Published online Apr 14, 2008. doi: 10.3748/wjg.14.2179
Revised: February 27, 2008
Published online: April 14, 2008
AIM: To compare the pharmacokinetics and tissue distribution of 5-fluorouracil administered intraperitoneally with two isotonic carrier solutions: HAES-steri (neotype 6% hydroxyethyl starch), a novel carrier solution with middle molecular weight and physiologic saline (0.9% sodium chloride solution), a traditional carrier solution for intraperitoneal chemotherapy, in rats.
METHODS: A total of 60 Sprague Dawley rats were randomized into groups according to the carrier solution administered. Each group was further randomized according to the intraperitoneal dwell period (1, 3, 6, 12, 18 and 24 h). At the end of the procedure the rats were killed, the peritoneal fluid was withdrawn completely and quantitated. Drug concentrations in peritoneal fluid, plasma, and tissues were determined by high-performance liquid chromatography.
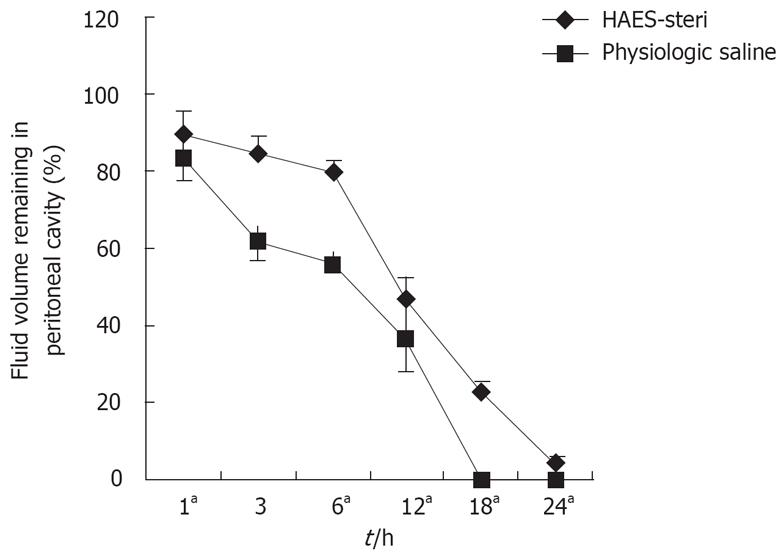
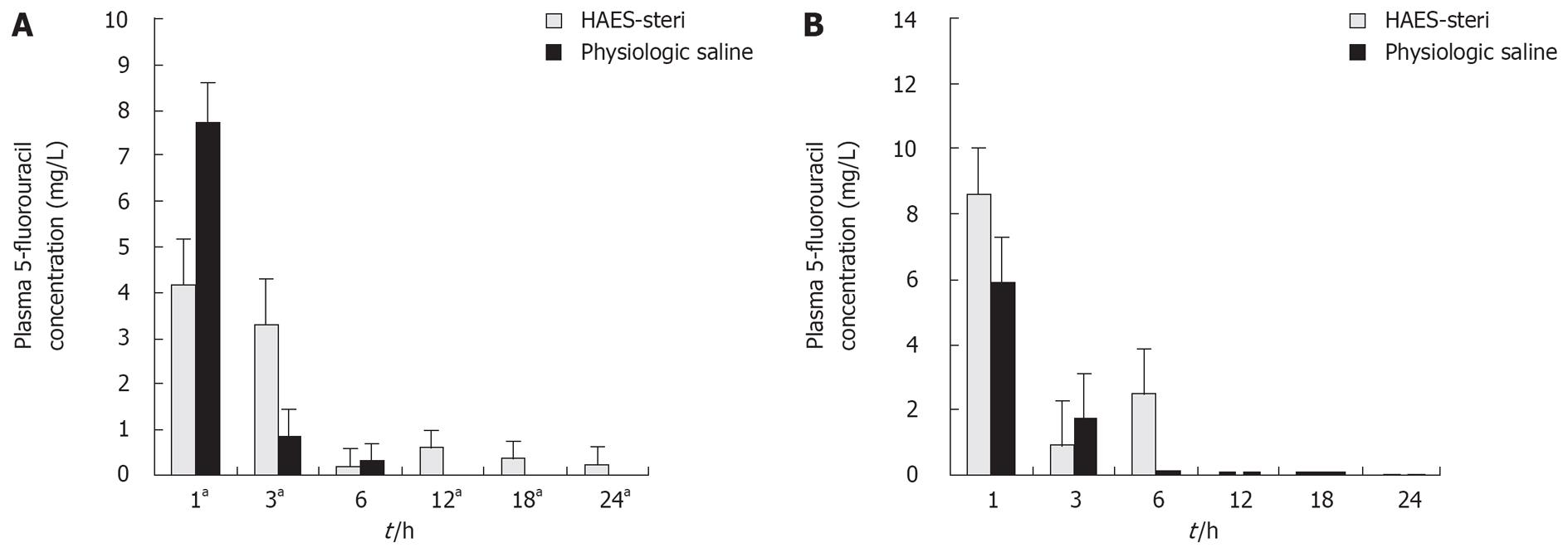
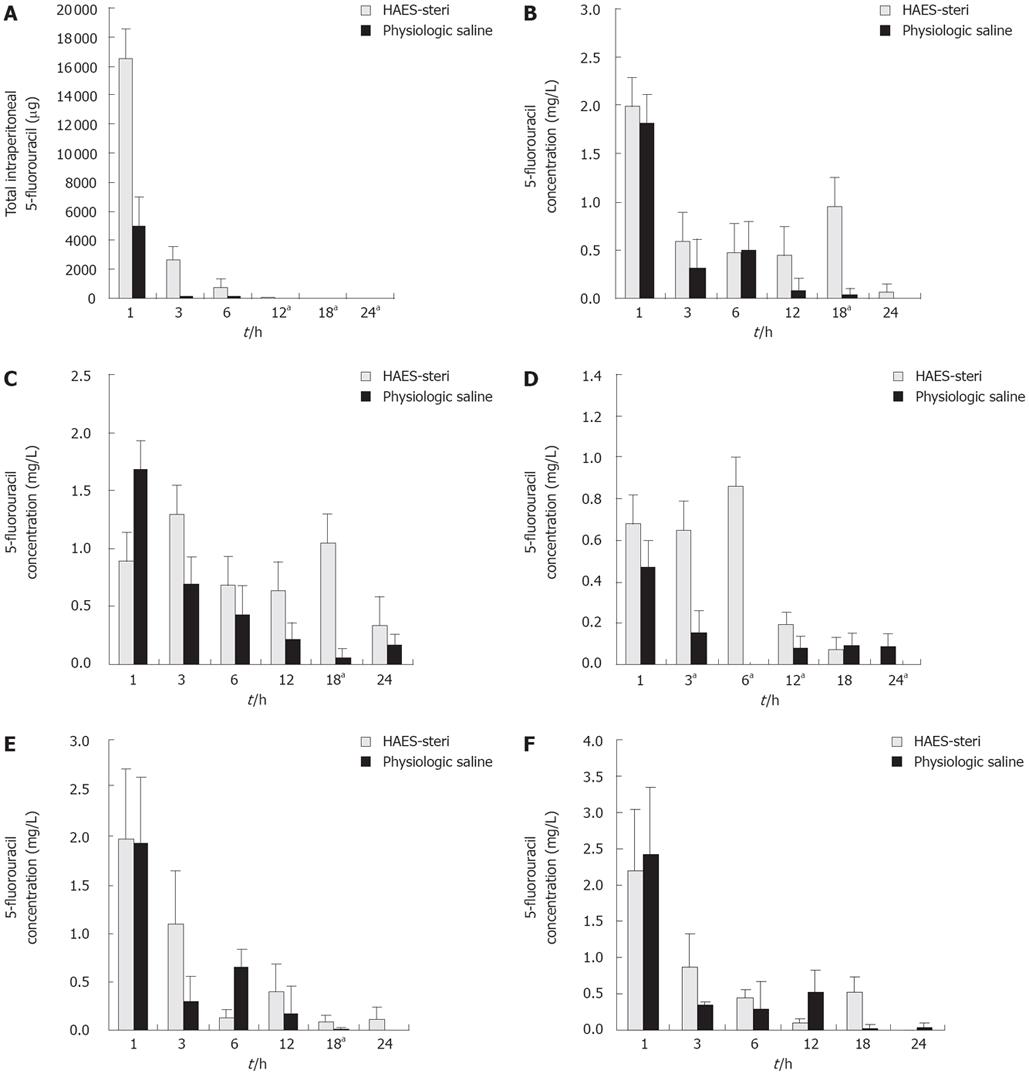
RESULTS: The mean volumes remaining in the peritoneal cavity were significantly higher with HAES-steri than those with physiologic saline at 1, 6, 12, 18, and 24 h (P = 0.047, 0.009, 0.005, 0.005 and 0.005 respectively, the percentages of remaining peritoneal fluid volume were 89.9 ± 5.6 vs 83.4 ± 4.9, 79.9 ± 2.8 vs 56.2 ± 15.7, 46.8 ± 5.5 vs 24.7 ± 9.7, 23.0 ± 2.8 vs 0.0 ± 0.0 and 4.2 ± 1.7 vs 0.0 ± 0.0 respectively). Mean concentrations in peritoneal fluid were significantly higher with HAES-steri than those with physiologic saline at 3, 12 and 18 h (P = 0.009, 0.009 and 0.005 respectively, the concentrations were 139.2768 ± 28.2317 mg/L vs mg/L, 11.5427 ± 3.0976 mg/L vs 0.0000 ± 0.0000 mg/L and 4.7724 ± 1.0936 mg/L vs 0.0000 ± 0.0000 mg/L respectively). Mean plasma 5-fluorouracil concentrations in portal vein were significantly higher with HAES-steri at 3, 12, 18 and 24 h (P = 0.009, 0.034, 0.005 and 0.019 respectively, the concentrations were 3.3572 ± 0.8128 mg/L vs 0.8794 ± 0.2394 mg/L, 0.6203 ± 0.9935 mg/L vs 0.0112 ± 0.0250 mg/L, 0.3725 ± 0.3871 mg/L vs 0.0000 ± 0.0000 mg/L, and 0.2469 ± 0.1457 mg/L vs 0.0000 ± 0.0000 mg/L respectively), but significantly lower at 1 h (P = 0.009, the concentrations were 4.1957 ± 0.6952 mg/L vs 7.7406 ± 1.2377 mg/L). There were no significant differences in the plasma 5-fluorouracil in inferior caval vein at each time-point. 