Published online May 21, 2008. doi: 10.3748/wjg.14.3101
Revised: April 10, 2008
Published online: May 21, 2008
Primary stomach lymphoblastic B-cell lymphoma (B-LBL) is a rare tumor. We describe a primary stomach B-LBL in a 38 years old female who presented with nonspecific complaints of fatigue and vomiting for 2 mo. Gastrofiberscopy revealed a large gastric ulcer, which was successfully resected. Pathology showed a lymphoblastic cell lymphoma arising from the stomach, and there was no evidence of disease at any extrastomach site. Immunohistochemical staining and gene rearrangement studies supported that the stomach tumor was a clonal B-cell lymphoma. Therefore, the diagnosis of B-LBL was made based on the stomach specimen.
- Citation: He MX, Zhu MH, Liu WQ, Wu LL, Zhu XZ. Primary lymphoblastic B-cell lymphoma of the stomach: A case report. World J Gastroenterol 2008; 14(19): 3101-3104
- URL: https://www.wjgnet.com/1007-9327/full/v14/i19/3101.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3101
Precursor B-cell lymphoblastic lymphoma (B-LBL) is a neoplasm of lymphoblasts committed to the B-cell lineage, which is an uncommon type of lymphoma and accounts for less than 10% of the total cases of lymphoblastic lymphoma. It usually affects people at a younger age. Most cases reported in a literature review are less than 18 years of age, some patients are under 35 years of age and the median age is 20 years[1]. Unlike precursor T-cell lymphoblastic lymphoma, precursor B-cell lymphoblastic lymphoma commonly involves lymph nodes or extranodal sites, mediastinal masses are infrequent. The most frequent sites of B-LBL lesions are the skin, bone, soft tissue, and lymph nodes[1–3].
Primary stomach lymphoma is defined as an extranodal non-Hodgkin’s lymphoma of any cell type, with no evidence of extrastomach dissemination. The majority of stomach cases reported in the English literature are extranodal marginal zone B cell lymphoma of mucosa-associated lymphoid tissue (MALToma), diffuse large B cell lymphoma (DLBCL), nasal type NK/T cell lymphoma, etc[3–5]. Primary stomach B-LBL is rare. Here we present a case of primary B-LBL involving the stomach.
The patient was a 38 years old female who had an unremarkable past medical history. She presented with nonspecific complaints of fatigue and vomiting for 2 mo. Gastrofiberscopy revealed a large gastric ulcer. During surgery, a large neoplastic ulcer was found in the stomach and gastric wall was diffusely thickened. The tumor was successfully resected with adjacent portions of the stomach. Good macroscopic margins were obtained. No other masses or enlarged lymph nodes were present. Examination of the bone marrow showed 13% immature lymphoid cells, the leukocyte count of peripheral blood was 12 × 109/L, and the percentage of peripheral blood lymphocytes was 35%.
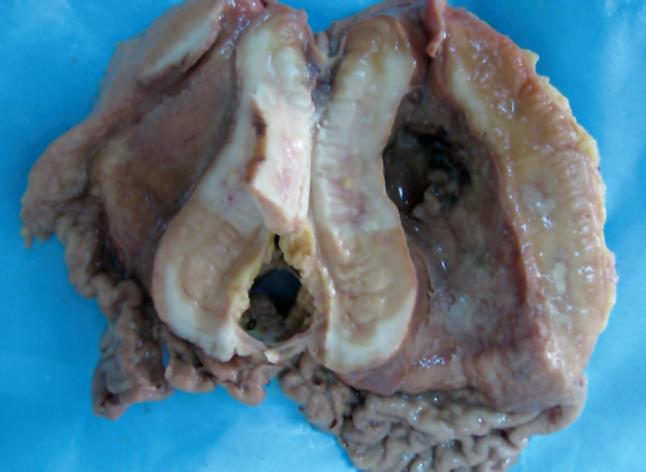
Grossly, the greater and lesser curvatures of the resected stomach measured 22 cm and 11 cm, respectively. There was a well circumscribed neoplastic ulcer measuring 10 cm × 8 cm × 2.5 cm in antro-pyloric region of the stomach (Figure 1). A small necrosis was found on surface of the neoplastic ulcer. The cut surface of the tumor was grey and firm. Tumor tissue was found in the gastric serosa. Portions of tumor tissue were fixed in formalin and embedded in paraffin and cut into sections which were stained with HE for routine histomorphology. Additional sections of paraffin-embedded tissue were used for immunohistochemical staining and gene rearrangement analysis.
