Published online Mar 14, 2008. doi: 10.3748/wjg.14.1592
Revised: November 8, 2007
Published online: March 14, 2008
AIM: To explore the inhibitory effects of pokeweed antiviral protein seed (PAP-S) and PAP encoded by a eukaryotic expression plasmid on hepatitis B virus (HBV) replication in vitro.
METHODS: HepG2 2.2.15 cells in cultured medium were treated with different concentrations of PAP-S. HBsAg, HBeAg and HBV DNA in supernatants were determined by ELISA and fluorescent quantitative PCR respectively. MTT method was used to assay for cytotoxicity. HepG2 were cotransfected with various amounts of PAP encoded by a eukaryotic expression plasmid and replication competent wild-type HBV 1.3 fold over-length plasmid. On d 3 after transfection, HBsAg and HBeAg were determined by using ELISA. Levels of HBV core-associated DNA and RNA were detected by using Southern and Northern blot, respectively.
RESULTS: The inhibitory effects of PAP-S on HBsAg, HBeAg and HBV DNA were gradually enhanced with the increase of PAP concentration. When the concentration of PAP-S was 10 &mgr;g/mL, the inhibition rates of HBsAg, HBeAg and HBV DNA were 20.9%, 30.2% and 50%, respectively. After transfection of 1.0 &mgr;g and 2.0 &mgr;g plasmid pXF3H-PAP, the levels of HBV nucleocapside-associated DNA were reduced by 38.0% and 74.0% respectively, the levels of HBsAg in the media by 76.8% and 99.7% respectively, and the levels of HBeAg by 72.7% and 99.3% respectively as compared with controls. Transfection with 2 &mgr;g plasmid pXF3H-PAP reduced the levels of HBV nucleocapside-associated RNA by 69.0%.
CONCLUSION: Both PAP-S and PAP encoded by a eukaryotic expression plasmid could effectively inhibit HBV replication and antigen expression in vitro, and the inhibitory effects were dose-dependent.
-
Citation: He YW, Guo CX, Pan YF, Peng C, Weng ZH. Inhibition of hepatitis B virus replication by pokeweed antiviral protein
in vitro . World J Gastroenterol 2008; 14(10): 1592-1597 - URL: https://www.wjgnet.com/1007-9327/full/v14/i10/1592.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1592
Pokeweed antiviral protein (PAP), a 29-kDa protein isolated from leaves or seeds of Phytolacca americana, inhibits translation by catalytically removing a specific adenine residue from the large rRNA of the 60S subunit of eukaryotic ribosomes[12]. The anti-viral activity of PAP has been described against a wide range of viruses, including HIV, herpes simplex virus, cytomegalovirus, influenza virus and polio virus[34]. Dose response studies indicate that the anti-HIV activity of PAP from PAP-S was comparable to Zidovudine (AZT)[5].
The purpose of the present study was to evaluate “in vitro” the potential usefulness of PAP in therapies against chronic hepatitis B. Hepatitis B virus (HBV) is a major cause of liver disease worldwide, ranging from acute and chronic hepatitis to cirrhosis and hepatocellular carcinoma[6]. Despite the availability of an effective and safe vaccine against HBV, infection by this virus is an important worldwide health problem[7]. Although several pharmacological strategies are currently being implemented to treat affected patients, no effective antiviral therapy against HBV infection has yet been fully developed[8]. Thus, new drugs to be used alone or in combination with existing treatments are needed. In this respect, “in vitro” screening of potentially active compounds is a useful step in the development of novel drugs.
In this study, we assessed anti-HBV activities of PAP from PAP-S by detecting HBsAg, HBeAg, and HBV DNA in the supernatant of HepG2 2.2.15 cell lines. We also constructed the eukaryotic expression plasmid encoding PAP, cotransfected it and the replication competent wild-type HBV 1.3 fold over-length plasmid into the HepG2 cells to investigate the inhibitory effect of the eukaryotic expression plasmid of PAP. PAP was found to be a remarkably potent and fast-acting antiviral agent against HBV replication in vitro in the absence of any obvious signs of toxicity. This is the first report of the anti-HBV effects of PAP. Our observations suggest that PAP deserves further investigation as a potential alternative or complementary anti-HBV agent.
