Published online May 21, 2008. doi: 10.3748/wjg.14.3021
Revised: April 9, 2008
Published online: May 21, 2008
AIM: To compare the costs and effectiveness of no screening and no eradication therapy, the population-based Helicobacter pylori (H pylori) serology screening with eradication therapy and 13C-Urea breath test (UBT) with eradication therapy.
METHODS: A Markov model simulation was carried out in all 237 900 Chinese males with age between 35 and 44 from the perspective of the public healthcare provider in Singapore. The main outcome measures were the costs, number of gastric cancer cases prevented, life years saved, and quality-adjusted life years (QALYs) gained from screening age to death. The uncertainty surrounding the cost-effectiveness ratio was addressed by one-way sensitivity analyses.
RESULTS: Compared to no screening, the incremental cost-effectiveness ratio (ICER) was $16 166 per life year saved or $13 571 per QALY gained for the serology screening, and $38 792 per life year saved and $32 525 per QALY gained for the UBT. The ICER was $477 079 per life year saved or $390 337 per QALY gained for the UBT compared to the serology screening. The cost-effectiveness of serology screening over the UBT was robust to most parameters in the model.
CONCLUSION: The population-based serology screening for H pylori was more cost-effective than the UBT in prevention of gastric cancer in Singapore Chinese males.
-
Citation: Xie F, Luo N, Lee HP. Cost effectiveness analysis of population-based serology screening and 13C-Urea breath test for
Helicobacter pylori to prevent gastric cancer: A markov model. World J Gastroenterol 2008; 14(19): 3021-3027 - URL: https://www.wjgnet.com/1007-9327/full/v14/i19/3021.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3021
Gastric cancer is the second leading cause of cancer death worldwide, which leads to a substantial burden of morbidity, mortality, and health care costs[12]. H pylori infection has been recognized as an important risk factor for cancer of gastric body and antrum (distal cancers)[34]. Approximately 50% of the world population has been affected by H pylori[5]. Although less than 1% of the infected will develop cancer, population-based H pylori screening in high-risk population has been proposed as a cost-effective strategy in the long term in Western countries[6–8].
The East Asian countries such as China and Japan have the highest incidence of distal gastric cancer, which is twice as common in males as in females[1]. H pylori infection was also found to be strongly linked to increased risk of gastric cancer in ethnic Chinese and Japanese[9]. Early detection and eradication of H pylori infection might be a useful way to reduce the risk of gastric cancer in Asian populations where prevalence of H pylori infection and gastric cancer are significantly higher than in the West[1]. However, it is unknown whether it is cost-effective to implement population-based H pylori screening in high-risk Asian populations. Moreover, two widely used screening programs demonstrated good sensitivity and specificity in detection of H pylori infection in Chinese[1011], therefore the question arises which screening program is more cost effective?
This study was aimed to evaluate the clinical and economic effects associated with no screening, population-based H pylori serology screening, and population-based 13C-Urea breath test (UBT) in Singapore Chinese males using a Markov model.