5-fluorouracil concentrations were significantly greater with HAES-steri at 18 h in gastric tissue (P = 0.016, the concentrations were 0.9486 ± 0.8173 mg/L vs 030392 ± 0.0316 mg/L), at 18 h in colon (P = 0.009, the concentrations were 0.1730 ± 0.0446 mg/L vs 0.0626 ± 0.0425 mg/L), at 3, 6, 12 and 24 h in liver (P = 0.009, 0.013, 0.034 and 0.013 respectively, the concentrations were 0.6472685 ± 0.5256 mg/L vs 0.1554 ± 0.1043mg/L, 0.8606826 ± 0.7155 mg/L vs 0.0014 ± 0.0029 mg/L, 0.0445 ± 0.0330 mg/L vs 0.0797 ± 0.1005 mg/L and 0.0863 ± 0.0399 mg/L vs 0.0034 ± 0.0075 mg/L respectively) and at 18 h in lung (P = 0.009, the concentrations were 0.0886 ± 0.0668 mg/L vs 0.0094 ± 0.0210 mg/L). There were no differences in 5-fluorouracil concentrations in renal tissue at each time-point.
CONCLUSION: The use of intraperitoneal 5-fluoro-uracil with HAES-Steri carrier solution provides a pharmacokinetic advantage for a local-regional killing of residual tumor cells and improve the accumulated penetrability of 5-fluorouracil with decreased systemic toxicity. Further clinical feasibility studies on the use of HAES-steri as carrier solution for intraperitoneal chemotherapy with 5-fluorouracil are warranted.
- Citation: Wei ZG, Li GX, Huang XC, Zhen L, Yu J, Deng HJ, Qing SH, Zhang C. Pharmacokinetics and tissue distribution of intraperitoneal 5-fluorouracil with a novel carrier solution in rats. World J Gastroenterol 2008; 14(14): 2179-2186
- URL: https://www.wjgnet.com/1007-9327/full/v14/i14/2179.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2179
The gastrointestinal cancer tumor is one of the most common clinical malignant tumors[1]. Although the surgical interventions have been advancing and radiotherapy, chemotherapy, biotherapy, immunotherapy and Chinese traditional medicine been developing all the time, the prognosis of patients with gastrointestinal cancer has not been improved obviously and the mortality not been decreased greatly so far, for which the main reason is that the regional recurrence and implantation metastasis can not be treated effectively. The recurrence and metastasis of gastrointestinal cancer often occurs in the resection place, peritoneal membrane surface and liver by turns[23]. Intraperitoneal chemotherapy, which aims at this biological behavior and makes the recurrence and metastasis places of gastrointestinal cancer exposed to anti-cancer drug directly for a long time, provides a new adjunctive therapy for intraperitoneal malignant tumors, especially