Published online May 14, 2008. doi: 10.3748/wjg.14.2900
Revised: March 21, 2008
Published online: May 14, 2008
AIM: To investigate the expression level and effects of leptin in human hepatocellular carcinoma cells in vitro and to explore the correlation between them.
METHODS: Human hepatocellular carcinoma cell line HepG2 was cultured in vitro, and (the expression level) mRNA of leptin and leptin receptors in HepG2 were assessed using reverse transcription polymerase chain reaction (RT-PCR). Effects of different concentrations of leptin (50 ng/mL, 100 ng/mL, 200 ng/mL) on HepG2 were detected with colorimetric assay by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) after incubation periods of 24 h, 48 h, and 72 h. Flow cytometry was performed to assess cell cycle progression of different concentrations of leptin as stated above after each 24 h incubation period.
RESULTS: mRNA of leptin and leptin receptors (including short and long isoforms) were expressed in HepG2. The 72 h incubation of leptin at different concentrations (50 ng/mL, 100 ng/mL, 200 ng/mL) promoted proliferation of HepG2 in a concentration- and time-dependent manner. The experimental group shows significant statistical differences when compared to the controlled group which contained 0 ng/mL of leptin. As the concentration of leptin increases, significant fewer cells were detected in G0-G1 phase and more cells in S and G2-M phases.
CONCLUSION: Leptin and leptin receptor are simultaneously expressed in human hepatocellular carcinoma cell line HepG2. Addition of leptin (0 ng/mL-200 ng/mL) in 72 h periods indicated there is a concentration- and time-dependent correlation in the stimulation of HepG2 cell proliferation. The effect of proliferation by leptin is due to promotion of DNA synthesis and enhancement of mitotic activity. The relationship between leptin and human hepatocellular carcinoma cells might indicate that adipokine could be associated with the progression of human hepatocellular carcinoma.
-
Citation: Zhou J, Lei W, Shen L, Luo HS, Shen ZX. Primary study of leptin and human hepatocellular carcinoma
in vitro . World J Gastroenterol 2008; 14(18): 2900-2904 - URL: https://www.wjgnet.com/1007-9327/full/v14/i18/2900.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2900
Leptin, a protein product encoded by the obese gene (ob gene)[1], is primarily derived from adipocytes which plays an important role in the regulation of food intake and the control of body weight[2]. It takes part in various physical conditions such as lipid metabolism, immune defense, neuroendocrine regulation, pituitary hormone secretion, and pubertal development[34]. In rodents and humans, leptin is related with many pathological syndromes including obesity, hyperphagia, hyperinsulinemia, reduced fertility, and cholelithiasis (including gallstone and hepatolithiasis)[5–8]. Studies have shown that individuals who are obese have increased risk for most cancers compared to individuals who are not obese[9]. Since leptin is closely associated with obesity, it may be the bridge conjoining obesity and cancer.
The biological functions of leptin on target cells and tissues are carried out through interaction with its specific receptors (ob-R) which belong to class I cytokine receptor family[10]. In rodents and humans, two leptin receptor isoforms predominate: the short leptin receptor isoform (ob-Ra) and the long leptin receptor isoform (ob-Rb)[1112]. They share the same extracellular domain, but they differ in the length of the transmembrane/cytoplasmic coding regions[13]. The physiologic significance of each isoform in relationship to obesity and cancer is still unknown.
Hepatocellular carcinoma (HCC) is one of the most malignant tumors in the world. It causes proximately one hundred and ten thousand deaths annually in China. HCC is extremely difficult to detect in prognosis due to its early metastasis and distant transmission. It has been reported that obesity was related to HCC complicated with cryptogenic cirrhosis and alcoholic liver disease[1415]. Therefore, we presume that leptin might be associated with HCC. In order to investigate the relationship between leptin and HCC, (the expression level) mRNA of leptin and its receptors in human HCC cell line HepG2 were detected by reverse transcription polymerase chain reaction (RT-PCR). In addition, the effects of leptin on HepG2 in vitro were also discussed in this study.