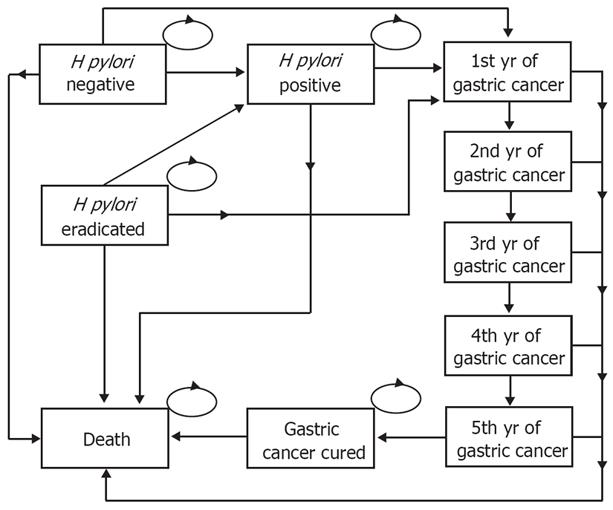
The decision analytical model compared three strategies: strategy 1, no screening and no eradication therapy; strategy 2, single serology screening for H pylori and treating those tested positive with eradication therapy; and strategy 3, single screening for H pylori using the UBT and treating those tested positive with the same eradication therapy as used in strategy 2. After the screening and treatment, both costs and outcomes of the strategies were evaluated using a Markov model (Figure 1)[1213], which, from the public healthcare provider’s perspective, estimated the costs, number of gastric cancer cases prevented, life years saved, and quality-adjusted life years (QALYs) gained from screening age to death (either died of gastric cancer or other causes, or achieved full life expectancy[14]). The distribution of people in the Markov states before the simulation started (i.e. cycle 0) was determined by the sensitivity and specificity of the screening strategies and prevalence of H pylori infection. The transition probabilities and corresponding plausible ranges in the model were obtained from a critical review of the published literature on target population where available (Table 1). Probabilities were converted from available rates using the formula recommended[13].
| Input variable | Base-case analysis | Range | Ref. |
| Incidence and prevalence rates | |||
| Age-specific prevalence of H pylori (%) | 20.0-43.3 | - | [10] |
| Age-specific prevalence of gastric cancer per 100 000 | 3-342 | - | [17] |
| Gastric cancer in distal stomach (%) | 60 | 50-80 | [7] |
| Relative risk of gastric cancer in persons with H pylori infection | 3.6 | 2-12 | [7] |
| Age-specific mortality from age of 25, per 1000 | 0.5-50.6 | - | [16] |
| Gastric cancer death in deaths from all causes (%) | 2.27 | 2.20-2.33 | [14,17] |
| Survival rate of gastric cancer after treatment (%) | [19] | ||
| 1-yr | 54.2 | 51-58 | |
| 2-yr | 41.8 | 38-45 | |
| 3-yr | 37.9 | 34-42 | |
| 4-yr | 34.0 | 30-38 | |
| 5-yr | 30.5 | 27-35 | |
| Screening and treatment variables (%) | |||
| H pylori serology screening sensitivity | 93 | 82-95 | [10] |
| H pylori serology screening specificity | 79 | 70-92 | [10] |
| H pylori13C-Urea breath test sensitivity | 97.9 | 90-100 | [11] |
| H pylori13C-Urea breath test specificity | 95.8 | 90-100 | [11] |
| Effectiveness of H pylori eradication | 92.0 | 87-98 | [21] |
| Probability of adverse effects related to eradication therapy necessitating medical intervention | 2.5 | 2-5 | [6] |
| Annual H pylori infection rate | 1.0 | 1-3 | [6,26] |
| Excess gastric cancer risk reduction attributable to H pylori eradication | 30 | 0-100 | [6] |
| Cost variables (2006USD)1 | |||
| H pylori serology screening | 26 | 10-50 | |
| H pylori13C-urea breath test | 83 | 60-100 | |
| H pylori eradication (triple therapy) | 30 | 20-50 | |
| Gastric cancer treatment per annum | 4358 | 328-59 000 | |
| Eradication-related adverse effects | 50 | 5-100 | |
| Other variables | |||
| Annual discount rate for costs and effectiveness (%) | 3 | 0-7 | [19,29] |
| Life expectancy, years | 77 | 76-80 | [14] |
| Utility | |||
| H pylori non-infected | 1.00 | 0.95-1.00 | [26] |
| H pylori infected | 0.90 | 0.80-1.00 | [26] |
| Gastric cancer | 0.38 | 0.13-0.65 | [26] |
One-way sensitivity analyses were conducted by altering individual variables within the aforementioned ranges. Based on the one-way sensitivity analyses, we additionally performed the best-case and the worst-case analyses, which included the most optimistic and pessimistic values for selected key variables.
