiling EMK, Cannon PR. Pathologic effects of elixir of sulfanilamide (diethylene glycol) poisoning. JAMA. 1938;111:919-926. |
| 4. | Okuonghae HO, Ighogboja IS, Lawson JO, Nwana EJ. Diethylene glycol poisoning in Nigerian children. Ann Trop Paediatr. 1992;12:235-238. |
| 5. | Hanif M, Mobarak MR, Ronan A, Rahman D, Donovan JJ Jr, Bennish ML. Fatal renal failure caused by diethylene glycol in paracetamol elixir: the Bangladesh epidemic. BMJ. 1995;311:88-91. |
| 6. | Ferrari LA, Giannuzzi L. Clinical parameters, postmortem analysis and estimation of lethal dose in victims of a massive intoxication with diethylene glycol. Forensic Sci Int. 2005;153:45-51. |
| 7. | Fatalities associated with ingestion of diethylene glycol-contaminated glycerin used to manufacture acetaminophen syrup--Haiti, November 1995-June 1996. MMWR Morb Mortal Wkly Rep. 1996;45:649-650. |
| 8. | O’Brien KL, Selanikio JD, Hecdivert C, Placide MF, Louis M, Barr DB, Barr JR, Hospedales CJ, Lewis MJ, Schwartz B. Epidemic of pediatric deaths from acute renal failure caused by diethylene glycol poisoning. Acute Renal Failure Investigation Team. JAMA. 1998;279:1175-1180. |
| 9. | Singh J, Dutta AK, Khare S, Dubey NK, Harit AK, Jain NK, Wadhwa TC, Gupta SR, Dhariwal AC, Jain DC. Diethylene glycol poisoning in Gurgaon, India, 1998. Bull World Health Organ. 2001;79:88-95. |
| 10. | Bowie MD, McKenzie D. Diethylene glycol poisoning in children. S Afr Med J. 1972;46:931-934. |
| 11. | Sun F, Su JD, Zheng H. Studies on the pharmacological activities and toxicities of armillarisin-A, a new choleretic drug. Yaoxue Xuebao. 1981;16:401-406. |
| 12. | Society of Infectious Diseases and Society of Liver Diseases of the Chinese Medical Association. Viral hepatitis prevention and treatment methods. Zhonghua Ganzangbing Zazhi. 2000;8:324-329. |
| 13. | Junod SW. Diethylene glycol deaths in Haiti. Public Health Rep. 2000;115:78-86. |
| 14. | Potter JJ, Rennie-Tankersley L, Mezey E. Endotoxin enhances liver alcohol dehydrogenase by action through upstream stimulatory factor but not by nuclear factor-kappa B. J Biol Chem. 2003;278:4353-4357. |
| 15. | Lenk W, Lohr D, Sonnenbichler J. Pharmacokinetics and biotransformation of diethylene glycol and ethylene glycol in the rat. Xenobiotica. 1989;19:961-979. |
| 16. | Winek CL, Shingleton DP, Shanor SP. Ethylene and diethylene glycol toxicity. Clin Toxicol. 1978;13:297-324. |
| 17. | Deisinger PJ, Guest D. Metabolic studies with diethylene glycol monobutyl ether acetate (DGBA) in the rat. Xenobiotica. 1989;19:981-989. |
| 18. | Heilmair R, Lenk W, Lohr D. Toxicokinetics of diethylene glycol (DEG) in the rat. Arch Toxicol. 1993;67:655-666. |
| 19. | Johnson KA, Baker PC, Kan HL, Maurissen JP, Spencer PJ, Marty MS. Diethylene glycol monobutyl ether (DGBE): two- and thirteen-week oral toxicity studies in Fischer 344 rats. Food Chem Toxicol. 2005;43:467-481. |
| 20. | Hebert JL, Auzepy P, Durand A. [Acute human and experimental poisoning with diethylene glycol]. Sem Hop. 1983;59:344-349. |
| 22. | Rollins YD, Filley CM, McNutt JT, Chahal S, Kleinschmidt-DeMasters BK. Fulminant ascending paralysis as a delayed sequela of diethylene glycol (Sterno) ingestion. Neurology. 2002;59:1460-1463. |
| 23. | Alfred S, Coleman P, Harris D, Wigmore T, Stachowski E, Graudins A. Delayed neurologic sequelae resulting from epidemic diethylene glycol poisoning. Clin Toxicol (Phila). 2005;43:155-159. |
| 24. | Hari P, Jain Y, Kabra SK. Fatal encephalopathy and renal failure caused by diethylene glycol poisoning. J Trop Pediatr. 2006;52:442-444. |
| 25. | Hasbani MJ, Sansing LH, Perrone J, Asbury AK, Bird SJ. Encephalopathy and peripheral neuropathy following diethylene glycol ingestion. Neurology. 2005;64:1273-1275. |
| 26. | Schwetz BA, Price CJ, George JD, Kimmel CA, Morrissey RE, Marr MC. The developmental toxicity of diethylene and triethylene glycol dimethyl ethers in rabbits. Fundam Appl Toxicol. 1992;19:238-245. |
| 27. | Kawamoto T, Matsuno K, Kayama F, Arashidani K, Yoshikawa M, Kodama Y. The effect of ethylene glycol monomethyl ether and diethylene glycol monomethyl ether on hepatic gamma-glutamyl transpeptidase. Toxicology. 1992;76:49-57. |