The plasmid PGEM-PAP containing the PAP gene was kindly supplied by Prof. YAN Bo (Institute of Biotechnology, Yunnan Academy of Agricultural Sciences, China). The replication competent wild-type HBV 1.3 fold over-length plasmid (genotype, ayw) and the plasmid pXF3H derived from the cytomegalovirus-driven vector pRK5 were both kindly supplied by Dr. Lei Yanchang. The coding gene of PAP (Gene Bank GI: 218010) was amplified from the plasmid PGEM-PAP by PCR using forward primer 5’-CGGGATCCAATAAATACAATCACCTTC GTAA-3’ and reverse primer 5’-CC AAGCTTAGGGAA CATGGCACTTTGGTAA-3’. The PCR product was cloned into BamHI/HindIII restriction sites of the CMV-driven expression vector fused with a hemagglutinin fusion epitope tag at its N-terminus (pXF3H).
PAP-S, purified from pokeweed (Phytolacca americana) seeds, was kindly supplied by Professor HU Zhong (Kunming Institute of Botany, The Chinese Academy of Sciences, China).
HepG2 and HepG2 2.2.15 cells were grown at 37°C with 50 mL/L CO2 in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS) (Invitrogen, CA, USA).
HepG2 cells were seeded on plastic dishes (3.5 cm in diameter) at a density of 4.5 × 105 cells per well in 6-well plates 18 h prior to transfection. Transfection of HepG2 cells was performed with lipofectamine 2000 (Invitrogen, CA, USA) following the manufacture’s instructions. The replication competent wild-type HBV 1.3 fold over-length plasmid pHBV1.3 and various amounts of a CMV-driven expression vector pXF3H-PAP were cotransfected into the HepG2 cells.
HepG2 2.2.15 cells were treated with PAP-S at various concentrations for 2 or 4 d in DMEM supplemented with 10% FCS in a 24-well plate at a density of 2 × 104 cells per well. The corresponding suspension was collected for analysis of the levels of HBsAg, HBeAg, and HBV DNA, in duple every 2 d for 4 d. The HepG2 2.2.15 cells as controls were washed twice with phosphate-buffered saline (PBS) and refed with culture medium every 2 d for 4 d.
The concentration of HBsAg or HBeAg in culture supernatants of HepG2 2.2.15 or the transfected HepG2 cells was detected by an enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Kehua Company, China) and quantified relative to a standard curve of serial dilutions of recombinant HBsAg or HBeAg. Inhibition rate (%) = (P/N values of the control wells-P/N values of the test wells)/(P/N values of the control wells-2.1) × 100%, and the median inhibition dose (ID50) represented the concentration of PAP-S when the inhibition rate was 50%. The median cytotoxin dose (CD50) represented the concentration of PAP-S when the number of the survival cells was 50%.
In order to determine the efficiency of transfection, Western blot with polyclonal anti-HA antibody was used to detect the expression of PAP in cotransfected HepG2 cells. For Western blot analysis, cytoplasmic lysates were incubated with 1 volume of 2 × loading buffer containing 10% beta-mercaptoethanol for 10 min at 95°C before loading onto a 12.5% SDS-PAGE. Proteins were transferred onto a nitrocellulose membrane via electroblot. The membranes were incubated with anti-hemagglutinin fusion epitope polyclonal antibody (Santa Cruz, USA) followed by horseradish peroxidase conjugated mouse anti-rabbit antibody. Proteins were visualized via enhanced chemiluminescence (Roche, Germany).
For HBV DNA from HepG2 2.2.15 cells, the DNA was detected by real-time PCR kit (DA AN GENE CO., Guangzhou, China) following the instruction manual provided. Amplification and detection were performed with an ICycler (Bio-Rad) Detection system. The program was optimized with denaturation at 94°C for 2 min followed by 40 cycles of amplification (at 94°C for 20 s, 55°C for 20 s, and 72°C for 20 s).
On d 3 after transfection, HepG2 cells were lysed with 0.8 mL of 0.01 mol/L Tris-HCl (pH 8.0), 0.05 mol/L NaCl, 5 mL/L NP-40, 1 mmol/L EDTA at room temperature for 10 min as previously described[9]. For the Southern hybridizations, 20 &mgr;g of total DNA was digested with HindIII, electrophoresed on a 1.4% agarose gel, and then transferred onto nylon membrane. The probe containing the full-length HBV genome was labeled with digoxigenin dUTP (DIG) by the DIG-high Prime DNA labeling and Detection Starter kitII according to the manufacturer’s protocol (Roche, Germany). The membrane was hybridized with HBV probe at 50°C overnight.