We evaluated all Singapore Chinese males aged from 35 to 44 as the prevalence of H pylori infection at this age group increased substantially compared to the younger age[1015]. Age-specific H pylori infection rate, gastric cancer incidence, and mortality were applied when the cohort aged in the model[101617]. The relative risk in developing gastric cancer in H pylori infected persons compared to the uninfected was obtained from published literature[318]. Proportion of gastric cancer death among deaths from all causes was derived from local reports[17]. The 1- to 5-year survival rates were estimated from a large prospective cohort study in Chinese[19]. Persons who survived for more than 5 years after diagnosis of gastric cancer were assumed to be cured and therefore achieved full life expectancy as the 5-year survival rate adequately reflected the curative success of gastric cancer treatment[720].
The screening strategies included 1 single serology screening by using enzyme-linked immunosorbent assay (ELISA) with a sensitivity and specificity of 93% and 79%, respectively (strategy 2)[10] and 1 single UBT using simple gas chromatograph-mass selective detector with a sensitivity and specificity of 97.9% and 95.8%, respectively (strategy 3)[11]. In both strategies, persons with positive test for H pylori (including both true and false positive) were treated with a triple therapy (i.e. rabeprazole 20 mg, amoxicillin 1000 mg, clarithro-mycin 500 mg, all twice a day for 4 d) with an eradication rate of 91%[2122]. This regimen was specifically chosen because it is safe and effective with less resistance rate in patients and is recommended by the Asia-Pacific consensus conference[23–25]. Persons who stopped the triple therapy due to side effects or did not comply with the regimen were considered as treatment failure and thus remained infected. Persons who remained infected despite attempts at eradication had life expectancies and other outcomes identical to the infected who did not undergo treatment. The reinfection rate of the persons whose infection had been successfully eradicated was assumed to be identical to the persons who had never been infected (i.e. 1% annually in the base-case analysis)[626]. Once the reinfection occurred, an individual’s gastric cancer risk was considered the same as that of an untreated, infected person of the same age.
An underlying assumption of the present study is that eradication of H pylori infection can reduce only the certain level of excess risk of distal gastric cancer (60% of all gastric cancers)[427]. We conservatively assumed that persons cured of H pylori infection will have 30% of excess risk reduction compared to those H pylori infected persons in the base-case analysis, while a wide range of excess risk reduction from 10% to 100% was tested in one-way sensitivity analysis.
The present study was done from the public healthcare provider’s perspective. Thus, the model included direct medical costs of serology screening, the UBT, and triple therapy. Adverse effects associated with the triple therapy that necessitated medical intervention were also included (Table 1). Annual direct medical costs associated with treatment of gastric cancer were estimated at the average level across different stages of the cancer[28]. Nonmedical direct costs and indirect costs were not included. The costs were accrued from the time of screening until death. All costs were reported in 2006 US dollars and annually discounted at 3% in all analyses[29].
Three health outcomes evaluated in this model included number of gastric cancer cases prevented, life years saved, and QALYs gained. All outcomes were also annually discounted at 3% in the base-case analysis[29].
ICER was expressed as US dollars per life year saved and US dollars per QALY gained, which were calculated for the two screening strategies compared to no screening strategy, as well as the UBT compared to the serology screening. The cost-effectiveness threshold was estimated at $28 000 per QALY gained in local context, which was derived from the conventional threshold of $50 000 per QALY gained used in the United States by comparing the gross national income per capita between the United States and Singapore[2830].
There were a total of 237 900 Chinese males aged between 35 and 44 in Singapore[31]. In the base-case scenario, compared to no screening and no eradication therapy, strategy 2 that implemented the serology screening on all cohort members with treatment for those with positive test cost $9.8 million, which saved 523 life years or gained 623 QALYs by preventing 272 gastric cancer cases. Strategy 3 that implemented the UBT on this cohort with treatment for those with positive test cost $23.0 million, which saved 550 life years or gained 656 QALYs by preventing 281 gastric cancer cases. A total of 875 and 847 persons were screened for each case of gastric cancer prevented in strategy 2 and 3, respectively. The serology screening avoided $1.4 million of discounted expenditures on treatment of gastric cancer, while the UBT avoided $1.5 million. The ICER were $16 166 per life year saved and $13 571 per QALY gained for the serology screening, and $38 792 per life year saved and $32 525 per QALY gained for the UBT (Table 2). When compared to serology screening, the ICER was $477 079 per life year saved or $390 337 per QALY gained for the UBT.
