Published online Apr 14, 2008. doi: 10.3748/wjg.14.2213
Revised: February 10, 2008
Published online: April 14, 2008
AIM: To investigate the significance of Thrombospondin-1 (TSP-1) expression and its relationship with angiogenesis during experimental fibrosis.
METHODS: Cirrhosis was induced in male Wistar rats by intraperitoneal administration of diethyl nitrosamine (DEN). The serial sections from liver tissues were stained with anti-CD34 and anti-TSP-1 antibodies before being quantitated by light microscopy.
RESULTS: Our results showed that of TSP-1 expression gradually increases according to the severity of fibrosis (GroupIvs group II, Group III and Group IV; Group II vs group III and group IV; group III vs group IV, P < 0.05). Moreover, TSP-1 expression was found to be correlated with angiogenesis (P < 0.05).
CONCLUSION: The correlative evidence of the link between TSP-1 and fibrosis or angiogenesis provided by this study suggests that besides its role as a strong promoter of transforming growth factor-β1 (TGF-β1), TSP-1 might have an additional role in liver fibrogenesis by stimulating angiogenesis and this protein could be a potential target to prevent fibrogenesis in chronic inflammatory diseases of the liver.
- Citation: Elpek G&, Gökhan GA, Bozova S. Thrombospondin-1 expression correlates with angiogenesis in experimental cirrhosis. World J Gastroenterol 2008; 14(14): 2213-2217
- URL: https://www.wjgnet.com/1007-9327/full/v14/i14/2213.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2213
Hepatic angiogenesis is frequently associated with inflammation and fibrogenesis during chronic liver injury[1–5]. Currently, it is not clear whether this process plays a beneficial role in the maintenance of homeostasis or contributes to liver damage during chronic inflammation. However, the fact that chronic inflammatory liver diseases respond poorly to immunosuppressive and anti-inflammatory therapy suggests that angiogenesis might be a promising therapeutic target in the prevention of fibrosis[6–8]. For this reason, attempts are being directed to evaluate the cellular and molecular mechanisms involved in the development of hepatic angiogenesis during chronic liver injury[3–5].
Thrombospondin 1 (TSP-1), one of the five members of the Thrombospondin gene family, is a matrix protein involved in complex processes including wound healing and angiogenesis[910]. The exact role of TSP-1 in angiogenesis is still controversial. TSP-1 can function as an inhibitor or as a promoter of angiogenesis, indicating that it might modulate this process in opposite directions[11–16]. In malignant and premalignant conditions of the liver, TSP-1 expression and its association with angiogenesis have been demonstrated[15–18]. Regarding non-neoplastic liver diseases, although the association of TSP-1 with latent transforming growth factor-β1 (TGF-β1) has been demonstrated in a few studies, the relationship between TSP-1 and angiogenesis during liver fibrogenesis has not been documented[19–21].
Therefore, this study was undertaken to investigate the significance of TSP-1 expression during diethyl nitrosamine (DEN) induced experimental liver fibrosis and to evaluate whether any relationship exists between TSP-1 expression and angiogenesis.
This animal study was approved by the local animal ethics committee of the Akdeniz University. Male adult Wistar rats weighing 250 g were used. They were maintained on a commercial diet and water in a room at 22 ± 2°C under normal laboratory lighting conditions.
Animal model: The rats received intra-peritoneal injections of DEN (Sigma, Saint Quentin Fallavier, France) at 100 mg/kg of body weight (n: 29) or 0.9% sodium chloride (n: 8) once a week. The injections were performed for 2 (n: 4), 4 (n: 5), 5 (n: 5), 6 (n: 5), 8 (n: 5) and 10 (n: 5) wk. The animals were sacrificed 2 wk after the last administrations and a hepatectomy was performed. Liver tissue samples were either frozen immediately in liquid nitrogen and stored at -70°C or fixed in 10% buffered formalin and embedded in paraffin.
Histology and immunohistochemistry: Four micro-meter thick serial sections from the liver tissues originally fixed in formalin and embedded in paraffin were prepared and stained with hematoxylin and eosin for the histopathological assessment. Masson trichrome staining was used in the evaluation of the extent of liver fibrosis.
