Published online May 14, 2008. doi: 10.3748/wjg.14.2894
Revised: March 15, 2008
Published online: May 14, 2008
AIM: To investigate the effect of herbal compound 861 (Cpd861) on the transforming growth factor-β1 (TGFβ1)/activin receptor-like kinase 1 (ALK1, type I receptor) signaling-pathway-related gene expression in the LX-2 cell line, and the inhibitory mechanism of Cpd861 on the activation of LX-2 cells.
METHODS: LX-2 cells were treated with TGFβ1 (5 ng/mL) Cpd861 (0.1 mg/mL), TGFβ1 (5 ng/mL) plus Cpd861 (5 ng/mL) for 24 h to investigate the effect of Cpd861 on the TGFβ1/ALK1 pathway. Real-time PCR was performed to examine the expression of α-SMA (α-smooth muscle actin), ALK1, Id1 (inhibitor of differentiation 1). Western blotting was carried out to measure the levels of α-SMA and phosphorylated Smad1, and immunocytochemical analysis for the expression of α-SMA.
RESULTS: In LX-2 cells, TGFβ1/ALK1-pathway-related gene expression could be stimulated by TGFβ1, which led to excessive activation of the cells. Cpd861 decreased the activation of LX-2 cells by reducing the expression of α-SMA mRNA and protein expression. This effect was related to inhibition of the above TGFβ1/ALK1-pathway-related expression of genes such as Id1 and ALK1, and phosphorylation of Smad1 in LX-2 cells, even with TGFβ1 co-treatment for 24 h.
CONCLUSION: Cpd861 can restrain the activation of LX-2 cells by inhibiting the TGFβ1/ALK1/Smad1 pathway.
- Citation: Li L, Zhao XY, Wang BE. Down-regulation of transforming growth factor β1/activin receptor-like kinase 1 pathway gene expression by herbal compound 861 is related to deactivation of LX-2 cells. World J Gastroenterol 2008; 14(18): 2894-2899
- URL: https://www.wjgnet.com/1007-9327/full/v14/i18/2894.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2894
Hepatic fibrosis is a reversible scarring process that is characterized by increased and altered deposition of extracellular matrix in the liver. During the past 20 years, much research has proved that the activation of hepatic stellate cells (HSCs) triggers fibrogenesis[1–3] and TGFβ1 has a pivotal regulating role in this process[145]. Currently, TGFβ1/ALK1/Smad1 signaling is found in HSCs and is involved in their activation[6–8].
Herbal compound 861 (Cpd861) is a traditional Chinese medicines that is used to treat liver diseases and has been demonstrated to have anti-fibrotic effects and to reverse cirrhosis, especially in the early stage[9–11]. Cpd861 is an extract of 10 herbs. A randomized, double-blinded, placebo-controlled clinical trial has demonstrated that Cpd861 can significantly delay and reverse the process of clinical hepatic fibrosis in patients with liver fibrosis and early cirrhosis due to HBV infection, as diagnosed with liver biopsy performed before and after treatment[1112].
Our previous studies have shown that Cpd861 can inhibit the activation of HSCs to exert its anti-fibrotic effect[13]. In this study, we selected the TGFβ1/ALK1/Smad1 signaling pathway in an attempt to elucidate the molecular mechanism of Cpd861 in the deactivation of LX-2 cells. The effect of Cpd861 on the expression of genes in this pathway, such as ALK1, Id1 and the protein level of phosphorylated Smad1 was investigated.