For Northern blot analysis, total RNA was extracted from the transfected cells with TRIZOL reagent (Gibco, USA) according to the manufacturers instructions. A total of 20 &mgr;g of RNA was resolved in 1.2% denatured gel and then transferred onto the nylon membrane and the membrane was hybridized with DIG-labeled HBV DNA fragment described above. For hybridization of glyceraldehyde-3-phosphate dehydrogenase (G3PDH), the full-length HBV DNA probe was removed from the membrane by washing twice at 37°C in 0.2 mol/L NaOH containing 1 mL/L sodium dodecyl sulfate (SDS) solution for 15 min and then re-hybridized with DIG-labeled probe for G3PDH.
HepG2 2.2.15 cells were used for determining the cytotoxicity of PAP-S. Cells were plated onto a 96-well plate at a density of 1 × 104 cells per 100 &mgr;L prior to drug treatment. PAP-S was added at concentrations of 0.1, 1.0, 10, 50 and 100 &mgr;g/mL and the cells were refed with drug-containing fresh medium every 2 d for up to 4 d, and then 20 &mgr;L of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl (MTT) was added to each well and further incubated in a CO2 incubator at 37°C for 90 min. The absorbance (A) at 490 nm was read. Data were calculated as a percentage of negative control cells that were not treated with PAP-S.
Data points were obtained from at least three different cell cultures, in which each condition was assayed in triplicate. Values were expressed as mean ± SD. To calculate the statistical significance of differences within or among groups, the paired t-test was used. Statistical significance was set at P < 0.05.
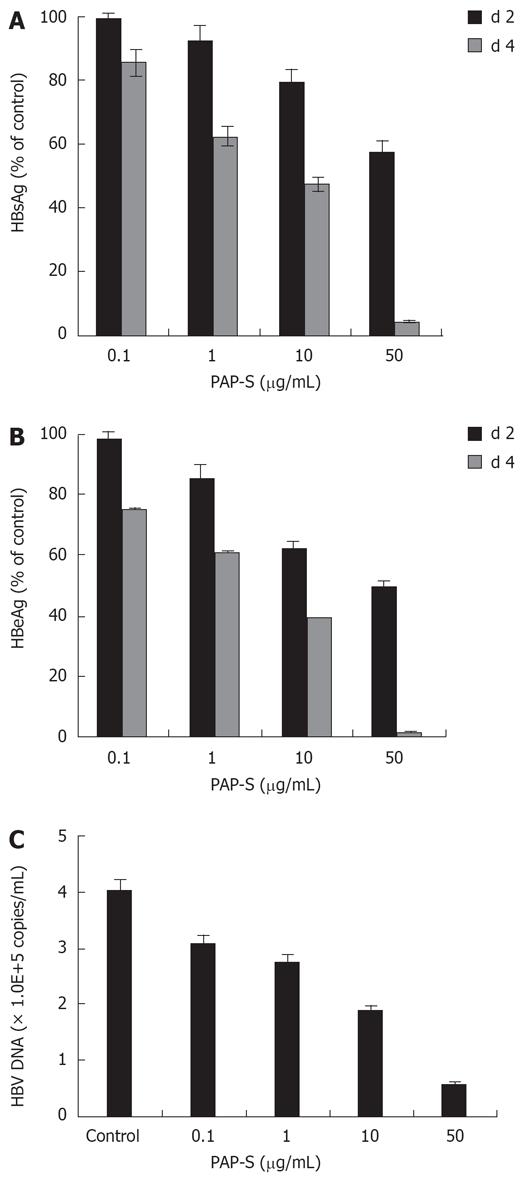
In HepG2 2.2.15 cells, MTT assay revealed that PAP-S had no inhibitory effect on cell proliferation up to 50 &mgr;g/mL. PAP-S inhibited the growth of HepG2 2.2.15 cells at concentrations above 50 &mgr;g/mL. The CD50 value was 29.3 &mgr;g/mL.
As shown in Figure 1A-C, treatment of above 1 &mgr;g/mL PAP-S could statistically significantly reduce HBsAg, HBeAg and HBV DNA in a dose dependent manner as compared with vehicle controls (P < 0.01). The ID50 of PAP-S to inhibit HBsAg and HBeAg production was 6.9 and 3.2 &mgr;g/mL on d 4, respectively. The therapeutic index (TI) for HBsAg and HBeAg was 4.3 and 9.3 on d 4, respectively. The data in this report clearly showed that the inhibitory activity of PAP-S on HBsAg and HBeAg was promoted time-dependently (P < 0.01). The suppression of HBV DNA was dose-dependent and approximately 53.3% inhibition was observed in the 4-d culture treated with PAP-S (10 &mgr;g/mL).