for gastrointestinal cancer now[45]. However, two pharmacokinetic problems appear to limit the effectiveness of intraperitoneal therapy: poor tumor penetration and no uniform intraperitoneal distribution by the drug-containing solution[6]. Several factors contribute to the drug distribution but intraperitoneal fluid volume is a dominant factor[7]. Rosenheim et al have demonstrated in monkeys that small volumes of fluid do not flow freely in the peritoneum, even with multiple position changes[8]. Volumes large enough to cause moderate abdominal distention result in more uniform intraperitoneal distribution. The ideal carrier solutions for intraperitoneal chemotherapy should expose cancerous surfaces or residual tumor cells within the peritoneal cavity to high levels of cytotoxic agent for as long as possible and make the agents distribute in the abdominal cavity uniformly[9].
Current techniques for intraperitoneal chemotherapy administration most often utilize isotonic micromolecule solutions such as physiological saline, however, the low molecular weight of this solution results in its rapid peritoneal absorption and cannot make the system toxicity from intraperitoneal chemotherapy under satisfactory controll[10]. A successful attempt has been made to prolong retention of intraperitoneal chemotherapy by using icodextrin[11–13], which is an isomolar glucose polymer-based dialysate solution. Another isomolar glucose polymer solution with a potentially long intraperitoneal dwell time is 6% hydroxyethyl starch[1415].
Slow clearance would benefit the use of cell cycle-specific drugs whose apoptotic effects enhance penetration in solid tumor. Agents undergoing extensive hepatic metabolism such as 5-fluorouracil and doxorubicin possess the greatest regional advantage with intraperitoneal instillation[16–18].
HAES-steri is a neotype hetastarch with middle molecule, which is commonly used for clinical volume expansion therapy. On the basis of its characteristic that it can stay in the blood vessel for a long time and the research about the solutions of the same kind, HAES-steri is promising to be an ideal carrier solution for intraperitoneal chemotherapy. The purpose of these animal experiments was to determine the pharmacokinetics and tissue concentrations of 5-fluorouracil after intraperitoneal perfusion with two isotonic carrier solutions: physiologic saline, a low molecular weight solution, and HAES-steri, a middle molecular weight solution.