LX-2 cells were a gift from Dr. Friedman of Mount Sinai School of Medicine, New York, USA. They are activated human HSCs that are generated by spontaneous immortalization in low-serum conditions[14]. Cpd861 powder (Radix Salviae Miltiorrhiae, Radix Astragali, Suberect Spatholobus, Flos Carthami, Rhizoma Chuanxiong, Radix Paeoniae Rubra, Rhizoma Cyperi, Pericarpium Citri Reticulatae, Radix Angelicae Sinensis, Radix bupleuri, patent No. 99103265.9) was from Jiangyin Pharmaceutical Company, JiangSu Province, China. Powder (2500 mg) was dissolved in 100 mL PBS, and the final concentration was 0.1 mg/mL in LX-2 cell-culture medium. After centrifugation at 3000 r/min for 10 min, the solution was sterilized at 105°C for 20 min. Dulbecco’s modified Eagle’s medium (DMEM), L-glutamine, streptomycin and fetal bovine serum (FBS) were purchased from Gibco, NY, USA. Penicillin was from Sigma, St. Louis, USA. Oligo (dT) primers, M-MLV (Moloney murine leukemia virus) reverse transcriptase, recombinant RNasin ribonuclease inhibitor and dNTP were acquired from Promega (Madison, WI, USA). TRIzol reagent was from Invitrogen, Carlsbad, CA, USA. Power SYBR Green PCR Master Mix was purchased from Applied Biosystems, Warrington, UK. The CO2 incubator was from SANYO, Sakata, Japan. Cell culture wells (6 cm2) were from Corning Incorporation, NY, USA. Phospho-Smad1 antibody (60 kDa) was from Cell Signaling Company, Danvers, MA, USA. Mouse anti-human α-SMA (42 kDa) was from Zhongshan Goldenbridge Biotechnology, Beijing, China.
LX-2 cells were cultivated in DMEM supplemented with 50 mL/L FBS, 200 mmol/L L-glutamine, penicillin G (100 U/mL) and streptomycin (100 U/mL) in a humidified incubator at (37°C, 5% CO2). Cells were cultivated in 25 cm2 culture flasks to 70% confluence or on glass coverslips in six-well culture dishes to 50% confluence. After serum starvation for 16 h, LX-2 cells were treated with Cpd861 0.1 mg/mL[13], TGFβ1 (5 ng/mL)[15] and TGFβ1 (5 ng/mL) plus Cpd861 0.1 mg/mL. Untreated cells served as a control group. All four groups were harvested after 24 h treatment to investigate the related gene expression and phosphorylation of Smad1 protein. Cells on glass coverslips in six-well culture dishes were fixed in 4% paraformaldehyde in PBS after 24 h for immunocytochemical analysis. Every group included six samples.
Cells were fixed in 4% paraformaldehyde and permeabilized with PBS containing 0.1% Triton X-100 for 15 min. They were incubated with blocking solution (5% Bovine Serum Albumin in PBS) for 30 min. Primary antibodies (mouse anti-human α-SMA, diluted 1:100 in blocking solution) were incubated with the cells overnight. After three washes with PBST (0.2% Tween 20 in PBS) for 15 min, cells were incubated with the secondary antibody (goat anti-mouse IgG labeled with biotin, with streptavidin labeled with horseradish peroxidase), and then with DAB (Diaminobenzidine), Beijing Zhongshan Goldenbridge Co.). PBS (0.01 mol/L) was substituted for primary antibody as a negative control. The α-SMA positive cells presented as brownish yellow. Cells were counterstained with hematoxylin to identify nuclei. Cells were viewed with an Olympus DP71 microscope (Tokyo, Japan) at 20 × magnification.
Total RNA was extracted from LX-2 cells using TRIzol reagent as the lysis buffer. cDNA was synthesized using the Reverse Transcription System (Promega). RNA (1 &mgr;g) in 7.7 &mgr;L nuclease-free water was added to 2.5 &mgr;L 10× transcriptase buffer, 2.5 &mgr;L 10 mmol/L dNTP, 0.5 &mgr;L RNase inhibitor, and 1 &mgr;L M-MLV reverse transcriptase. The reaction was performed for 60 min at 42°C (cDNA synthesis), and 5 min at 95°C (enzyme denaturation).