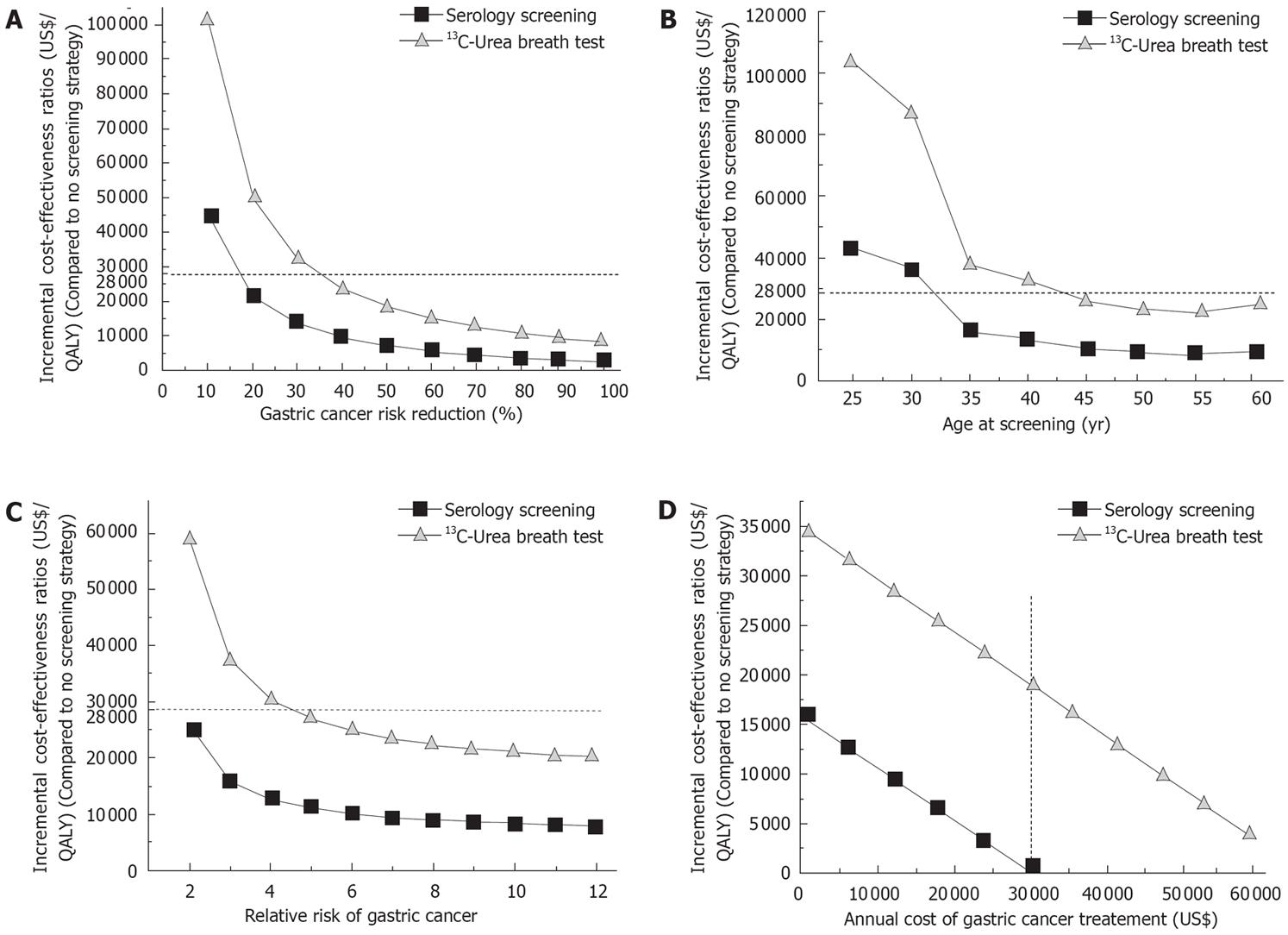
In the one-way sensitivity analyses, the level of excess gastric cancer risk reduction attributable to H pylori eradication varied from 10% to 100%[67]. Using a $28 000 per QALY gained as a threshold, the serology screening would be cost-effective if H pylori eradication reduced more than 15% of excess gastric cancer risk. In contrast, the UBT could be cost-effective only when the excess gastric cancer risk was reduced by 35% or more (Figure 2A).