Male and female Sprague Dawley rats weighing between 200 and 300 g were obtained from a single breeding colony (Laboratory Animal center of Southern Medical University). Animals were individually housed and were allowed free access to food and water. These experiments were conducted after approval by Laboratory Animal Center of Southern Medical University. (the license No. is SY × K 2006-0074).
All rats were briefly anesthetized by inhalation of ether (ether, Guanghua Chemistry Co., Ltd. Guangdong, China). Using a 50 mL injection syringe, the cytotoxic agent plus the carrier solution was administered intraperitoneally. The volume of solution administered was 0.1 mL/g body weight. Rats were returned to their cages to recover and were allowed free access to food and water. Rats were anesthetized by inhalation of ether for one minute before the end of the dwell-time. Through a midline thoracolaparotomy the peritoneal fluid was withdrawn completely and quantitated. The blood samples of portal vein and inferior caval vein were taken with a 5 mL injection syringe and 2 mL syringe respectively, and tissue samples were taken from the stomach, colon, liver, kidney and lung.
A total of 60 rats were randomized into two groups according to the carrier solution administered. 5-fluorouracil (Nantong Jinghua Pharmaceutical Co., Ltd., China) was administered in tamed iodine. The dose of drug used in this study was chosen at 100 mg/kg, which exceeds the intraperitoneal dosage used in humans and was meant to be above the analytic detection limit in fluid samples. Based on this dosage, 5-fluorouracil was administered at a concentration of 1000 &mgr;g/mL. Two isotonic carrier solutions (0.1 mL/g body weight) were used: physiologic saline (Shenzhou Pharmaceutical Co., Ltd, Guangzhou, China) and 6% hetastarch (Beijing Fresenius Kabi Pharmaceutical Co., Ltd.). Each group was further randomized according to the length of the dwell period of chemotherapy (1, 3, 6, 12, 18 or 24 h). At the end of the procedure the rats were killed. A midline thoracoabdominal incision was made and all peritoneal fluid removed. The volume of peritoneal fluid was recorded and a 0.5 mL sample was retained for analysis. Blood and tissue were also sampled. 5-fluorouracil concentrations in plasma, peritoneal fluid, and tissue samples were analyzed by high-performance liquid chromatography (HPLC).
5-fluorouracil levels were determined in plasma, peritoneal fluid and tissue samples, using the HPLC procedures. The HPLC system consisted of a P200 high pressure constant flow pump, an UV-VIS detector set at 265 nm UV, along with an EC2000 color spectrum workstation, an EC2000 Chromatopac data processor. A reversed-phase Diamonsil C18 5 &mgr;m silica column 250 mm × 4.6 mm was used, coupled to a guard column of the same chemical consistency (Dikma Technologies, Beijing, China). The mobile phase consisted of an mixture of acetonitrile and ultrapure water (1:19, v/v), run at a flow rate of 1.0 mL/min. Sample injections were 20 &mgr;L with 5-bromouracil asinternal standard. All solvents used were HPLC grade (Merck, KGaA).
Blood samples were centrifuged and the plasma was separated from the cells. Using a 15-mL polypropylene conical tube, a 500 &mgr;L sample of plasma was treated with 100 &mgr;L 5-bromouracil as internal standard and 2 mL acetoacetate (Guanghua Chemistry Co., Ltd., Guangdong, China) and mixed thoroughly in a vortex mixer. After centrifugation, the acetoacetate was transferred to another polypropylene tube and evaporated at approximately 40°C by blowing with a gentle stream of nitrogen. The residue was resuspended in 100 &mgr;L mobile phase and filtered through a 0.45 &mgr;m syringe filter before HPLC injection. Peritoneal fluid samples were treated as blood sample before HPLC injection.
Tissue samples were processed after drying surface moisture with filter paper. A sample of tissue was accurately weighed with electronic balance and homogenized in ultrapure water with the volume as 3 times as the weight of the sample (v:w = 3 mL/g) with a homogenizing machine. The tissue sample site was consistent for all animals. The homogenate was centrifuged and the supernatant fluid was removed and the following was used as the blood sample.
The main parameters of pharmacokinetics and areas under the concentration-time curve were determined using DAS 2.0 (Drug and Statistics Software, Anhui, China). All pharmacokinetic data were compared between groups at each time-point with Mann-Whitney test (two-tailed) using SPSS 10.0. For all statistical procedures, P values < 0.05 were considered significant.
Measurements of peritoneal fluid volume at each time-point showed slower clearance from the peritoneal cavity of HAES-steri when compared to physiologic saline (Figure 1). The mean percentage of fluid volume remaining in the peritoneal cavity was significantly higher with HAES-steri at 1, 6, 12, 18 and 24 h (P = 0.047, 0.009, 0.005, 0.005 and 0.005 respectively). No excess peritoneal fluid remained at 18 h with physiologic saline. At 24 h, the percentage of remaining peritoneal fluid volume with HAES-steri was 4.2%.