The cDNA samples were analyzed with the Applied Biosystems 7300/7500 real-time PCR system (Applied Biosystems, Foster city, CA, USA) for detecting the gene expression of α-SMA, ALK1 and Id1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. The primer sequences were obtained from SaiBaiSheng Biocompany, Beijing, China (Table 1). Each experiment was performed in 20 &mgr;L reaction volume. The PCR program consisted of an initial activation step at 50°C for 2 min, followed by 95°C for 10 min, then 40 cycling steps of denaturing for 15 s at 95°C, annealing and extension at 60°C for 1 min (data collected at this stage). The PCR data were analyzed using SDS 2.1 software (Applied Biosystems). mRNA levels were normalized relative to GAPDH values. Fold expression changes and standard errors were calculated using the equation 2-ΔΔCt (Ct, threshold cycle). Each group had six wells of cells for examining the relative fold change of gene expression. Three replicate reactions per sample and endogenous control were used to ensure statistical significance.
| Gene | Oligonucleotide primer | Fragment sequence size (bp) |
| α-SMA (target) | ||
| Sense | CGCATCCTCATCCTCCCT | 268 |
| Anti-sense | GGCCGTGATCTCCTTCTG | |
| ALK1 (target) | ||
| Sense | AGACCCCCACCATCCCTA | 67 |
| Anti-sense | CGCATCATCTGAGCTAGG C | |
| Id1 (target) | ||
| Sense | CCAGAACCGCAAGGTGAG | 62 |
| Anti-sense | GGTCCCTGATGTAGTCGATGA | |
| Gapdh (household) | ||
| Sense | GGCTCTCCAGAACATCATCC | 187 |
| Anti-sense | GCTTCACCACCTTCTTGATG |
Cells were lysed on ice by 80 &mgr;L lysis buffer for 30 min. The cell lysate was centrifuged at 10 000 r/min for 10 min and the supernatant was collected for Western blot analysis. Protein concentration was measured using BCA Protein Assay kit (Pierce Company, Rockford, IL, USA) following the manufacturer’s instructions. Protein samples (40 &mgr;g/lane) were subjected to 10% SDS-PAGE and then transferred onto a PVDF nitrocellulose membrane by electro-blotting. The membrane was incubated in 25 mL blocking buffer for 1 h at room temperature. After blocking, the membrane was incubated in 10 mL blocking buffer containing 1:1000 dilution of rabbit anti-phospho-Smad1 or anti-SMA at 4°C overnight. After gentle washing with blocking buffer, the membrane was incubated in rabbit anti-β-actin polyclonal antibody (1:200 dilution). After vigorous washing, the membrane was incubated with a 1:5000 dilution of horseradish peroxidase-conjugated anti-rabbit IgG antibody. The intensity of the bands was determined by scanning video densitometry. The levels of phospho-Smad1 and SMA were normalized to the level of β-actin protein. Six independent experiments were performed. The intensity of the bands was determined using Gel-Pro Analyzer Version 3.0 (Media Cybernetics, Silver Spring, MD, USA).
Data were expressed as mean ± SD and the statistical significance was assessed by one-way analysis of variance followed by Student-Newman-Keul tests. P < 0.05 was considered to be significant.