Human hepatocellular carcinoma cell line HepG2 was obtained from Shanghai Cell Biology Institute of Chinese Academy of Sciences. Human recombinant leptin was the product from Sigma (St. Louis, Missouri, USA). Roswell Park Memorial Institute-1640 (RPMI-1640), fetal bovine serum (FBS) and Trizol reagent were the products of Invitrogen (Carlsbad, California, USA). Trypsin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), ethylene diamine tetraacetic acid (EDTA) and dimethyl sulfoxide (DMSO) were purchased from Amresco (Cleveland, Ohio, USA). Revert aid first strand cDNA synthesis kit and TaqDNA polymerase were the products of Fermentas (Burlington, Iowa, USA). All other reagents unless indicated were from Sigma (St. Louis, Missouri, USA).
Cell culture: HepG2 were grown as a monolayer in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 100 kU/L penicillin, and 0.1 g/L streptomycin. They were cultured in T-75 cm2 culture flasks and maintained at 37°C in 5% CO2 humidified atmosphere. At the beginning of the experiment, cells in the exponential growth phase were detached from the flask with 0.25% trypsin and 0.02% EDTA solution.
RT-PCR: mRNA of leptin, ob-Ra and ob-Rb in cell line HepG2 were assessed using RT-PCR as described previously[16]. Total RNA was isolated from confluent cells using Trizol reagent according to the manufacture’s instructions. The concentration of RNA was quantitated by absorbance change at 260 nm and 280 nm. Total RNA was suspended in DEPC-treated water and stored at -80°C.
Single-stranded cDNA was synthesized from 1 &mgr;g of RNA using revert aid first strand cDNA synthesis kit with random hexamer primer. The housekeeping gene β-actin was amplified to set a control for RNA loading and also to minimize variations in cDNA synthesis to ensure efficiency. Primer sequences for β-actin, leptin, ob-Ra and ob-Rb have been reported in the previous paper[17]. Following are the primers for: 1) β-actin: sense strand is 5’-ACCCACACTGTGCCCATCTA-3’ and anti-sense strand is 5’-CGGAACCGCTCATTGCC-3’ (encodes a 289-bp fragment); 2) leptin: sense strand is 5’-GTGCGGATTCTTGTGGCTTT-3’ and anti-sense strand is 5’-GGAATGAAGTCCAAACCGGTG-3’ (encodes a 174-bp fragment); 3) ob-Ra: sense strand is 5’-TTGTGCCAGTAATTATTTCCTCTT-3’ and anti-sense strand is 5’-AGTTGGCACATTGGGTTCAT-3’ (encodes a 200-bp fragment); 4) ob-Rb: the sense strand is 5’-TTGTGCCAGTAATTATTTCCTCTT-3’ and antisense strand is 5’-CTGATCAGCGTGGCGTATTT-3’ (encodes a 439-bp fragment).
Amplification of the resulting cDNA sequence was carried out using polymerase chain reaction (PCR). 3 µL cDNA was combined with 25 pmol oligonucleotide primers specific for β-actin, leptin, ob-Ra, ob-Rb and TaqDNA polymerase 1 U, PCR buffer (5 &mgr;L), 25 mmol/L MgCl2 (4 &mgr;L), 10 mmol/L dNTP mixture (1 &mgr;L), and ddH2O (34 &mgr;L in a 50 &mgr;L solution). PCR was performed in a thermal cycler (Gene Amp PCR system 2400, Perkin-Elmer Corp., Uberlingen, Germany). The condition for the reaction were the following: 1 min at 94°C (predenaturation), followed by 30 cycles of 1 min (denaturation) at 52°C (for β-actin), 55°C (for leptin) or 50°C (for ob-Ra and ob-Rb); 1 min at 72°C (annealing), and then 5 min at 72°C (extension). PCR products were electrophoresed on 2% agarose gels stained with ethidium bromide, and the length were estimated to be 100-bp. Imaging was performed on JS-380 instrument (Peiqing Inc., Shanghai, China).