At each time-point drug concentrations were determined within the peritoneal cavity (Figure 2). The mean peritoneal fluid 5-fluorouracil concentration was significantly greater at 3, 12, 18 and 24 h (P = 0.009, 0.009, 0.005 and 0.005 respectively). There was no significant difference in 5-fluorouracil concentrations of peritoneal fluid between carrier solutions at other time-points.
Plasma 5-fluorouracil concentrations were significantly lower when the drug was administered with HAES-steri at 1 h (P = 0.009) and were significantly higher at 3, 12, 18 and 24 h (P = 0.009, 0.034, 0.005 and 0.019 respectively) (Figure 3A).
There were no significant differences in plasma 5-fluorouracil concentration in inferior caval vein between different carrier solutions at each time-point (Figure 3B).
The mean total quantity of 5-fluorouracil in the peritoneal fluid decreased with time for both hetastarch and peritoneal dialysis solution, but was significantly greater with hetastarch at 12 h (P = 0.008), 18 h (P = 0.009) and 24 h (P = 0.009) (Figure 4A). No measurable drug or excess peritoneal fluid was present at 18 h when physiologic saline was used. When HAES-steri was used, the mean volume of peritoneal fluid remaining at 24 h was 4.2% ± 1.7% (± SD) of the initial peritoneal fluid volume. The mean total quantity of 5-fluorouracil in this fluid was extremely low (0.7458 ± 0.1954 &mgr;g).
The area under the concentration over time curve (AUC) ratio with HAES-steri was 1551.095 for peritoneal fluid, 17.49 for plasma in portal vein and 19.466 for plasma in inferior caval vein. The AUC ratio for physiologic saline was 824.054 for peritoneal fluid, 14.516 for plasma in portal vein and 20.275 for plasma in inferior caval vein. The AUC ratio of peritoneal fluid to plasma in portal vein was 88.68 for HAES-steri, and 56.76 for physiologic saline. The AUC ratio of peritoneal fluid to plasma in inferior caval vein was 79.68 for HAES-steri, and 40.64 for physiologic saline. There was an increase of 156% in the AUC ratio of peritoneal fluid to plasma in portal vein and 196% in inferior caval vein with HAES-steri (88.68 vs 56.76; 79.68 vs 40.64).
Mean tissue concentrations of 5-fluorouracil were greater in gastric tissue with HAES-steri. These differences were significant at 18 h (P = 0.016). No significant differences were seen in gastric tissue concentrations of 5-fluorouracil at other time-points (Figure 4B).
Tissue concentrations of 5-fluorouracil were significantly greater in colon tissue with HAES-steri at 18 h (P = 0.009) (Figure 4C). There were no significant differences in colon tissue concentrations of 5-fluorouracil at other time-points.
Tissue concentrations of 5-fluorouracil were significantly greater in liver tissue with HAES-steri at 3, 6, 12 and 24 h in liver (P = 0.009, 0.013, 0.034 and 0.013 respectively) (Figure 4D). There were no significant differences in liver tissue concentrations of 5-fluorouracil at other time-points.
Tissue concentrations of 5-fluorouracil were significantly higher in lung tissue with HAES-steri at 18 h (P = 0.009) (Figure 4E). There were no significant differences in lung tissue concentrations of 5-fluorouracil at other time-points.
No significant differences were seen in renal tissue concentrations of 5-fluorouracil with the two carrier solutions at each time-point (Figure 4F).
Intraperitoneal chemotherapy has shown certain benefits as a treatment for peritoneal surface malignancies and regional recurrence of gastrointestinal cancer after operation so far[19–21]. However, no standard treatment in terms of schedule, dwell-time, drug or carrier solution has been established[9]. It remains an unrealized goal that intraperitoneal chemotherapy makes a great stride in improving the survival and prognosis of patients with gastrointestinal cancer. The lack of ideal carrier solutions is one of the main problems which prevent the progress of intraperitoneal chemotherapy. After radical or palliative surgery for gastrointestinal cancer, tumor recurrence within the peritoneal cavity may be due to residual tumor nodules on the peritoneal surface or to implantation of free cancer cells circulating within peritoneal fluid. Preventing recurrence effectively requires that the tumor nodules have prolonged exposure to the cytotoxic drug. It is necessary for chemotherapy solutions to distribute evenly throughout the entire peritoneal cavity for a prolonged period in order to treat peritoneal and visceral surfaces safely and successfully.