Immunolabeling was performed using polyclonal antibodies directed anti rat CD34 (sc- 7045 goat, dilution: 1:500, Santa Cruz, CA, USA) and thrombospondin-1 (sc-12312 goat, IgG, dilution 1:200, Santa Cruz, CA, USA). An avidin-biotin-peroxidase technique (sc-2023, anti-goat ABC staining Kit; Santa Cruz, CA, USA) was used for labeling. For CD34, sections from paraffin embedded tissue blocks were dried in a hot air oven at 55°C overnight and dewaxed. Microwave antigen retrieval (750 W, 4 × 5 min in citrate buffer 0.01 mol/L, pH 6) was performed. TSP-1 staining was applied to 5 &mgr;m thick air dried (30 min) cryostat sections, fixed in acetone (10 min). Endogenous peroxidase was blocked by using 3% hydrogen peroxide in methanol for 30 min. Each step of incubation was followed thorough washing of the slides in phosphate buffered saline (PBS). After incubation with primary antibody against CD34 and TSP-1 (each 30 min), sections were reacted with secondary biotinylated antibody (30 min) and AB enzyme reagent (avidin and biotinylated horseradish peroxidase) for 30 min. Finally, all slides were treated with DAB reagent to develop color and counterstained with Mayer’s hematoxylin. Normal goat and rabbit IgG instead of primary antibodies were used as negative control at the same dilution.
The vascular density in portal and periportal areas was assessed by determining the count of CD34 labeled vessel sections at higher magnification (× 400) with the use of an ocular grid subdivided into 100 areas. For each subject vascular density (VD) was noted.
For quantitative evaluation of TSP-1 expression, in each section positive and negative cells were counted in systematically randomly selected 10 to 15 microscopic fields by using an ocular grid at high magnification (× 400). The positive staining was calculated as the percentage of positive cells to total number of counted cells. Positive cells touching the left and lower edge of the grid were not included.
All analysis were performed using Statistical Package for Social Science (SPSS 15.0 for Windows, USA). Mann-Whitney-U test was used to establish the difference between groups. Friedman test was used to determine the relationship between quantitative parameters. Data were expressed as mean ± SD and P < 0.05 was considered significant.
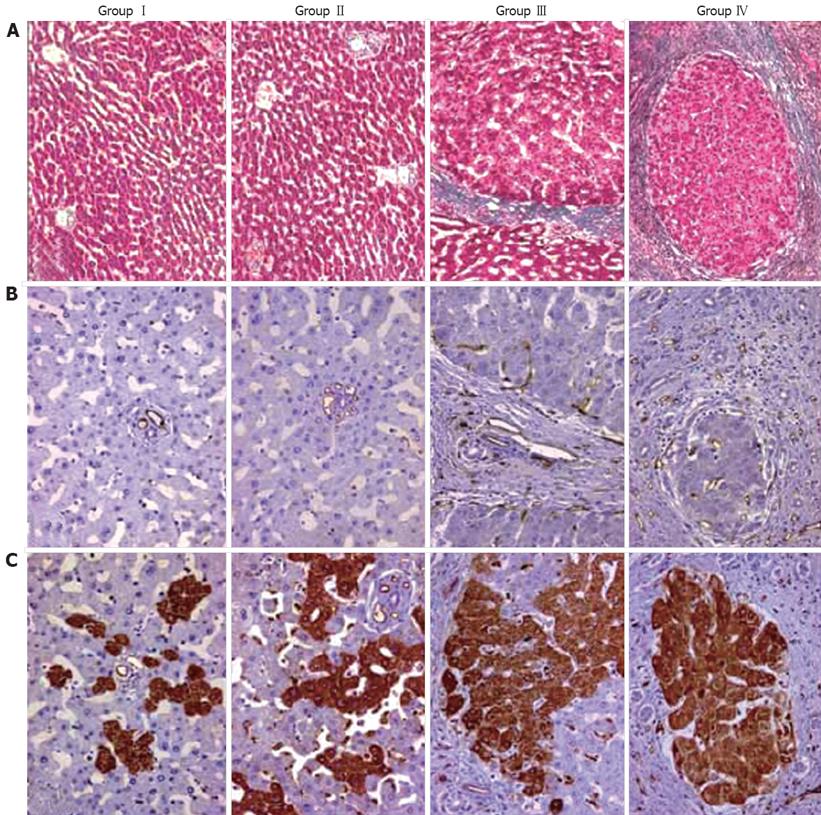
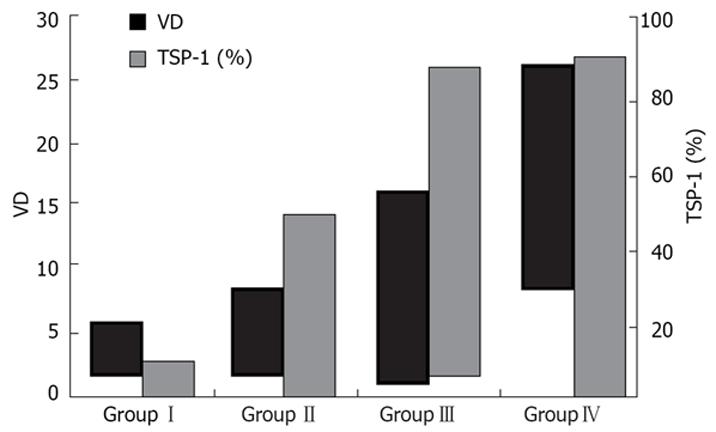
In this study, fibrogenesis was not observed in the control group. In DEN treated rats, fibrous septa were detected after 5 wk. The liver was cirrhotic in all cases after 8 wk. According to the severity of fibrosis, cases were divided in following groups: GroupI: normal livers, group II: non-fibrotic livers (2 and 4 wk), group III: fibrotic livers (5 and 6 wk) and group IV: cirrhotic livers (8 and 10 wk) (Figure 1A). In groupI, CD34 staining was restricted to the endothelium of portal vessels. While in non-fibrotic livers CD34 expression was noted in a few vascular structures around portal areas, numerous CD34-labeled vessels were detected in fibrotic livers. In group IV, CD34 staining revealed a dense vascular plexus surrounding the cirrhotic nodules (Figure 1B). Parallel to this finding, VD values were increased together with the progression of fibrosis (Figure 2). DEN-treated cases (group II, III and IV) had higher VD than the control group (P < 0.05). The difference between VD values of group II, III and IV was also statistically significant (P < 0.05) (Figure 2 and Table 1).