MTT for cell proliferation: MTT colorimetric assay was used to detect the effects of leptin on proliferation of cell line HepG2. Cells were washed extensively with filtered and sterilized phosphate-buffered saline (PBS) to remove the dead cells. HepG2 cells were suspended at a concentration of 1.0 × 104/mL, and then they were seeded into 96-well microplate at 150 &mgr;L/well and incubated to adhere overnight. RPMI-1640 medium containing different concentrations of human recombinant leptin were added in 0 ng/mL, 50 ng/mL, 100 ng/mL and 200 ng/mL to HepG2 cells with each concentration account for 6 wells. MTT assay was performed after incubation periods of 24 h, 48 h, and 72 h. MTT 20 &mgr;L (5 mg/mL) stock solution in PBS was added to each well. The microplate was incubated for 4 h and 100 &mgr;L DMSO was added (to each well). Optical density (A) value was measured by an ELISA plate reader (Digiscan Lab instruments, Austria) at wavelength of 492 nm. Each variant group was performed in triplicate wells for measurement accuracy.
Flow cytometric analysis: HepG2 cells in the exponential growth phase were detached by 0.25% trypsin and 0.02% EDTA. The cell suspension was then seeded uniformly into four T-75 cm2 culture flasks and incubated to adhere overnight. RPMI-1640 medium containing different concentrations of human recombinant leptin were added in 0 ng/mL, 50 ng/mL, 100 ng/mL, and 200 ng/mL to HepG2 cells. Flow cytometric analysis was performed after incubated for 24 h. The cells were briefly washed twice with PBS, fixed in ice-cold ethanol (70% vol/vol in ddH2O) and stained with propidium iodide (PI) solution (25 &mgr;g/mL PI, 180 U/ml RNase, 0.15% Triton X-100, and 30 mg/mL polyethylene glycol in 4 mmol/L critrate buffer w/pH = 7.8). DNA contents were detected using a FACScan flow cytometer (Becton Dickinson Co., San Jose, CA). The relative percentages of each phase of the cell cycle were analyzed using Cell Quest Software (Becton Dickinson Co., San Jose, CA). For the measurement accuracy, each variant group was tested in triplicate.
The data were presented as mean ± SD. ANOVA was used to analysis the results. P < 0.05 were considered to indicate statistically significant differences. Entire data were analyzed with the statistical software SPSS 11.5.
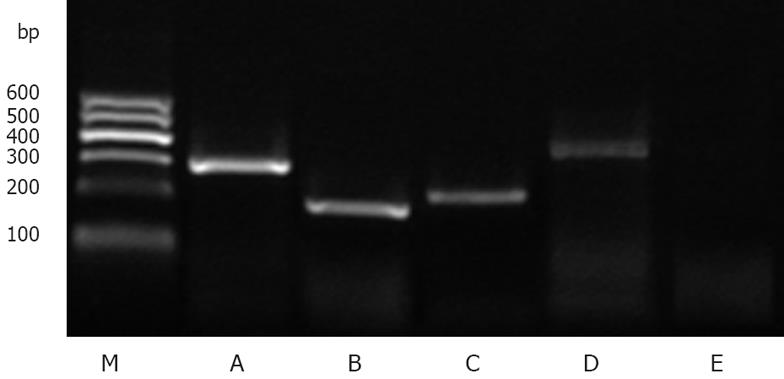
Total RNA extracted from HepG2 cells were reversely transcribed to check whether mRNA of leptin, ob-Ra and ob-Rb were expressed. RT-PCR detection with β-actin primers revealed a 289-bp fragment in HepG2. The 174-bp cDNA band indicated that the cells expressed leptin mRNA. PCR products for both the short isoform (ob-Ra, 200-bp) and the long isoform (ob-Rb, 439-bp) of leptin receptor were also detectable. No fragment was detected in the negative control that PCR product without the cDNA (Figure 1).