However, isotonic salt solutions, the traditional carrier solutions in use for intraperitoneal chemotherapy, tend to be rapidly absorbed due to their low molecular weight. Pestieau and colleagues have proved that isotonic 0.9% sodium chloride was cleared more rapidly from the peritoneal cavity than high molecular weight solutions and hypertonic sodium chloride, when used as carrier solutions for intraperitoneal chemotherapy with 5-fluorouracil and gemcitabine in an animal model[9]. The inability of isotonic salt or dextrose solutions to maintain a prolonged high intraperitoneal fluid volume limits their effectiveness as carrier solutions for intraperitoneal chemotherapy. The osmolality of the solution may play a role in prolonging the dwell time of intraperitoneal chemotherapy. In a study by Litterst et al, it was shown that slightly hypertonic carrier solutions can prolong the peritoneal retention of chemotherapeutic agents within the peritoneal cavity, probably by inducing a fluid shift inward to the peritoneal cavity[22]. Although the increased accumulation of drugs in tumor cells and enhanced cytotoxicity of cisplatin in hypotonic solution have been confirmed in vitro and in vivo[2324], the clinical success with hypotonic solutions as carrier solutions for intraperitoneal chemotherapy has been limited[2526]. It has been shown that high molecular weight carrier solutions such as Icodextrin and hetastarch have the ability to maintain high intraperitoneal volume for a longer period. Preliminary data receiving intraperitoneal chemotherapy with 7.5% Icodextrin showed that a similar quantity of fluid was drained from the peritoneal cavity as was originally instilled 24 h after administration[27]. However, net fluid flow into the peritoneal cavity may occur when 7.5% icodextrin solutions were used, which could decrease the concentration of drug exposed to cancerous surfaces[6]. A clinical study on the fluid dynamics of 4% icodextrin as carrier solution for intraperitoneal chemotherapy showed that it maintained its instilled volume for up to 48 h, and half the instilled volume remained after 72 and 96 h[28]. Another high molecular weight carrier solution, 6% hetastarch, has also been used in a recent clinical study, which showed reduced clearance of hetastarch from the peritoneal cavity when compared with 1.5% dextrose peritoneal dialysis solution[29].
In the study reported here, the use of HAES-steri, a starch-based carrier solution with middle molecular weight, reduced the clearance of chemotherapy solution from the peritoneal cavity when compared to physiologic saline (0.9% sodium chloride solution). The mean percentage of fluid volume remaining in the peritoneal cavity was significantly higher with HAES-steri at 1, 6, 12, 18 and 24 h. The total quantity of intraperitoneal drug at each time-point was also higher with HAES-steri, especially at 12 h and 18 h. By delaying the clearance of intraperitoneal fluid and thereby maintaining a large distribution, HAES-steri may maximize exposure of cancerous surfaces and optimize intraperitoneal chemotherapy treatments. At the 3, 12 and 18 h time-point, a significantly increased concentration of intraperitoneal 5-fluorouracil was demonstrated. It indicated that HAES-steri could reduce the clearance of 5-fluorouracil from peritoneal cavity and increase the drug concentration exposed to peritoneal surfaces at the same time as this solution maintained high volume in peritoneal cavity for a long time. Accordingly, a larger number of residual tumor cells, minute nodules or free cancer cells in peritoneal cavity can be attacked by high concentrations of anti-cancer drug for over a given time period, which may improve the effectiveness of intraperitoneal chemotherapy for peritoneal regional recurrence of gastrointestinal cancer after surgery.
An important parameter for pharmacokinetic analyses of a drug is the AUC, which represents the total drug exposure integrated over time[10]. The AUC is traditionally the relationship between time and plasma concentration, but can also be applied to concentration of drug in peritoneal fluid for intraperitoneal chemotherapy. Cancer chemotherapy pharmacokinetics assumes a definite relationship of drug response to drug dose. Following intraperitoneal administration of a drug, the AUC reflects the degree of exposure of peritoneal surfaces to chemotherapeutic agent. It is the best estimate of drug delivery and a predictor of response. By comparing the AUC of a drug after intraperitoneal administration in HAES-steri to the AUC after administration in physiologic saline, an estimate of the optimum carrier solution that will prolong contact of peritoneal surfaces and residual tumor cells with chemotherapy solution can be obtained. The ratio of the AUC of 5-fluorouracil in peritoneal fluid to that in plasma of inferior caval vein after intraperitoneal administration reflects exposure of peritoneal surfaces to chemotherapy solution in relation to plasma concentrations of drug, which influences systemic toxicity. The higher AUC ratio of peritoneal fluid to plasma 5-fluorouracil concentration with HAES-steri suggests that better regional exposure of 5-fluorouracil and lower systemic toxicity can be achieved than with physiologic saline. Using intraperitoneal 5-fluorouracil with HAES-steri provides a potential for a favorable antitumor effect on small peritoneal surface tumor deposits or microscopic residual disease.
Another advantage of intraperitoneal chemotherapy is to prevent liver metastasis of gastrointestinal cancer after operation with chemotherapeutics of high concentration in portal vein and liver. In this study, although plasma 5-fluorouracil concentrations were significantly lower when the drug was administered with HAES-steri than with physiologic saline at 1 h, those were significantly higher at 3, 12, 18 and 24 h. Moreover, concentrations of 5-fluorouracil were significantly greater in liver tissue with HAES-steri at 3, 6, 12 and 24 h. This shows that HAES-steri has advantages over physiologic saline as carrier solutions for intraperitoneal chemotherapy to kill free cancer cells in portal vein, consequently, using HAES-steri as carrier solution for intraperitoneal chemotherapy with 5-fluorouracil may improve the effectiveness to prevent liver metastasis of gastrointestinal cancer after surgery.