In normal livers (groupI), TSP-1 expression was restricted to the endothelium of portal vessels and to a few hepatocytes (Figure 1C). However, in non-fibrotic group TSP-1 expression was higher than normal livers with more positive hepatocytes and perisinusoidal cells (P < 0.05). TSP-1 expression continued to increase in fibrotic livers and was more widespread in cirrhotic livers. The expression of TSP-1 in DEN-treated rat groups was significantly different from each other (P < 0.05) (Figure 1C, Figure 2 and Table 1).
Friedman test showed that there was a significant correlation between VD and TSP-1 expression (P < 0.05).
Results of the recent studies emphasized that hepatic angiogenesis is associated with fibrogenesis in the wound healing response to chronic liver injury[1–5]. In our study, parallel to this finding, angiogenesis, assessed as VD, was increased with the progression of fibrosis (P < 0.05). Besides, in group II, despite the absence of overt fibrosis, VD was higher than that of normal livers, suggesting that angiogenesis is an early event which might take place before the onset of fibrosis during chronic liver damage.
It is well known that angiogenesis does not involve a single pathway, but is a complex event regulated by many angiogenic and antiangiogenic factors, including TSP-1[910]. In neoplastic and premalignant conditions of the liver, the relationship between TSP-1 expression and angiogenesis has been studied[15–17]. However, in non-neoplastic liver diseases the association of TSP-1 expression with angiogenesis and its role in this complex event has not been documented. Because TSP-1 is also a known activator of TGF-β1, a key mediator in tissue fibrogenesis, a few studies has been focused to evaluate the effect of TSP-1 in hepatic activation of TGF-β1[19–21]. It was concluded that TSP-1 may act in the pathogenesis of liver fibrogenesis as a strong promoter of TGF-β1. Although in the present study TGF-β1 expression was not evaluated, we observed an increase of TSP-1 expression parallel to the severity of fibrosis. TSP-1 expression of normal livers was restricted to the endothelium of portal vessels and to a few hepatocytes. However, this value was 3.61 fold higher in non-fibrotic group. The percentage of TSP-1 expressing cells continued to increase in fibrotic and cirrhotic livers (P < 0.05). The present data support the contribution of TSP-1 expression in the wound healing response to chronic liver injury[19–21]. Moreover, in this study, a strong correlation between TSP-1 expression and angiogenesis was observed (P < 0.05). This finding suggests that TSP-1 is not only involved in fibrogenesis by the hepatic activation of TGF-β1 but also might play another role in the remodeling of the liver architecture by contributing to the development of angiogenesis.