MTT assay indicated the cell proliferate conditions of HepG2. As shown in Table 1, leptin significantly stimulated HepG2 cells growth in a concentration- and time-dependent manner. Leptin caused significant growth potency on HepG2 within 72 h. There were significant statistical differences between concentration groups of 0 ng/mL vs 50 ng/mL (P < 0.01), 0 ng/mL vs 100 ng/mL (P < 0.01), 0 ng/mL vs 200 ng/mL (P < 0.01), 50 ng/mL vs 100 ng/mL (P < 0.05) and 50 ng/mL vs 200 ng/mL (P < 0.01). Meanwhile, significant statistical differences also existed between duration groups of 24 h vs 48 h (P < 0.01) and 24 h vs 72 h (P < 0.01).
The effects of leptin on cell cycle progressions of HepG2 as determined by flow cytometry analysis are shown in Table 2. In the 24 h frame, the results indicate that as the concentration of leptin increases (e.g. 50 ng/mL, 100 ng/mL and 200 ng/mL), the proportion of the HepG2 cells in G0-G1 phase gradually reduces and the number of cells in S and G2-M phases gradually increases. In comparison with control group, the data indicates a significant reduction of cells in G0-G1 phase (P < 0.01) and a significant increase of cells in S phase (P < 0.01) and G2-M phase (P < 0.01) in response to treatment of leptin.
The relationship between obesity and cancer has been excessively documented for cancers or adenocarcinoma in endometrium, breast, prostate, renal cells, pancreas, colon, and esophagus[1819]. Epidemiological observations revealed that obesity was a risk factor of HCC complicated with cryptogenic cirrhosis and alcoholic liver disease[1415], and in addition, clinical investigations identified that the leptin level of serum had increased significantly in alcoholic and post-hepatitis liver cirrhosis patients with or without HCC as compared to control subjects w/o the complication[20–24]. Still, there were few studies on the involvement of leptin in HCC.
It has been reported that leptin participates in an auto/paracrine manner in the pituitary gland, and it plays regulations roles in the pig[25]. One study reported that the proteins of leptin and ob-R had been detected in 72.22% and 30.56% of HCC, respectively[26]. Present study indicated that the mRNA of leptin, and as well as the short and the long leptin receptor isoforms were all expressed in HepG2. These findings suggested that leptin might act as an auto/paracrine growth factor towards hepatocytes, and together with its receptors, they could play role in HCC initiation and progression.
Serum leptin level fluctuations are also detected in some non-physiological conditions. For example, leptin levels up to 400 ng/mL have been reported in children with chronic renal failure[27]. When obese, but otherwise healthy subjects treated with leptin (1 mg/kg per day), serum leptin levels rose up to 736 ng/mL[28]. However, serum leptin level in normal physiological situation is less than 11.4 ng/mL. Thus, a relatively higher concentration of leptin (200 ng/mL) was used in our study. There are some divaricate results among the studies of leptin and cancer. Leptin not only stimulated proliferation of some human cancer cell lines, including breast cancer cell lines (ZR75-1, MCF-7), esophageal cancer cell lines (KYSE 410) and prostate cancer cell lines (PC-3, DU 145), but also it inhibited the growth of other human cancer cell lines, such as pancreatic cancer cell lines (Mia-Paca, PANC-1)[18]. Data regarding leptin’s effect on HCC cells are rarely reported, and they appear to be contradictory. In one in vitro study, leptin was found to have little effect on the proliferation of liver cancer cell line SMMC-7721[26], while in another study, leptin had shown anti-tumor activity[29]. In our in vitro study, exogenous leptin had effects on the proliferation of HCC cell line HepG2 in a concentration- and time-dependent manner. We might conclude that human cancer cell lines exhibit differential responses to the leptin treatments, depending upon the biological characteristics of the cancer cells and the organ of derivation of the cell lines. Because our study was to interfere HCC cell line with exogenous leptin in vitro, results may differ in concentration threshold and activation initiation time with in vivo HCC cells in tumor tissues with endogenous leptin. Therefore, it will be necessary to continue the research on leptin’s effects on HCC with targeted animal models.