Tissue concentrations of 5-fluorouracil were significantly higher in gastric and colon tissue at 18-h time-point when HAES-steri was used as carrier solution. This would suggest that HAES-steri increases the accumulated penetrated activity of 5-fluorouracil when used as carrier solution for intraperitoneal chemotherapy, which may benefit the eliminating internal cancer cells in residual tumor nodules. No significant differences were seen in renal tissue concentrations of 5-fluorouracil with the two carrier solutions at each time-point and there were no significant differences in lung tissue concentrations of 5-fluorouraci at time-points except 3-h. These may indicate again that HAES-steri makes the systemic toxicity of intraperitoneal chemotherapy under control when used as carrier solution.
Another advantage of HAES-steri as carrier solution for intraperitoneal chemotherapy is that maintenance of an expanded intraperitoneal space with the use of HAES-steri may additionally ensure separation of loops of bowel to allow direct contact of chemotherapy solution with bowel surfaces prone to adhesion formation and subsequent disease recurrence[30]. The reduction in adhesion formation has been shown with the use of intraperitoneal 4.5% icodextrin lavage and instillation after laparoscopic gynecologic surgery[18]. The further pathologic study of the impact on the peritoneum and the healing of the operative incision when HAES-steri is used intraperitoneally is needed.
In brief, this study suggests that HAES-steri, by remaining longer in the peritoneal cavity, provides wider intraperitoneal distribution of 5-fluorouracil, and an increased exposure of peritoneal surfaces to anti-cancer drug with lower systemic toxicity than physiologic saline. HAES-steri is a promising carrier solution for intraperitoneal chemotherapy and further clinical feasibility studies on the use of HAES-steri as carrier solution for intraperitoneal chemotherapy with 5-fluorouracil are warranted.
The principal pharmacokinetic advantage of intraperitoneal chemotherapy over intravenous chemotherapy is the high local drug concentration with low systemic toxicity. The traditional carrier solutions for intraperitoneal chemotherapy fail to optimize this advantage, because these are prone to be cleared from peritoneal cavity rapidly. The ideal carrier solutions should expose cancerous surfaces or residual tumor cells within the peritoneal cavity to high levels of the cytotoxic agent as long as possible.
It has been shown in published articles that high molecular weight solutions have the ability to maintain high intraperitoneal volume for a longer period, which may offer a number of advantages over low molecular weight solutions. Further study on the pharmacokinetics of intraperitoneal chemotherapy with middle or high molecular weight solutions may solve the problem that there are no ideal carrier solutions for intraperitoneal chemotherapy.
In this article, we studied and compared the pharmacokinetics and tissue distribution of intraperitoneal 5-fluorouracil with HEAS-steri, a neotype 6% hydroxyethyl starch as carrier solution, and physiologic saline (0.9% sodium chloride solution), a traditional carrier solution, in rats, which may provide a promising carrier solution for intraperitoneal chemotherapy.
This study on the pharmacokinetics and tissue distribution of intraperitoneal 5-fluorouracil with HEAS-steri and physiologic saline offers experimental data for further clinical study and application of HEAS-steri as carrier solution for intraperitoneal chemotherapy.
Intraperitoneal chemotherapy, a treatment in which anticancer drugs are put directly into the abdominal cavity through a thin tube, has obvious pharmacokinetic advantages over intravenous chemotherapy when used in palliative therapy for peritoneal carcinomatosis from gastrointestinal carcinoma and prevention of the postoperative peritoneal recurrences and metastases of gastrointestinal cancer.
In the present study the effect HAES-steri, as a promising carrier solution for intraperitoneal chemotherapy, was investigated. The study showed that HAES-steri delayed the clearance of intraperitoneal fluid and increased the concentration of 5-fluorouracil in the peritoneal fluid, tissue (especially liver) and portal vein. The manuscript deals with an interesting topic and conclusive results.