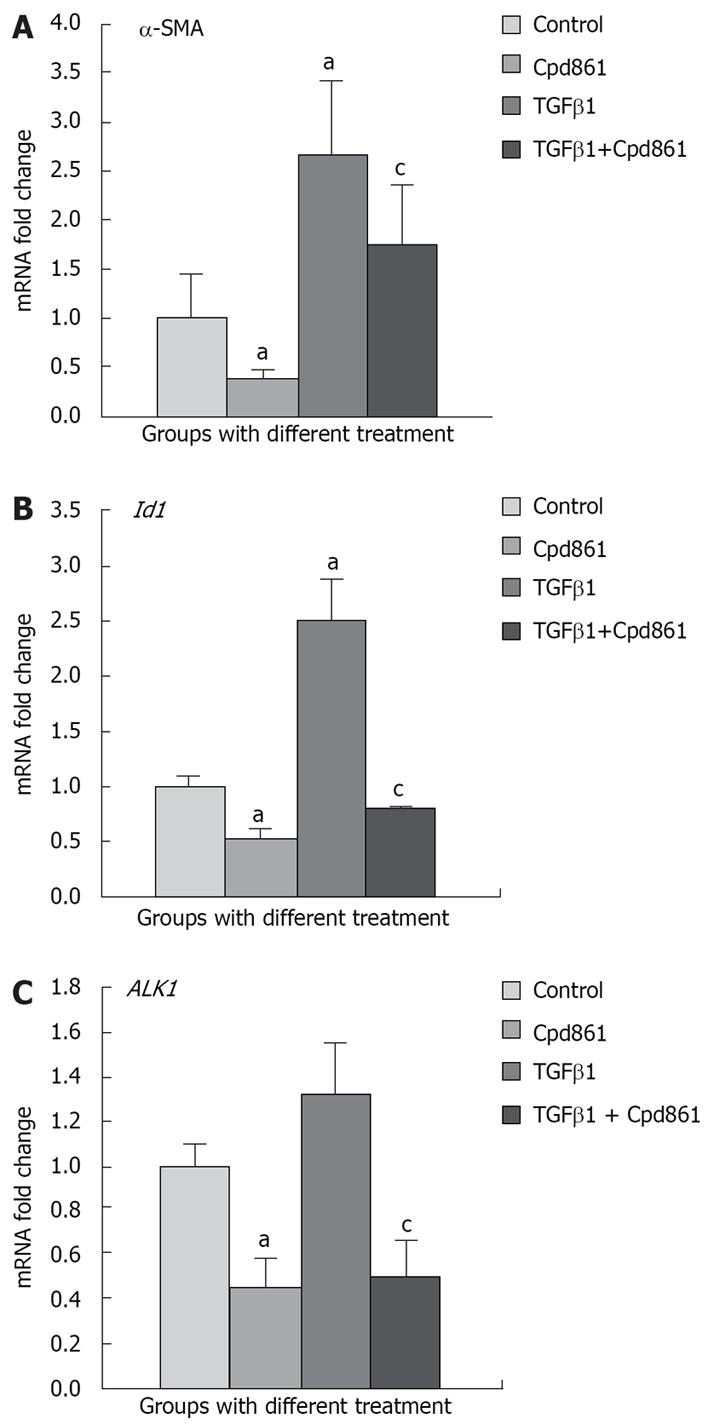
Changes in gene or protein expression of α-SMA are often used to study the extent of HSC activation[1617]. In this study, the effect of TGFβ1 and Cpd861 on the activation of the LX-2 cell line was investigated by examining changes in gene and protein expression of α-SMA.
Real-time PCR analysis showed that after exogenous TGFβ1 (5 ng/mL) stimulation for 24 h, α-SMA mRNA expression reached 2.65 fold (P < 0.05) compared with that in control LX-2 cells, whereas Cpd861 inhibited the expression to 0.38-fold (24 h, P < 0.05) (Figure 1A). α-SMA mRNA expression in Cpd861 and TGFβ1 co-treated cells was 1.73-fold compared to that in the controls.
Western blot analysis confirmed the trend of α-SMA gene expression changes (Figure 2A and B). In the TGFβ1 treatment group, α-SMA protein expression increased significantly compared with that in the controls (photodensity: 0.49 in TGFβ1 group and 0.34 in control respectively), whereas its expression was inhibited by Cpd861 (0.19 vs 0.49), even in the presence of TGFβ1 (0.20 vs 0.34) (Figure 2).