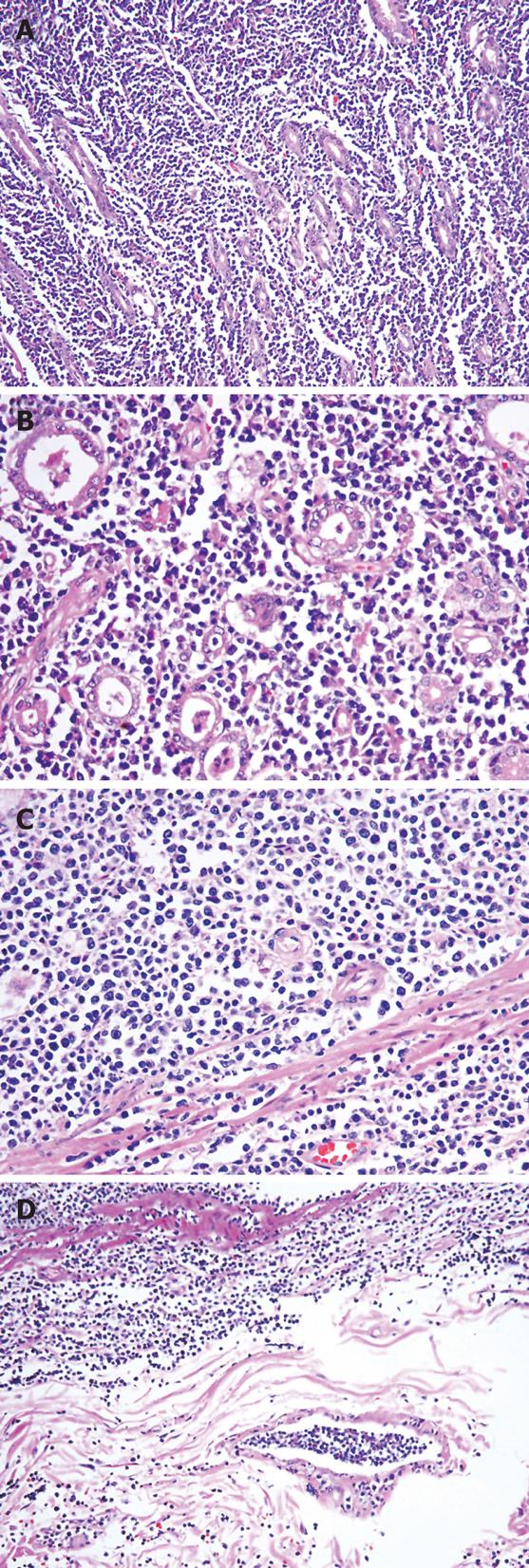
Microscopically, the tumor cells were uniform, medium-sized immature lymphoid cells, their nuclei contained evenly dispersed nuclear chromatin with a high nuclear/cytoplasmic ratio. The nuclei were round or oval or convoluted in shape. A large number of mitotic figures were appreciated. The tumor cells were diffusely distributed in the gastric glands, and found in all layers of the gastric wall. There were tumor emboli within the gastric wall lymphatic vessels. Lymphoepithlial lesions were not found (Figure 2).
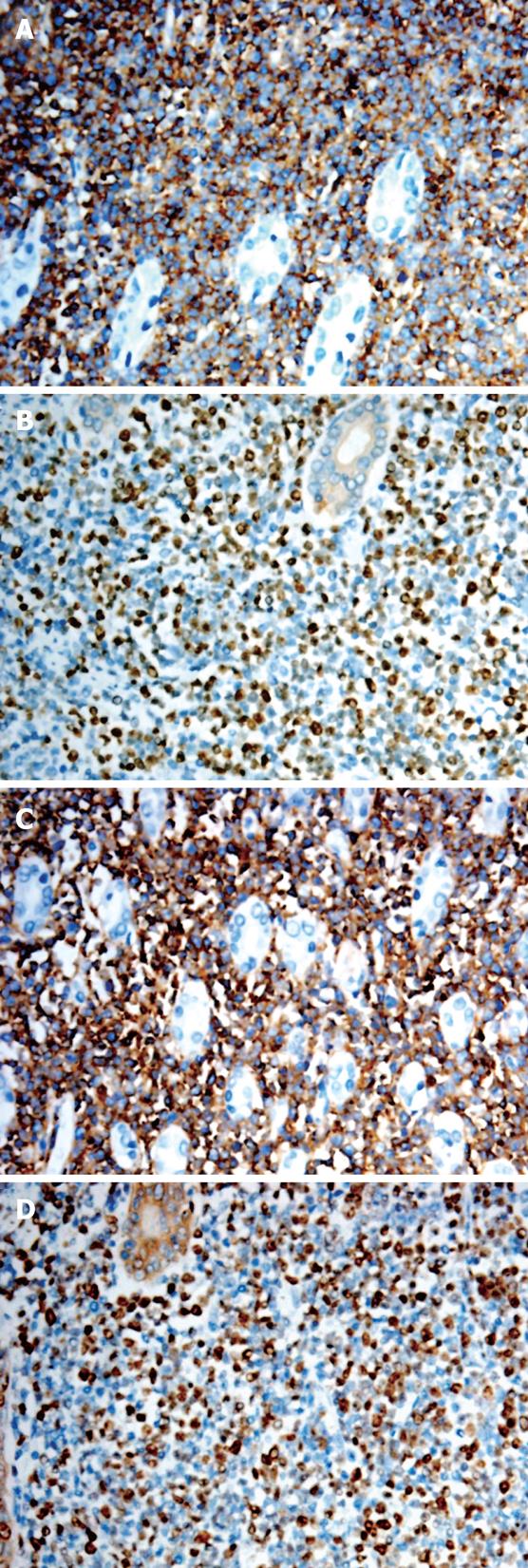
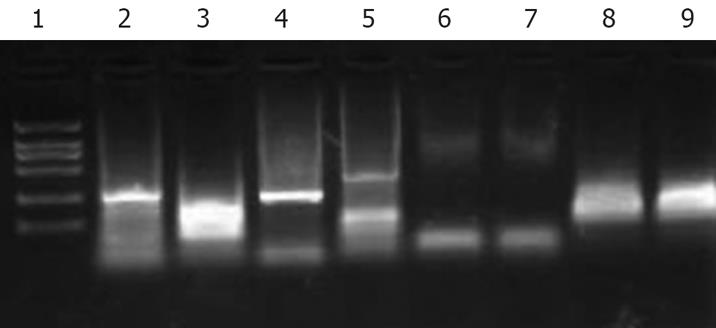
Immunohistochemistry analysis revealed immature lymphoid cells positive for cytoplasmic CD10 and CD79a, nuclear TdT and CD99 antigens, and negative for CD20 antigen. About 50%-70% of the tumor cells were reactive for Ki-67 (Figure 3). Gene rearrangement analysis showed monoclonal immunoglubin high chain gene rearrangement (Figure 4).
The morphology, immunophenotype, and gene rearrangement of the neoplastic cells supported the diagnosis of stomach precursor B-LBL. There was no evidence that supported the diagnosis of precursor B lymphoblastic leukemia in the bone marrow and peripheral blood.
Primary stomach lymphomas are in the minority, most of which are mucosa- associated lymphoid tissue B cell lymphoma, diffuse large B cell lymphoma, extranodal nasal type NK/T cell lymphoma, etc. Primary stomach B-LBL is rare[1–5].
Ninety percent of lymphoblastic lymphomas are of precursor T cell lineage and only 10% are of precursor B cell lineage[12]. Precursor B lymphoblastic lymphoma (B-LBL)/leukemia (B-ALL) are originated from B cell lineage. ALL and LBL represent different clinical presentations of the same neoplasm and are grouped in the category of precursor B-cell