The ICER was sensitive to age at which population-based screening was carried out as shown in Figure 3. When screening age was more than 32 years, the ICER was less than $28 000 per QALY gained for the serology screening. The UBT appeared cost-effective when the screening age was more than 45 years (Figure 2B).
Relative risk of gastric cancer for H pylori infected population had a significant impact on the ICER. When H pylori eradication was assumed to reduce 30% of excess gastric cancer risk (as in the base-case analysis), the serology screening appeared cost-effective over the full range of the relative risk (i.e. from 2 to 12). In contrast, the UBT appeared cost-effective only with the relative risk above 5 (Figure 2C).
Cost of annual gastric cancer treatment imposed a substantial impact on the cost-effectiveness of the strategies. For both strategies, the cost had an approximately linear relation with the ICER that decreased dramatically with the increase in annual cost of the cancer treatment (Figure 2D). When the annual cost was $30 075, the one-time expenditure on serology screening and treatment of those with positive test would be fully offset by the savings in preventing gastric cancers (Figure 2D). Cost of the serology screening and the UBT also had a moderate impact on the ICER. Each $5 increment in cost of the serology screening and the UBT augmented the ICER by $2000 and $1800, respectively (data not shown).
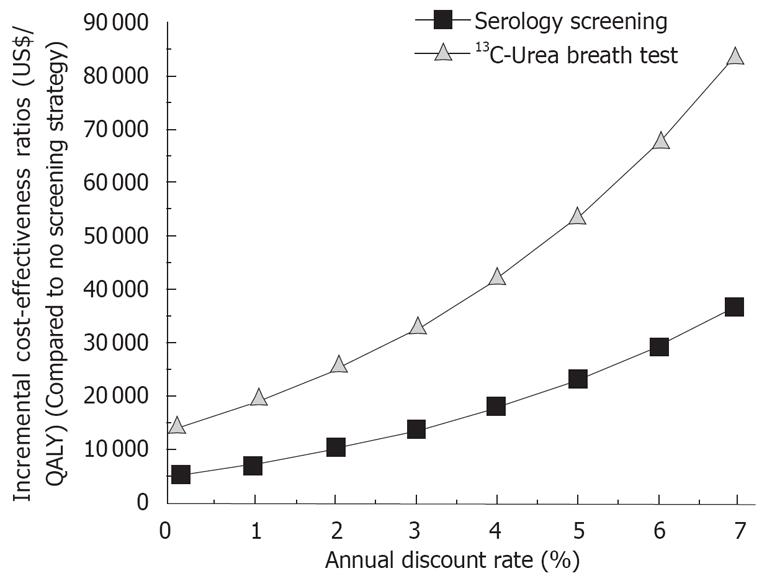
The ICER was also sensitive to the annual discount rate. With the increase in the annual discount rate, the ICER appeared less favorable for both strategies.
Other variables had little impact on the cost-effective-ness within the ranges listed in Table 1, which included sensitivity and specificity of the serology screening and the UBT, effectiveness of H pylori eradication, probability and costs of adverse effects related to eradication therapy necessitating medical intervention, and utilities of each health state.
In all these sensitivity analyses, the ICER was extremely less favorable for the UBT compared to the serology screening.