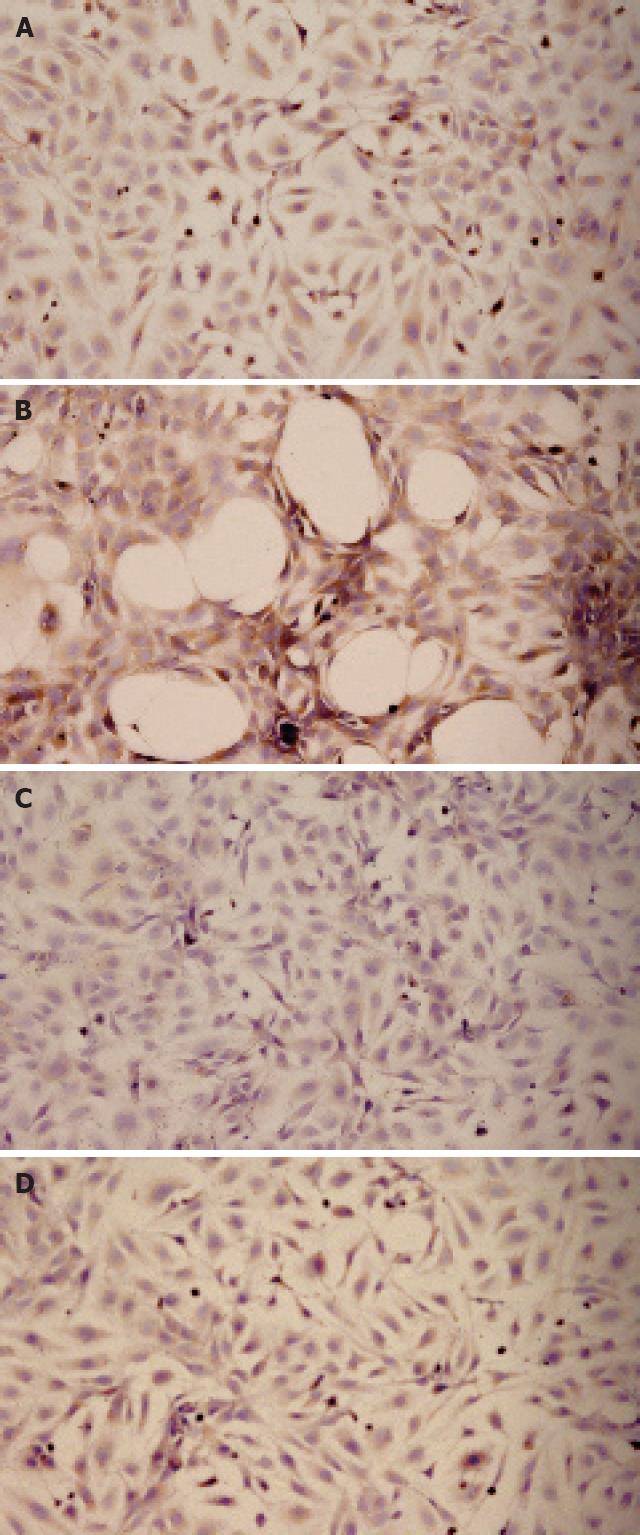
Immunocytochemical analysis showed that α-SMA protein was expressed in the cytoplasm, which was stained brown in low-power fields (Figure 3A). An interesting phenomenon was that after TGFβ1 treatment for 24 h, many various-sized, round spaces were present. Cells around the spaces were fusiform in shape, with dark brown cytoplasm. We presumed that it was a result of contraction of adjacent cells, which acquired the contractive property from excessive activation (Figure 3B). Cells treated with Cpd861, however, had a lighter brown cytoplasm (Figure 3C). In the Cpd861 and TGFβ1 co-treatment group, the cytoplasm presented lighter brown than in the TGFβ1-treated group and no spaces were seen (Figure 3D).
These results confirmed that gene and protein expression and function of α-SMA in LX-2 cells, markers of activation of LX-2 cells, were enhanced by TGFβ1 and inhibited by Cpd861. This is consisted with a previous study, which showed that Cpd861 can inhibit activation of LX-2 cells[13].
TGFβ1/ALK1/Smad1 signaling has been found in HSCs[1819]. In this pathway, ALK1 phosphorylated Smad1 leads to gene expression of Id1[20]. Idl proteins have a helix-loop-helix (HLH) domain but lack ability to bind DNA that acts as an important dominant negative antagonist of the bHLH family of transcription factors[2122]. Recently, the Id1 gene was identified as a novel target gene that promotes the expression and polymerization of α-SMA, therefore involving the transdifferentiation of HSCs.