lymphoblastic leukemia/lymphoma by the revised World Health Organization classification of lymphoid neoplasms[1]. Because of the biologic unity of B-ALL and B-LBL, the criteria for distinguishing B-LBL from B-ALL are also arbitrary and applied inconsistently. In some studies[267], patients with B-LBL and acute leukemia are included, but in other studies[346], patients with leukemic involvement are excluded. According to the new WHO classification of lymphoid neoplasms, when the process is confined to a mass lesion without any or minimal evidence of blood and marrow involvement, the diagnosis is lymphoma; when extensive marrow and blood are involved, lymphoblastic leukemia is the appropriate term; if a patient presents with a mass lesion and the number of lymphoblasts is less than 25% in the marrow, the diagnosis of lymphoma is preferred[13–5]. Precursor B lymphoblastic B-LBL most commonly involves the skin, bone, soft tissue, and lymph nodes, whereas stomach B-LBL is uncommon[68]. Leukemic presentation with involvement of peripheral blood and bone marrow is most common. Extramedullary involvement is frequent with particular pre-direction for the central nervous system, lymph nodes, spleen, liver, and gonads. The leukocyte count may be decreased, normal or markedly elevated. The etiology of B-LBL is unknown. However, there is evidence that suggests a genetic factor in some cases[910]. The neoplastic cells of B-LBL are morphologically indistinguishable from those of B-ALL and typically small to medium size blast cells, with scant cytoplasm, moderately condensed to dispersed fine chromatin, inconspicuous nucleoli, and a high mitotic rate. Immunophenotypically, the neoplastic cells express terminal deoxynucleotidyl transferase (TdT) and B-cell antigens, such as CD79a, CD10, CD19, and CD22[129].