By adopting the techniques of flow cytometry, we were able to significantly expand the analysis of cell cycle progression. With respect to the cell cycle, we were able to accurately determine the relative proportion of cells in each phase of the cycle progression. From our data, it was evident that as the concentration of exogenous leptin increased from 0 to 200 ng/mL, it gradually reduced the relative proportion of the HepG2 cells in G0-G1 phase and gradually increased the number of the cells in S and G2-M phases. Since the S phase of the cell cycle is the synthetic phase of DNA, and G2-M phase represents a later synthetic phase of DNA and the splitting of the cells through mitotic phase, data from our study demonstrate that leptin increase cell proliferation of HepG2 by promoting of DNA synthesis and enhancing mitotic activity.
It has been reported that leptin replacement is a very promising therapeutic approach for managing the complications of lipodystrophy. In addition, leptin may have therapeutic potential in the treatment of epilepsy[3031]. The primary finding of our study on leptin and HCC cell line indicates that adipokine could be associated with the progression of human hepatocellular carcinoma. Administration of the leptin antagonists or receptors in the targeted cells may open a new way in HCC prevention and treatment. Further studies are warranted and ongoing.
Leptin is the protein product encoded by obese gene, which plays an important role in the regulation of food intake, and the control of body weight. It is related with many pathological syndrome including obesity, hyperphagia, hyperinsulinemia, reduced fertility, and cholelithiasis. There are considerable researches about the effects of leptin.
Recently, the researches of relationships between leptin and tumors are hotspots. But there are few studies on the involvement of leptin in human hepatocellular carcinoma.
In this study, we found mRNA of leptin and leptin receptors (including short and long isoforms) were expressed in human hepatocellular carcinoma cell line HepG2. Leptin (0 ng/mL-200 ng/mL) in 72 h could stimulate proliferation of HepG2 in vitro, and that effect was due to promotion of DNA synthesis and enhancement of mitotic activity.
The primary study of leptin and HepG2 may indicate that adipokine could be associated with the progression of human hepatocellular carcinoma, and thus may offer new aim for further understanding of the biological function of leptin and new therapeutic targets.
The manuscript written by Zhou J et al describes a possible role of leptin in hepatocarcinogenesis and promotion of the disease. The data are interesting and potentially important. It’s recommended the additional experiments using another HCC cell lines which could make this study’s conclusions more confidential.
| 1. | Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425-432. |
| 2. | Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683-1686. |
| 3. | Janeckova R. The role of leptin in human physiology and pathophysiology. Physiol Res. 2001;50:443-459. |
| 4. | Shimizu F, Matsuzaki T, Iwasa T, Tanaka N, Minakuchi M, Kuwahara A, Yasui T, Furumoto H, Irahara M. Transition of leptin receptor expression during pubertal development in female rat pituitary. Endocr J. 2008;55:191-198. |
| 5. | Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903-908. |
| 6. | Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632-635. |
| 7. | Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398-401. |
| 8. | Lei ZM, Ye MX, Fu WG, Chen Y, Fang C, Li J. Levels of serum leptin, cholecystokinin, plasma lipid and lipoprotein differ between patients with gallstone or/and those with hepatolithiasis. Hepatobiliary Pancreat Dis Int. 2008;7:65-69. |
| 9. | Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625-1638. |
| 10. | Tartaglia LA, Dembski M, Weng X, Deng N, Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263-1271. |