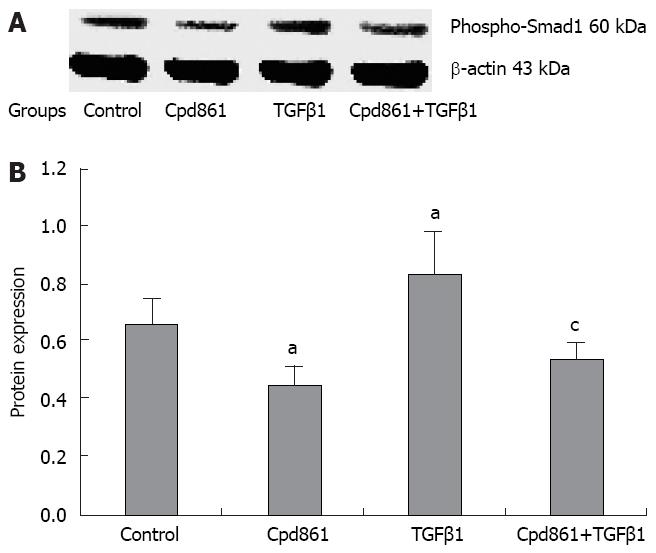
Here, we examined the level of phosphorylated Smad1 (the marker of activation of the pathway) using Western blot analysis. The level of phosphorylated Smad1 increased after TGFβ1 treatment and was reduced by Cpd861 or TGFβ1 and Cpd861 co-treatment (photodensity was 0.66 in control, 0.45 in Cpd861-treated cells, 0.84 in TGFβ1-treated cells, and 0.55 in TGFβ1 and Cpd861 co-treated cells, Figure 4).
Stimulation of LX-2 cells with exogenous TGFβ1 strongly induced Id1 gene expression by 2.5-fold, whereas Cpd861 reduced Id1 expression by 0.53-fold (P < 0.05). The fold change of Id1 expression in Cpd861 and TGFβ1 co-treatment was 0.79 compared with that in the controls, but it was significantly lower than that with TGFβ1 treatment alone (Figure 1B).
The ALK1 gene was constitutively expressed in LX-2 cells and TGFβ1 did not further increase its expression, but Cpd861 reduced it significantly (Figure 2C). The change in gene expression of the receptor might be an important inhibitory mechanism of Cpd861 in the pathway.
Our data indicated that the TGFβ1/ALK1/Smad1 pathway could be activated by TGFβ1 and abrogated by Cpd861 in LX-2 cells. The TGFβ1/ALK1/Smad1 pathway is one of the TGFβ superfamily signaling branches[23]. Several studies have indicated that activation of the pathway might play an important role in development of organ fibrosis[24–27]. Recent research has demonstrated that the existence of ALK1 and the TGFβ1/ALK1/Smad1 pathway is critical during the transdifferentiation process of primary rat HSCs to myofibroblast (MFB)[20]. The present study demonstrated that activation of the TGFβ1/ALK1/Smad1 pathway may be enhanced by TGFβ1 in LX-2 cells. It was shown that Cpd861 inhibited activation of the TGFβ1/ALK1/Smad1 pathway, and this might have been mediated by reducing ALK1 expression. However, effects of Cpd861 on ALK1 protein expression and on the affinity of TGFβ1 for ALK1 need further investigation.
Cpd861 has been demonstrated to be effective for treatment of patients with hepatic fibrosis[28]. Previous studies have shown that Cpd861 has multiple anti-fibrotic mechanisms, such as stimulating apoptosis of activated HSCs[29], down-regulating tissue inhibitor of metalloproteinase 1 gene expression[30], reducing expression of collagen and fibrosis-related cytokines. This study presented another possible anti-fibrotic mechanism of Cpd861, and also showed that the TGFβ1/ALK1/Smad1 pathway may represent a potential target for antifibrotic therapy. However, which components in the compound play the inhibitory roles needs to be further investigated.