Our review of the literature revealed few case reports of stomach B-LBL lymphoma. In this report, we describe an exceptional primary stomach precursor B-LBL with no evidence of disease at any extrastomach site. The number of lymphoblasts was less than 25% in the marrow and there was no evidence in the peripheral blood. Since immunohistochemical study showed the neoplastic lymphoid cells of precursor B-cell type, the diagnosis of B-LBL was made in this stomach case[19]. Precursor B-LBL, a potentially curable disease, an aggressive therapy is very important[71112]. In this report, after surgical resection and final pathologic diagnosis of B-LBL, the patient was treated with chemotherapy. Now, she is followed-up at the outpatient clinic and in complete remission half a year after the initial diagnosis.
The differential diagnosis of precursor B-LBL includes myeloid lymphoma/leukemia, Burkitt’s lymphoma, and precursor T-LBL, etc. The tumor morphology and immunophenotype help differential diagnosis. Meanwhile, the precursor B-ALL should be excluded[113].
| 1. | Precursor B lymphoblastic leukemia/lymphoblastic lymphoma. In: Jaffe ES, Harris NL, Stein H, Stein H, Vardiman JW. World Health Organization classification of tumour, Pathology and genetics of tumours of haematopoietic and lymphoid tissues. International agency for reseach on cancer. Lyon, France: IARC Press 2001; 109-117. |
| 2. | Soslow RA, Baergen RN, Warnke RA. B-lineage lymphoblastic lymphoma is a clinicopathologic entity distinct from other histologically similar aggressive lymphomas with blastic morphology. Cancer. 1999;85:2648-2654. |
| 3. | Lin P, Jones D, Dorfman DM, Medeiros LJ. Precursor B-cell lymphoblastic lymphoma: a predominantly extranodal tumor with low propensity for leukemic involvement. Am J Surg Pathol. 2000;24:1480-1490. |
| 4. | Maitra A, McKenna RW, Weinberg AG, Schneider NR, Kroft SH. Precursor B-cell lymphoblastic lymphoma. A study of nine cases lacking blood and bone marrow involvement and review of the literature. Am J Clin Pathol. 2001;115:868-875. |
| 5. | Burkhardt B, Zimmermann M, Oschlies I, Niggli F, Mann G, Parwaresch R, Riehm H, Schrappe M, Reiter A. The impact of age and gender on biology, clinical features and treatment outcome of non-Hodgkin lymphoma in childhood and adolescence. Br J Haematol. 2005;131:39-49. |
| 6. | Bassi D, Lentzner BJ, Mosca RS, Alobeid B. Primary cardiac precursor B lymphoblastic lymphoma in a child: a case report and review of the literature. Cardiovasc Pathol. 2004;13:116-119. |
| 7. | Zinzani PL, Bendandi M, Visani G, Gherlinzoni F, Frezza G, Merla E, Manfroi S, Gozzetti A, Tura S. Adult lymphoblastic lymphoma: clinical features and prognostic factors in 53 patients. Leuk Lymphoma. 1996;23:577-582. |
| 8. | Kim JY, Kim YC, Lee ES. Precursor B-cell lymphoblastic lymphoma involving the skin. J Cutan Pathol. 2006;33:649-653. |
| 9. | Medeiros LJ, Carr J. Overview of the role of molecular methods in the diagnosis of malignant lymphomas. Arch Pathol Lab Med. 1999;123:1189-1207. |
| 10. | Hojo H, Sasaki Y, Nakamura N, Abe M. Absence of somatic hypermutation of immunoglobulin heavy chain variable region genes in precursor B-lymphoblastic lymphoma: a study of four cases in childhood and adolescence. Am J Clin Pathol. 2001;116:673-682. |
| 11. | Le Gouill S, Lepretre S, Briere J, Morel P, Bouabdallah R, Raffoux E, Sebban C, Lepage E, Brice P. Adult lymphoblastic lymphoma: a retrospective analysis of 92 patients under 61 years included in the LNH87/93 trials. Leukemia. 2003;17:2220-2224. |
| 12. | Kantarjian HM, O’Brien S, Smith TL, Cortes J, Giles FJ, Beran M, Pierce S, Huh Y, Andreeff M, Koller C. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18:547-561. |