In the best-case and worst-case analyses, the most critical variables, including level of excess gastric cancer risk reduction, relative risk of gastric cancer in H pylori infected population, annual cost of gastric cancer treatment, cost of the serology screening and the UBT, and annual discount rate, were simultaneously varied. Both strategies achieved more health benefits (i.e. life years gained or QALYs) at a lower cost compared to no screening, and the UBT also received more health benefit at a lower cost compared to the serology screening in the best-case scenario (i.e. dominant) (Table 2). In contrast, the ICER was more than $300 000 for all comparisons in the worse-case scenario. The UBT achieved the same gaining in QALYs but at an extra cost of $11 290 897 compared to the serology screening in the worst case analysis (Table 2).
The present study modeled the life-time cost and effectiveness associated with population-based H pylori screening and treatment for those with positive test in Chinese males. Compared to no screening and no eradication therapy strategy, the serology screening was cost-effective, while the UBT was not cost-effective based on the threshold of $28 000 per QALY gained. The UBT gained very little extra health benefits in terms of either life years saved or QALYs gained but at a substantially higher cost compared to the serology screening. This suggests that the population-based serology screening for H pylori infection be adopted in this specific population, especially under the circumstances that the cost of gastric cancer treatment keeps arising due to the advances in new technologies. Also with this model, future clinical advances on the efficacy of H pylori eradication in prevention of gastric cancer can be easily translated into the cost-effectiveness ratio, which is now playing an increasingly important role in informing medical decision making.
The serology screening was found to be cost-effective in the present study, which is similar to the published studies using the similar model to estimate the economic and clinical effects of H pylori screening[67]. Nevertheless, the model used in the present study had several improvements which are worth noting. First, we have a health state to identify the persons who were H pylori positive and successfully eradicated by the triple therapy (i.e. ‘H pylori eradicated’ in Figure 1). This is a health state in the Markov model which can allow for successful capturing of the economic and health benefits resulted from the screening strategies. Second, in line with the important assumption that the persons who survived more than 5 years after diagnosis of gastric cancer were assumed to be cured[720], we used five tunnel states, instead of a single gastric cancer health state, to represent the status for each of the first five years since diagnosed with gastric cancer. The mortalities for these tunnel states were different from each other based on the epidemiological evidence[19]. This refinement may better simulate the real progress of gastric cancer and thus obtain more accurate estimations in cost and effectiveness. Third, this model is life-time estimation and every person remained in the model until death. Thus some parameters are time-sensitive including H pylori incidence, gastric cancer incidence, and mortality (Table 1). Instead of fixed point estimates, age-specific estimates may be more appropriate and accurate to reflect the changes in these important parameters with the aging of the cohort in the model.
Besides, some differences between these two studies and the present study are notable. The cost and effectiveness of the screening strategies essentially stemmed from the actual number of gastric cancer cases prevented by the strategies. Therefore, given the certain level of excess gastric cancer risk reduction by the eradication, cost of gastric cancer treatment and relative risk of gastric cancer in H pylori infected persons are deemed to have a very important and significant impact on the estimated ICER. The screening strategies would save more money if the cost needed to treat a gastric cancer case increased and prevent more gastric cancer cases if relative risk of gastric cancer in H pylori infected persons increased. Furthermore, the economic and health benefits of prevention of gastric cancer cases may only occur in the future rather than in the present, which highlights the important role of discount rate used: the larger the discount rate used the less the benefits obtained (Figure 3). However, these parameters were not examined in some previous study[6], or only little impact of these parameters was reported[7].