Activation of hepatic stellate cells (HSCs) triggers fibrogenesis and TGFβ1/ALK1/Smad1 signaling has been shown to be involved in the transdifferentiation of HSCs. Herbal compound 861 (Cpd861) can inhibit activation of HSCs to exert its anti-fibrotic effect, but the mechanism is not very clear.
The effect of TGFβ1/ALK1/Smad1 signaling on the activation of HSCs has recently been reported. This study chose the pathway as a means to establishing the molecular mechanism of Cpd861 in the deactivation of LX-2 cells.
This is believed to be the first attempt to describe the molecular mechanism of the traditional herbal medicine Cpd861, which has been demonstrated to have anti-fibrotic effects and to reverse liver disease caused by HBV infection. This is believed to be the first time that TGFβ1/ALK1/Smad1 has been selected as a pathway to study the effects of deactivation of LX-2 HSCs.
This study presents another possible anti-fibrotic mechanism of Cpd861 and shows that the TGFβ1/ALK1/Smad1 pathway may represent a potential target for the anti-fibrotic therapy.
ALK1 is activin receptor-like kinase 1, a type I receptor, which is typically activated by the bone morphogenetic proteins, and then phosphorylates Smad1, Smad5 and Smad8 for signal transduction. Id1 is inhibitor of differentiation 1, which has an helix-loop-helix (HLH) domain but lacks a DNA-binding domain. It was first shown to act as a dominant negative antagonist of the basic HLH family of transcription factors, which positively regulate differentiation in many cell lineages. It is an important part of signaling pathways involved in development, cell cycle and tumorigenesis.
This study demonstrates that Cpd861 can down-regulate the TGFβ1/ALK1 pathway, which results in the deactivation of HSCs. The study presents novel findings on the anti-fibrotic mechanism of Cpd861 and its possible application in anti-fibrotic therapy.
| 1. | Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76-99. |
| 2. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. |
| 3. | Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427-436. |
| 4. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. |
| 5. | Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22 Suppl 1:S79-S84. |
| 6. | Ouyang XS, Wang X, Ling MT, Wong HL, Tsao SW, Wong YC. Id-1 stimulates serum independent prostate cancer cell proliferation through inactivation of p16(INK4a)/pRB pathway. Carcinogenesis. 2002;23:721-725. |
| 7. | Lindert S, Wickert L, Sawitza I, Wiercinska E, Gressner AM, Dooley S, Breitkopf K. Transdifferentiation-dependent expression of alpha-SMA in hepatic stellate cells does not involve TGF-beta pathways leading to coinduction of collagen type I and thrombospondin-2. Matrix Biol. 2005;24:198-207. |
| 8. | Shen H, Fan J, Burczynski F, Minuk GY, Cattini P, Gong Y. Increased Smad1 expression and transcriptional activity enhances trans-differentiation of hepatic stellate cells. J Cell Physiol. 2007;212:764-770. |
| 9. | Jia JD, Bauer M, Cho JJ, Ruehl M, Milani S, Boigk G, Riecken EO, Schuppan D. Antifibrotic effect of silymarin in rat secondary biliary fibrosis is mediated by downregulation of procollagen alpha1(I) and TIMP-1. J Hepatol. 2001;35:392-398. |
| 10. | Kayano K, Sakaida I, Uchida K, Okita K. Inhibitory effects of the herbal medicine Sho-saiko-to (TJ-9) on cell proliferation and procollagen gene expressions in cultured rat hepatic stellate cells. J Hepatol. 1998;29:642-649. |
| 11. | Yin SS, Wang BE, Wang TL, Jia JD, Qian LX. [The effect of Cpd 861 on chronic hepatitis B related fibrosis and early cirrhosis: a randomized, double blind, placebo controlled clinical trial]. Zhonghua Ganzangbing Zazhi. 2004;12:467-470. |