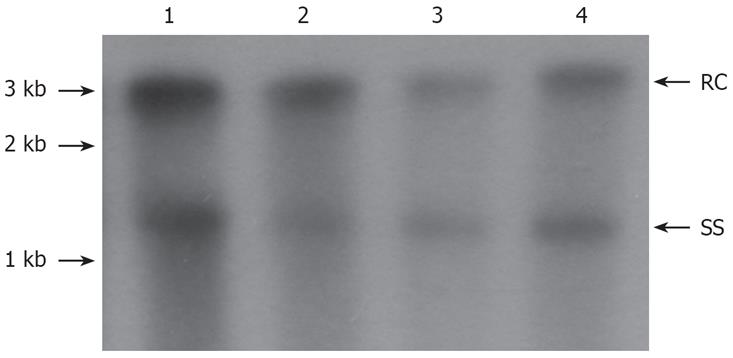
On d 3 after transfection, levels of HBV core-associated DNA were detected by Southern blot. As shown in Figure 2, Western blot showed that PAP was expressed in cotransfected HepG2 cells. As compared with controls, the levels of HBV nucleocapside-associated DNA were reduced by 38.0% and 74.0% respectively after transfection of 1.0 and 2.0 &mgr;g plasmid pXF3H-PAP into HepG2 cells. The levels of HBV nucleocapside-associated DNA were reduced by 63.0% when the transfected HepG2 cells were exposed to 20.0 &mgr;mol/L 3TC (Figure 3). The present results indicated that PAP reduced the HBV nucleocapside-associated DNA in a dose-dependent manner.
As compared with controls, the levels of HBsAg in the media were reduced by 76.8% and 99.7% respectively on d 3 after transfection of 1.0 &mgr;g and 2.0 &mgr;g plasmid pXF3H-PAP into HepG2 cells. The levels of HBsAg were reduced by 93.7% when the transfected HepG2 cells were exposed to 20.0 &mgr;mol/L 3TC. The levels of HBeAg in the media were reduced by 72.7% and 99.3% respectively after transfection of 1.0 &mgr;g and 2.0 &mgr;g plasmid pXF3H-PAP into HepG2 cells. The levels of HBeAg were reduced by 82.4% when the transfected HepG2 cells were exposed to 20.0 &mgr;mol/L 3TC. The present results indicated that PAP reduced the levels of HBsAg and HBeAg in a dose-dependent manner.
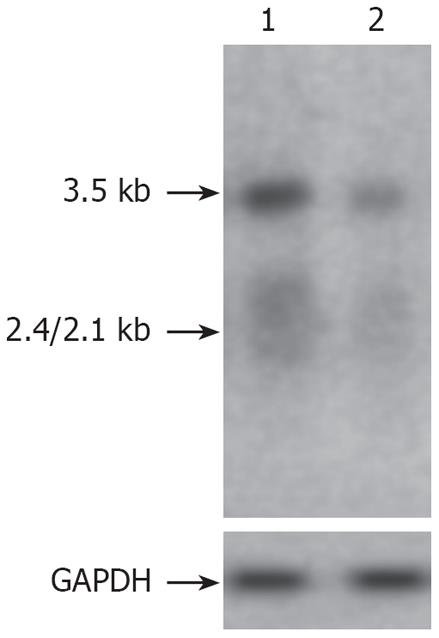
Northern blot indicated that the HBV core-associated RNA levels of 3.5 kb, 2.4/2.1 kb in cotransfected HepG2 cells were significantly suppressed by PAP (Figure 4). After transfection of 2.0 &mgr;g plasmid pXF3H-PAP, the levels of HBV core-associated RNA were reduced by 69% as compared with controls.
The anti-viral activity of PAP has been described against numerous pathogenic viruses[34], which included poliovirus, HIV, herpes simplex virus, cytomegalovirus, influenza virus and now, the double-strand DNA virus, hepatitis B virus. The ability of PAP to inhibit viral protein synthesis and depurinate viral RNA and DNA[1011] as well as capped rRNA and mRNAs[12] and its ability to inhibit ribosomal frame shifting and retransposition, make it an ideal candidate for anti-viral strategies.