The cost-effectiveness of the serology screening over the UBT study was robust to most of the parameters through the one-way sensitivity analyses. Nevertheless, some findings are worth attention. As shown in Figure 3, the screening strategies would be more cost-effective if the starting age increased, which might be explicitly explained by the fact that both H pylori infection rate and gastric cancer incidence would increase with age. However, a recent large randomized controlled trial in Chinese revealed that persons with precancerous lesions (gastric atrophy, intestinal metaplasia, and dysplasia) significantly reduced the efficacy of H pylori eradication in prevention of gastric cancer compared to those without the lesions[32]. As the precancerous lesions increased significantly with age in Chinese[33], this could be important evidence to support the younger screening age. Thus, we suggested that the optimal screening age could be 35 years where there would be a substantial improvement on the ICER compared to younger age but only slight improvement compared to older age. Otherwise, if an older screening age was chosen, despite the increase in H pylori infection rate and gastric cancer incidence, the level of excess gastric cancer risk reduction (i.e. the efficacy of the eradication) would remain at the far lower end of the spectrum, favoring no screening against the serology screening (Figure 2).
Prevention of gastric cancer will save the medical expenditures for treatment of cancer and increase the life years and QALYs. However, this health benefit could be associated with additional medical expenditures (even the expenditures on daily living for extended life years) incurred during the extended life years, which will not occur in case of premature death. As including this cost component remains controversial, we did not take it into consideration in the present study. We also acknowledged that some parameters used in the model (e.g. survival rate of gastric cancer) were not available for Chinese males in Singapore, which may limit the accuracy of point estimates for cost and effectiveness. Finally, the threshold for ICER used in the present study was estimated from the US threshold using the ratio of gross national income between two countries, which is relative arbitrary and warrants further empirical local studies on this important topic.
In summary, the population-based serology screening for H pylori infection was more cost-effective than the UBT in prevention of gastric cancer in Singapore Chinese males.
H pylori infection has been recognized as an important risk factor for gastric cancer. Screening for H pylori has been proposed as a cost-effective strategy in prevention of gastric cancer.
A number of screening strategies are currently available. However, it is unknown which screening strategy is more cost-effective in high-risk populations, especially in Asian populations.
A separate health state was used to identify the persons who were H pylori positive and successfully eradicated by the triple therapy. This state can allow for successful capturing of the economic and health benefits resulted from the screening strategies. Five tunnel states, instead of a single gastric cancer health state, were used in line with the important assumption that the persons who survived more than 5 years after diagnosis of gastric cancer were assumed to be cured.
The findings in this study will be useful and important for decision makers to efficiently allocate scarce health resources for population-based H pylori screening.
The authors studied a clinically relevant issue. The manuscript is well written and is worth of publication in the Journal as is. This study has a substantial element of novelty. There is no data in literature concerning cost-effectiveness of serology-based screening strategy, particularly in countries with high prevalence of the infection, where the gastric cancer is a problem of special importance.
| 1. | Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol. 2003;56:1-9. |
| 2. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. |
| 3. | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302-1305. |
| 4. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. |
| 5. | An international association between Helicobacter pylori infection and gastric cancer. The EUROGAST Study Group. Lancet. 1993;341:1359-1362. |