Our results showed TSP-1 might be a stimulator of angiogenesis during liver injury. TSP-1 is generally recognized as an antiangiogenic agent[111215]. However results of the some studies have been demonstrated that TSP-1 might be a stimulator of angiogenesis[13–16]. For this reason the actual role played by TSP-1 in angiogenesis has been investigated in previous studies with several different conclusions. Some studies demonstrated that the effect of TSP-1 may depend on its concentration[1322], the type of domain being activated[23] and the type of receptors present on endothelial cells[24]. It has been also speculated that the actual role of TSP-1 might be related to number of its receptors[25]. Although it is not possible to conclude, based on our findings, which factor determines the angiogenic effect of TSP-1 during chronic liver injury, our data reinforce its dual role in the modulation of angiogenesis in opposite directions.
In conclusion, the results of this descriptive study reveal that in experimental liver fibrogenesis TSP-1 expression gradually increases according to the severity of fibrosis and strongly correlates with angiogenesis. Our data suggest that TSP-1 might contribute to the wound healing response to liver injury not only as a strong promoter of TGF-β1, but also as an inducer of angiogenesis. In the light of this observation, it would be of interest to evaluate the mechanism triggered by TSP-1 in hepatic angiogenesis with further experimental models, in order to completely clarify if TSP-1 could be a potential target in the manipulation of angiogenesis in chronic inflammatory liver diseases ending with cirrhosis.
Angiogenesis progresses together with fibrogenesis in the wound healing response to chronic liver injury. Thrombospondin-1 (TSP-1) is a matricellular protein which is involved in complex processes including wound healing and angiogenesis. TSP-1 might modulate angiogenesis in opposite directions. In malignant and premalignant conditions of the liver the relationship between TSP-1 expression and angiogenesis have been demonstrated. Regarding non-neoplastic liver diseases, although the association of TSP-1 with latent transforming growth factor-β1 (TGF-β1) has been demonstrated in a few studies, the relationship between TSP-1 and angiogenesis during liver fibrogenesis has not been documented.
At present it is not possible to ascertain the exact pathogenic role of angiogenesis in liver fibrogenesis. However, the fact that chronic liver diseases respond poorly to conventional therapies suggests that manipulation of angiogenesis could be a promising approach to treatment. For this reason, the cellular and molecular mechanisms that are involved in the development of angiogenesis during liver fibrogenesis have been a topic of intensive investigations in the recent years.
This study demonstrated that in experimental liver fibrogenesis TSP-1 expression gradually increases according to the severity of fibrosis and strongly correlates with angiogenesis.
Based on the results of this research, TSP-1 might contribute to the wound healing response to liver injury not only as a strong promoter of TGF-β1, but also as an inducer of angiogenesis and could be a potential target in the manipulation of angiogenesis in chronic inflammatory liver diseases ending with cirrhosis.
TSP-1 is a high molecular weight glycoprotein (450 kDa) which is composed of three identical subunits cross-linked by disulfide bonds. Each subunit is composed of several domains interacting with different surface receptors. TSP-1 is involved in various processes such as cell motility, inflammation and wound healing. It also modulates endothelial cell adhesion, motility and growth.
This is quite an interesting investigational paper. This study demonstrated that in experimental liver fibrogenesis, TSP-1 expression gradually increases according to the severity of fibrosis and strongly correlates with angiogenesis. Further study would focus on evaluating the mechanism of TSP-1 in hepatic angiogenesis.