In this study, we evaluated the effects of PAP from PAP-S on hepatitis B virus in vitro by the HepG2.2.15 cell line transfected with hepatitis B virus DNA. Furthermore, we constructed the eukaryotic expression plasmid encoding PAP, cotransfected it and the replication competent wild-type plasmid pHBV1.3 into the HepG2 hepatoma cells in order to explore the inhibitory effect of PAP on HBV replication.
Previous studies suggested that the antiviral activity of PAP was attributed to its ability to inhibit protein synthesis by catalytically cleaving a specific adenine base from the highly conserved alpha-sarcin/ricin loop (SRL) of the large ribosomal RNA[13]. However, animal studies have suggested that the antiviral action of PAP may not be contributed to its inactivation of ribosomes[1415]. PAP produced only 30% inhibition of total protein synthesis in herpes simplex virus-infected cells, whereas it inhibited virus production by 90%[16]. Similarly, it has been reported that PAP inhibits HIV-1 production of p24 in both T cells and macrophages at concentrations that do not adversely affect protein synthesis[17], which suggested that antiviral activity of PAP can be dissociated from its toxicity[18–21].
Further research discovered that not only rRNA but also polynucleotide, single-stranded DNA, double-stranded DNA and mRNA, can be depurinated by PAP[2223]. Rajamohan[1124] treated HIV-1 RNA with various concentrations of PAP. The depurinating activity of test PAP was determined by measuring the amount of adenine and guanine released from the HIV-1 RNA using a quantitative HPLC method, which confirmed that PAP caused a concentration-dependent depurination from HIV-1 RNA. These findings indicate that PAP should be capable of recognizing and depurinating viral RNA.
In this study, it was found that both PAP-S and PAP encoded by a eukaryotic expression plasmid (pXF3H-PAP) could inhibit the levels of HBsAg, HBeAg and HBV DNA in a dose-dependent manner in vitro. At the RNA level, PAP might be capable of recognizing and depurinating HBV-mRNA, which included 3.5 kb pregenome mRNA and 2.4/2.1 kb pregenome mRNA, so that the levels of HBV mRNA were reduced. At the DNA level, on one hand, because the 3.5 kb pregenome mRNA was inhibited, the levels of HBcAg/HBeAg and HBV polymerase translated from 3.5 kb pregenome mRNA and the minus strand HBV DNA transcribed from the 3.5 kb pregenome mRNA template were reduced accordingly. On the other hand, PAP might be capable of recognizing and depurinating the new synthesized HBV DNA, so that the levels of HBV DNA were reduced. At the protein level, on one hand, because HBV-mRNA was inhibited, the HBcAg and HBeAg translated from 3.5 kb mRNA and the HBsAg and pre-S antigen translated from 2.4 kb or 2.1 kb mRNA were inhibited accordingly. On the other hand, PAP might inhibit HBV protein translation by depurinating rRNA.
Further research demonstrated that PAP cleaved supercoiled pBR322 dsDNA, generating relaxed and linear molecules[25]. PAP could identify the characteristic spatial conformation of supercoiled DNA and hydrolyze AMP in specific area, turn the supercoiled DNA into nicked circle DNA or linear DNA so that the supercoiled DNA exerted irreversible topological inactivation[26]. HBV covalently closed circle (cccDNA) is also supercoiled DNA and has a characteristic spatial conformation, so we speculated that PAP could inhibit HBV by identifying and inactivating the HBV cccDNA template.
Several nucleoside analogs are under clinical development for use against HBV. Lamivudine (3TC), a nucleoside analog, and adefovir dipivoxil (ADV), an acyclonucleotide analog, are clinically approved. However, long-term treatment can induce viral resistance, and following the cessation of therapy, viral rebound is frequently observed[2728]. There continues to be a need for new antiviral agents with novel mechanisms of action. The anti-HBV mechanism of PAP might be different from 3TC that targeted the viral polymerase. A previous study had reported that PAP exhibited potent in vivo activities against nucleoside reverse transcriptase inhibitor-resistant HIV-1 in a surrogate human peripheral blood lymphocyte (Hu-PBL) SCID mouse model of human AIDS[29]. More work is needed, however, to determine the mechanism of anti-HBV activity of PAP.