| 1. | Ebert M, Xing X, Burgermeister E, Schmid R, Rocken C. Perspectives of clinical proteomics in gastrointestinal cancer. Expert Rev Anticancer Ther. 2007;7:465-469. |
| 2. | Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236-242. |
| 3. | Yonemura Y, Bandou E, Kinoshita K, Kawamura T, Takahashi S, Endou Y, Sasaki T. Effective therapy for peritoneal dissemination in gastric cancer. Surg Oncol Clin N Am. 2003;12:635-648. |
| 4. | Sugarbaker PH. Adjuvant intraperitoneal chemotherapy: a review. Recent Results Cancer Res. 2007;169:75-82. |
| 5. | Cortesi E, Martelli O, Padovani A. Role of intraperitoneal chemotherapy. Minerva Ginecol. 2001;53:29-33. |
| 6. | Mohamed F, Sugarbaker PH. Carrier solutions for intraperitoneal chemotherapy. Surg Oncol Clin N Am. 2003;12:813-824. |
| 7. | Myers CE, Collins JM. Pharmacology of intraperitoneal chemotherapy. Cancer Invest. 1983;1:395-407. |
| 8. | Rosenshein N, Blake D, McIntyre PA, Parmley T, Natarajan TK, Dvornicky J, Nickoloff E. The effect of volume on the distribution of substances instilled into the peritoneal cavity. Gynecol Oncol. 1978;6:106-110. |
| 9. | Pestieau SR, Schnake KJ, Stuart OA, Sugarbaker PH. Impact of carrier solutions on pharmacokinetics of intraperitoneal chemotherapy. Cancer Chemother Pharmacol. 2001;47:269-276. |
| 10. | Rowinsky EK, Donehower RC, Jones RJ, Tucker RW. Microtubule changes and cytotoxicity in leukemic cell lines treated with taxol. Cancer Res. 1988;48:4093-4100. |
| 11. | Kerr DJ, Young AM, Neoptolemos JP, Sherman M, Van-Geene P, Stanley A, Ferry D, Dobbie JW, Vincke B, Gilbert J. Prolonged intraperitoneal infusion of 5-fluorouracil using a novel carrier solution. Br J Cancer. 1996;74:2032-2035. |
| 12. | McArdle CS, Kerr DJ, O'Gorman P, Wotherspoon HA, Warren H, Watson D, Vinke BJ, Dobbie JW, el Eini DI. Pharmacokinetic study of 5-fluorouracil in a novel dialysate solution: a long-term intraperitoneal treatment approach for advanced colorectal carcinoma. Br J Cancer. 1994;70:762-766. |
| 13. | Dobbie JW. New principles, better practices, and clearer perceptions in intraperitoneal chemotherapy: clinical experience using icodextrin 20 as a carrier solution. Adv Perit Dial. 1997;13:162-167. |
| 14. | Mohamed F, Marchettini P, Stuart OA, Sugarbaker PH. Pharmacokinetics and tissue distribution of intraperitoneal paclitaxel with different carrier solutions. Cancer Chemother Pharmacol. 2003;52:405-410. |
| 15. | Mohamed F, Stuart OA, Sugarbaker PH. Pharmacokinetics and tissue distribution of intraperitoneal docetaxel with different carrier solutions. J Surg Res. 2003;113:114-120. |
| 16. | Kraft AR, Tompkins RK, Jesseph JE. Peritoneal electrolyte absorption: analysis of portal, systemic venous, and lymphatic transport. Surgery. 1968;64:148-153. |
| 17. | Markman M. Intraperitoneal chemotherapy. Semin Oncol. 1991;18:248-254. |
| 18. | Mohamed F, Marchettini P, Stuart OA, Yoo D, Sugarbaker PH. A comparison of hetastarch and peritoneal dialysis solution for intraperitoneal chemotherapy delivery. Eur J Surg Oncol. 2003;29:261-265. |
| 19. | Sugarbaker PH. Managing the peritoneal surface component of gastrointestinal cancer. Part 2. Perioperative intraperitoneal chemotherapy. Oncology (Williston Park). 2004;18:207-219; discussion 220-222, 227-228, 230. |
| 20. | Yan TD, Black D, Sugarbaker PH, Zhu J, Yonemura Y, Petrou G, Morris DL. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol. 2007;14:2702-2713. |
| 21. | Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24:4011-4019. |
| 22. | Litterst CL, Torres LJ, Sikic BI. Absorption of antineoplastic drugs following large volume administration to rats. Cancer Treat Rep. 1980;66:147-155. |
| 23. | Groose E, Walker L, Masters JR. The influence of osmolarity on drug cytotoxicity in vitro. Br J Cancer. 1986;54:181. |
| 24. | Los G, Verdegaal EM, Mutsaers PH, McVie JG. Penetration of carboplatin and cisplatin into rat peritoneal tumor nodules after intraperitoneal chemotherapy. Cancer Chemother Pharmacol. 1991;28:159-165. |
| 25. | Tsujitani S, Fukuda K, Saito H, Kondo A, Ikeguchi M, Maeta M, Kaibara N. The administration of hypotonic intraperitoneal cisplatin during operation as a treatment for the peritoneal dissemination of gastric cancer. Surgery. 2002;131:S98-S104. |
| 26. | Elias D, El Otmany A, Bonnay M, Paci A, Ducreux M, Antoun S, Lasser P, Laurent S, Bourget P. Human pharmacokinetic study of heated intraperitoneal oxaliplatin in increasingly hypotonic solutions after complete resection of peritoneal carcinomatosis. Oncology. 2002;63:346-352. |
| 27. | McArdle CS, Kerr DJ, O'Gorman P, Wotherspoon HA, Warren H, Watson D, Vinke BJ, Dobbie JW, el Eini DI. Pharmacokinetic study of 5-fluorouracil in a novel dialysate solution: a long-term intraperitoneal treatment approach for advanced colorectal carcinoma. Br J Cancer. 1994;70:762-766. |
| 28. | Hosie K, Gilbert JA, Kerr D, Brown CB, Peers EM. Fluid dynamics in man of an intraperitoneal drug delivery solution: 4% icodextrin. Drug Deliv. 2001;8:9-12. |
| 29. | diZerega GS, Verco SJ, Young P, Kettel M, Kobak W, Martin D, Sanfilippo J, Peers EM, Scrimgeour A, Brown CB. A randomized, controlled pilot study of the safety and efficacy of 4% icodextrin solution in the reduction of adhesions following laparoscopic gynaecological surgery. Hum Reprod. 2002;17:1031-1038. |