| 1. | Lai WK, Adams DH. Angiogenesis and chronic inflammation; the potential for novel therapeutic approaches in chronic liver disease. J Hepatol. 2005;42:7-11. |
| 2. | Medina J, Arroyo AG, Sanchez-Madrid F, Moreno-Otero R. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185-1195. |
| 3. | Drixler TA, Vogten MJ, Ritchie ED, van Vroonhoven TJ, Gebbink MF, Voest EE, Borel Rinkes IH. Liver regeneration is an angiogenesis- associated phenomenon. Ann Surg. 2002;236:703-711; discussion 711-712. |
| 4. | Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010-1021. |
| 5. | Rosmorduc O, Wendum D, Corpechot C, Galy B, Sebbagh N, Raleigh J, Housset C, Poupon R. Hepatocellular hypoxia-induced vascular endothelial growth factor expression and angiogenesis in experimental biliary cirrhosis. Am J Pathol. 1999;155:1065-1073. |
| 6. | Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Yamazaki M. Vascular endothelial growth factor and receptor interaction is a prerequisite for murine hepatic fibrogenesis. Gut. 2003;52:1347-1354. |
| 7. | Wang YQ, Ikeda K, Ikebe T, Hirakawa K, Sowa M, Nakatani K, Kawada N, Kaneda K. Inhibition of hepatic stellate cell proliferation and activation by the semisynthetic analogue of fumagillin TNP-470 in rats. Hepatology. 2000;32:980-989. |
| 8. | Vogten JM, Drixler TA, te Velde EA, Schipper ME, van Vroonhoven TJ, Voest EE, Borel Rinkes IH. Angiostatin inhibits experimental liver fibrosis in mice. Int J Colorectal Dis. 2004;19:387-394. |
| 9. | Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929-934. |
| 10. | Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol. 2001;17:25-51. |
| 11. | Fontanini G, Boldrini L, Calcinai A, Chine S, Lucchi M, Mussi A, Angeletti CA, Basolo F, Bevilacqua G. Thrombospondins I and II messenger RNA expression in lung carcinoma: relationship with p53 alterations, angiogenic growth factors, and vascular density. Clin Cancer Res. 1999;5:155-161. |
| 12. | Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;155:441-452. |
| 13. | Kasper HU, Ebert M, Malfertheiner P, Roessner A, Kirkpatrick CJ, Wolf HK. Expression of thrombospondin-1 in pancreatic carcinoma: correlation with microvessel density. Virchows Arch. 2001;438:116-120. |
| 14. | Straume O, Akslen LA. Expresson of vascular endothelial growth factor, its receptors (FLT-1, KDR) and TSP-1 related to microvessel density and patient outcome in vertical growth phase melanomas. Am J Pathol. 2001;159:223-235. |
| 15. | Kawahara N, Ono M, Taguchi K, Okamoto M, Shimada M, Takenaka K, Hayashi K, Mosher DF, Sugimachi K, Tsuneyoshi M. Enhanced expression of thrombospondin-1 and hypovascularity in human cholangiocarcinoma. Hepatology. 1998;28:1512-1517. |
| 16. | Poon RT, Chung KK, Cheung ST, Lau CP, Tong SW, Leung KL, Yu WC, Tuszynski GP, Fan ST. Clinical significance of thrombospondin 1 expression in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4150-4157. |
| 17. | Yerian LM, Anders RA, Tretiakova M, Hart J. Caveolin and thrombospondin expression during hepatocellular carcinogenesis. Am J Surg Pathol. 2004;28:357-364. |
| 18. | Hayashi K, Kurohiji T, Shirouzu K. Localization of thrombospondin in hepatocellular carcinoma. Hepatology. 1997;25:569-574. |
| 19. | El-Youssef M, Mu Y, Huang L, Stellmach V, Crawford SE. Increased expression of transforming growth factor-beta1 and thrombospondin-1 in congenital hepatic fibrosis: possible role of the hepatic stellate cell. J Pediatr Gastroenterol Nutr. 1999;28:386-392. |
| 20. | Breitkopf K, Sawitza I, Westhoff JH, Wickert L, Dooley S, Gressner AM. Thrombospondin 1 acts as a strong promoter of transforming growth factor beta effects via two distinct mechanisms in hepatic stellate cells. Gut. 2005;54:673-681. |
| 21. | Kondou H, Mushiake S, Etani Y, Miyoshi Y, Michigami T, Ozono K. A blocking peptide for transforming growth factor-beta1 activation prevents hepatic fibrosis in vivo. J Hepatol. 2003;39:742-748. |
| 22. | Qian X, Tuszynski GP. Expression of thrombospondin-1 in cancer: a role in tumor progression. Proc Soc Exp Biol Med. 1996;212:199-207. |
| 23. | Chandrasekaran L, He CZ, Al-Barazi H, Krutzsch HC, Iruela-Arispe ML, Roberts DD. Cell contact-dependent activation of alpha3beta1 integrin modulates endothelial cell responses to thrombospondin-1. Mol Biol Cell. 2000;11:2885-2900. |
| 24. | Taraboletti G, Morbidelli L, Donnini S, Parenti A, Granger HJ, Giavazzi R, Ziche M. The heparin binding 25 kDa fragment of thrombospondin-1 promotes angiogenesis and modulates gelatinase and TIMP-2 production in endothelial cells. FASEB J. 2000;14:1674-1676. |
| 25. | Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503-506. |