| 12. | Wang BE. Treatment of chronic liver diseases with traditional Chinese medicine. J Gastroenterol Hepatol. 2000;15 Suppl:E67-E70. |
| 13. | Wang L, Wang J, Wang BE, Xiao PG, Qiao YJ, Tan XH. Effects of herbal compound 861 on human hepatic stellate cell proliferation and activation. World J Gastroenterol. 2004;10:2831-2835. |
| 14. | Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142-151. |
| 15. | Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000;31:1094-1106. |
| 16. | Abergel A, Sapin V, Dif N, Chassard C, Darcha C, Marcand-Sauvant J, Gaillard-Martinie B, Rock E, Dechelotte P, Sauvant P. Growth arrest and decrease of alpha-SMA and type I collagen expression by palmitic acid in the rat hepatic stellate cell line PAV-1. Dig Dis Sci. 2006;51:986-995. |
| 17. | Pan Q, Li DG, Lu HM, Wang YQ, Zhang WZ, Xu QF. A new immortalized rat cell line, hepatic stellate cell-PQ, exhibiting characteristics of hepatic stellate cell. Hepatobiliary Pancreat Dis Int. 2005;4:281-284. |
| 18. | Shen H, Huang GJ, Gong YW. Effect of transforming growth factor beta and bone morphogenetic proteins on rat hepatic stellate cell proliferation and trans-differentiation. World J Gastroenterol. 2003;9:784-787. |
| 19. | Shen H, Huang G, Hadi M, Choy P, Zhang M, Minuk GY, Chen Y, Gong Y. Transforming growth factor-beta1 downregulation of Smad1 gene expression in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G539-G546. |
| 20. | Wiercinska E, Wickert L, Denecke B, Said HM, Hamzavi J, Gressner AM, Thorikay M, ten Dijke P, Mertens PR, Breitkopf K. Id1 is a critical mediator in TGF-beta-induced transdifferentiation of rat hepatic stellate cells. Hepatology. 2006;43:1032-1041. |
| 21. | Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol. 2003;13:410-418. |
| 22. | Damdinsuren B, Nagano H, Kondo M, Yamamoto H, Hiraoka N, Yamamoto T, Marubashi S, Miyamoto A, Umeshita K, Dono K. Expression of Id proteins in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Int J Oncol. 2005;26:319-327. |
| 23. | Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187:265-276. |
| 24. | Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573-3584. |
| 25. | Abe H, Matsubara T, Iehara N, Nagai K, Takahashi T, Arai H, Kita T, Doi T. Type IV collagen is transcriptionally regulated by Smad1 under advanced glycation end product (AGE) stimulation. J Biol Chem. 2004;279:14201-14206. |
| 26. | Takahashi T, Abe H, Arai H, Matsubara T, Nagai K, Matsuura M, Iehara N, Yokode M, Nishikawa S, Kita T. Activation of STAT3/Smad1 is a key signaling pathway for progression to glomerulosclerosis in experimental glomerulonephritis. J Biol Chem. 2005;280:7100-7106. |
| 27. | Matsubara T, Abe H, Arai H, Nagai K, Mima A, Kanamori H, Sumi E, Takahashi T, Matsuura M, Iehara N. Expression of Smad1 is directly associated with mesangial matrix expansion in rat diabetic nephropathy. Lab Invest. 2006;86:357-368. |
| 28. | Wang BE, Wang TL, Jia JD, Ma H, Duan ZP, Li XM, Li J, Wang AM, Qian LX. Experiment and clinical study on inhibition and reversion of liver fibrosis with integrated Chinese and Western Medicine. CJIM. 1999;5:6-11. |
| 29. | You H, Wang B, Wang T. [Proliferation and apoptosis of hepatic stellate cells and effects of compound 861 on liver fibrosis]. Zhonghua Ganzangbing Zazhi. 2000;8:78-80. |
| 30. | Yin C, Ma H, Wang A, Ma X, Jia J, Wang B. [Effect of compound 861 on tissue inhibitor of metalloprotenase 1 gene expression of HSC-T6 cells]. Zhonghua Ganzangbing Zazhi. 2002;10:197-199. |