| 6. | Fendrick AM, Chernew ME, Hirth RA, Bloom BS, Bandekar RR, Scheiman JM. Clinical and economic effects of popu-lation-based Helicobacter pylori screening to prevent gastric cancer. Arch Intern Med. 1999;159:142-148. |
| 7. | Parsonnet J, Harris RA, Hack HM, Owens DK. Modelling cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: a mandate for clinical trials. Lancet. 1996;348:150-154. |
| 8. | Roderick P, Davies R, Raftery J, Crabbe D, Pearce R, Patel P, Bhandari P. Cost-effectiveness of population screening for Helicobacter pylori in preventing gastric cancer and peptic ulcer disease, using simulation. J Med Screen. 2003;10:148-156. |
| 9. | Miwa H, Go MF, Sato N. H. pylori and gastric cancer: the Asian enigma. Am J Gastroenterol. 2002;97:1106-1112. |
| 10. | Kang JY, Yeoh KG, Ho KY, Guan R, Lim TP, Quak SH, Wee A, Teo D, Ong YW. Racial differences in Helicobacter pylori seroprevalence in Singapore: correlation with differences in peptic ulcer frequency. J Gastroenterol Hepatol. 1997;12:655-659. |
| 11. | Lee HS, Gwee KA, Teng LY, Kang JY, Yeoh KG, Wee A, Chua BC. Validation of [13C]urea breath test for Helicobacter pylori using a simple gas chromatograph-mass selective detector. Eur J Gastroenterol Hepatol. 1998;10:569-572. |
| 12. | Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics. 1998;13:397-409. |
| 13. | Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322-338. |
| 14. | World Health Organization. Mortality Country Fact Sheet 2006 Singapore. Geneva: World Health Organization. 2006;. |
| 15. | The Committee on Epidemic Diseases. Seroprevalence of Helicobacter pylori infection in Singapore. Epidemiological News Bulletin. 1996;22:31-32. |
| 16. | Singapore Department of Statistics. Yearbook of Statistics 2006 Singapore. Singapore: Department of Statistics. 2006;. |
| 17. | Seow A, Koh WP, Chia KS, Shi LM, Lee HP, Shanmugaratnam K. Trends in Cancer Incidence in Singapore 1968-2002. Singapore: Singapore Cancer Registry Report No. 6. 2004;. |
| 19. | Tian J, Wang XD, Chen ZC. Survival of patients with stomach cancer in Changle city of China. World J Gastroenterol. 2004;10:1543-1546. |
| 20. | Koga S, Kaibara N, Kishimoto H, Nishidoi H, Kimura O, Okamoto T, Tamura H. Comparison of 5- and 10-year survival rates in operated gastric cancer patients. Assessment of the 5-year survival rate as a valid indicator of postoperative curability. Langenbecks Arch Chir. 1982;356:37-42. |
| 21. | Yang KC, Wang GM, Chen JH, Chen TJ, Lee SC. Comparison of rabeprazole-based four- and seven-day triple therapy and omeprazole-based seven-day triple therapy for Helicobacter pylori infection in patients with peptic ulcer. J Formos Med Assoc. 2003;102:857-862. |
| 22. | Gambaro C, Bilardi C, Dulbecco P, Iiritano E, Zentilin P, Mansia C, Usai P, Vigneri S, Savarino V. Comparable Helicobacter pylori eradication rates obtained with 4- and 7-day rabeprazole-based triple therapy: a preliminary study. Dig Liver Dis. 2003;35:763-767. |
| 23. | Danese S, Armuzzi A, Romano A, Cremonini F, Candelli M, Franceschi F, Ojetti V, Venuti A, Pola P, Gasbarrini G. Efficacy and tolerability of antibiotics in patients undergoing H pylori eradication. Hepatogastroenterology. 2001;48:465-467. |
| 24. | Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol. 1998;13:1-12. |
| 25. | Stack WA, Knifton A, Thirlwell D, Cockayne A, Jenkins D, Hawkey CJ, Atherton JC. Safety and efficacy of rabeprazole in combination with four antibiotic regimens for the eradication of Helicobacter pylori in patients with chronic gastritis with or without peptic ulceration. Am J Gastroenterol. 1998;93:1909-1913. |
| 26. | Wang Q, Jin PH, Lin GW, Xu SR, Chen J. Cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer: Markov decision analysis. Zhonghua Liuxingbingxue Zazhi. 2003;24:135-139. |
| 27. | Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. 1999;94:2373-2379. |
| 28. | Dan YY, So JB, Yeoh KG. Endoscopic screening for gastric cancer. Clin Gastroenterol Hepatol. 2006;4:709-716. |
| 29. | Lipscomb J, Weinstein MC, Torrance GW. Time preference. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press 1996; 214-246. |
| 30. | World Bank. World Development Indicators Database.: World Bank. 2006;. |
| 31. | Department of Statistics. Census of Population 2000 Statistical Release 1: Demographic Characteristics. Department of Statistics: Singapore. 2001;. |
| 32. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. |
| 33. | You WC, Blot WJ, Li JY, Chang YS, Jin ML, Kneller R, Zhang L, Han ZX, Zeng XR, Liu WD. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res. 1993;53:1317-1321. |