| 11. | Fei H, Okano HJ, Li C, Lee GH, Zhao C, Darnell R, Friedman JM. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc Natl Acad Sci USA. 1997;94:7001-7005. |
| 12. | Friedman JM. Leptin, leptin receptors, and the control of body weight. Nutr Rev. 1998;56:s38-s46; discussion s54-s75. |
| 13. | Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413-437. |
| 14. | Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36:150-155. |
| 15. | Roth MJ, Baer DJ, Albert PS, Castonguay TW, Dorgan JF, Dawsey SM, Brown ED, Hartman TJ, Campbell WS, Giffen CA. Relationship between serum leptin levels and alcohol consumption in a controlled feeding and alcohol ingestion study. J Natl Cancer Inst. 2003;95:1722-1725. |
| 16. | Zarkesh-Esfahani H, Pockley G, Metcalfe RA, Bidlingmaier M, Wu Z, Ajami A, Weetman AP, Strasburger CJ, Ross RJ. High-dose leptin activates human leukocytes via receptor expression on monocytes. J Immunol. 2001;167:4593-4599. |
| 17. | Yuan SS, Chung YF, Chen HW, Tsai KB, Chang HL, Huang CH, Su JH. Aberrant expression and possible involvement of the leptin receptor in bladder cancer. Urology. 2004;63:408-413. |
| 18. | Somasundar P, Yu AK, Vona-Davis L, McFadden DW. Differential effects of leptin on cancer in vitro. J Surg Res. 2003;113:50-55. |
| 19. | Somasundar P, Riggs D, Jackson B, Vona-Davis L, McFadden DW. Leptin stimulates esophageal adenocarcinoma growth by nonapoptotic mechanisms. Am J Surg. 2003;186:575-578. |
| 20. | Wang YY, Lin SY. Leptin in relation to hepatocellular carcinoma in patients with liver cirrhosis. Horm Res. 2003;60:185-190. |
| 21. | Lin SY, Wang YY, Sheu WH. Increased serum leptin concentrations correlate with soluble tumour necrosis factor receptor levels in patients with cirrhosis. Clin Endocrinol (Oxf). 2002;57:805-811. |
| 22. | Testa R, Franceschini R, Giannini E, Cataldi A, Botta F, Fasoli A, Tenerelli P, Rolandi E, Barreca T. Serum leptin levels in patients with viral chronic hepatitis or liver cirrhosis. J Hepatol. 2000;33:33-37. |
| 23. | Comlekci A, Akpinar H, Yesil S, Okan I, Ellidokuz E, Okan A, Ersoz G, Tankurt E, Batur Y. Serum leptin levels in patients with liver cirrhosis and chronic viral hepatitis. Scand J Gastroenterol. 2003;38:779-786. |
| 24. | Greco AV, Mingrone G, Favuzzi A, Capristo E, Gniuli D, Addolorato G, Brunani A, Cavagnin F, Gasbarrini G. Serum leptin levels in post-hepatitis liver cirrhosis. J Hepatol. 2000;33:38-42. |
| 25. | Siawrys G, Kaminski T, Smolinska N, Przala J. Expression of leptin and long form of leptin receptor genes and proteins in pituitary of cyclic and pregnant pigs. J Physiol Pharmacol. 2007;58:845-857. |
| 26. | Wang XJ, Yuan SL, Lu Q, Lu YR, Zhang J, Liu Y, Wang WD. Potential involvement of leptin in carcinogenesis of hepatocellular carcinoma. World J Gastroenterol. 2004;10:2478-2481. |
| 27. | Daschner M, Tonshoff B, Blum WF, Englaro P, Wingen AM, Schaefer F, Wuhl E, Rascher W, Mehls O. Inappropriate elevation of serum leptin levels in children with chronic renal failure. European Study Group for Nutritional Treatment of Chronic Renal Failure in Childhood. J Am Soc Nephrol. 1998;9:1074-1079. |
| 28. | Fujioka K, Patane J, Lubina J, Lau D. CSF leptin levels after exogenous administration of recombinant methionyl human leptin. JAMA. 1999;282:1517-1518. |
| 29. | Elinav E, Abd-Elnabi A, Pappo O, Bernstein I, Klein A, Engelhardt D, Rabbani E, Ilan Y. Suppression of hepatocellular carcinoma growth in mice via leptin, is associated with inhibition of tumor cell growth and natural killer cell activation. J Hepatol. 2006;44:529-536. |
| 30. | Asterholm IW, Halberg N, Scherer PE. Mouse Models of Lipodystrophy Key reagents for the understanding of the metabolic syndrome. Drug Discov Today Dis Models. 2007;4:17-24. |
| 31. | Diano S, Horvath TL. Anticonvulsant effects of leptin in epilepsy. J Clin Invest. 2008;118:26-28. |