Moreover, we have detected the cytotoxicity of PAP on the cell and found that the tested concentration of PAP exerted little growth inhibition. The findings demonstrated that repetitive intravaginal administration of PAP at concentrations as high as 2000 times its in vitro anti-HIV IC50 value was not associated with local or systemic toxicity[30]. PAP may be useful as an anti-HBV agent.
In conclusion, the present study demonstrated that the eukaryotic expression plasmid pXF3H-PAP and PAP-S could effectively inhibit HBV antigen secretion and HBV replication in a dose-dependent manner in vitro. The present anti-HBV medicine cannot eliminate HBV cccDNA in the hepatocytes so that they cannot clear HBV thoroughly. PAP is therefore worthy to be further investigated as an excellent candidate for potential clinical studies.
Hepatitis B virus (HBV) is a major cause of liver disease worldwide, ranging from acute and chronic hepatitis to cirrhosis and hepatocellular carcinoma. The anti-HBV medicines such as interferon and nucleoside analogs are currently being implemented to treat affected patients. However, long-term treatment can induce viral resistance, and following the cessation of therapy, viral rebound is frequently observed. There continues to be a need for new antiviral agents with novel mechanisms of action.
The aim of this study is to explore the inhibitory effects of Pokeweed antiviral protein (PAP) on HBV replication in vitro.
This study clearly showed that PAP could effectively inhibit HBV replication and antigen expression in a dose-dependent manner in vitro. This is the first report of the anti-HBV effects of PAP.
PAP might be useful as a potential alternative or complementary anti-HBV agent. The perspective of future application: the further study for the exact mechanisms of anti-HBV activity of PAP.
This is an interesting study. PAP has not been studied against HBV previously, so the manuscript provides new information to confirm that PAP, as expected, has an antiviral effect against HBV. The data and anti-HBV mechanisms are novel.
| 1. | Kurinov IV, Uckun FM. High resolution X-ray structure of potent anti-HIV pokeweed antiviral protein-III. Biochem Pharmacol. 2003;65:1709-1717. |
| 2. | Rajamohan F, Ozer Z, Mao C, Uckun FM. Active center cleft residues of pokeweed antiviral protein mediate its high-affinity binding to the ribosomal protein L3. Biochemistry. 2001;40:9104-9114. |
| 3. | Parikh BA, Tumer NE. Antiviral activity of ribosome inactivating proteins in medicine. Mini Rev Med Chem. 2004;4:523-543. |
| 4. | D'Cruz OJ, Waurzyniakt B, Uckun FM. A 13-week subchronic intravaginal toxicity study of pokeweed antiviral protein in mice. Phytomedicine. 2004;11:342-351. |
| 5. | Van Oijen MG, Preijers FW. Rationale for the use of immunotoxins in the treatment of HIV-infected humans. J Drug Target. 1998;5:75-91. |
| 6. | Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118-1129. |
| 7. | The EASL Jury. EASL International Consensus Conference on Hepatitis B. 13-14 September, 2002: Geneva, Switzerland. Consensus statement (short version). J Hepatol. 2003;38:533-540. |
| 8. | Gish RG, Perrillo RP, Jacobson IM. Customizing the management of chronic hepatitis B virus infection. Semin Liver Dis. 2007;27 Suppl 1:9-17. |
| 9. | Lu X, Hazboun T, Block T. Limited proteolysis induces woodchuck hepatitis virus infectivity for human HepG2 cells. Virus Res. 2001;73:27-40. |
| 10. | Rajamohan F, Engstrom CR, Denton TJ, Engen LA, Kourinov I, Uckun FM. High-level expression and purification of biologically active recombinant pokeweed antiviral protein. Protein Expr Purif. 1999;16:359-368. |
| 11. | Rajamohan F, Kurinov IV, Venkatachalam TK, Uckun FM. Deguanylation of human immunodeficiency virus (HIV-1) RNA by recombinant pokeweed antiviral protein. Biochem Biophys Res Commun. 1999;263:419-424. |
| 12. | Hudak KA, Bauman JD, Tumer NE. Pokeweed antiviral protein binds to the cap structure of eukaryotic mRNA and depurinates the mRNA downstream of the cap. RNA. 2002;8:1148-1159. |
| 13. | Mansouri S, Nourollahzadeh E, Hudak KA. Pokeweed antiviral protein depurinates the sarcin/ricin loop of the rRNA prior to binding of aminoacyl-tRNA to the ribosomal A-site. RNA. 2006;12:1683-1692. |
| 14. | Qi L, Nett TM, Allen MC, Sha X, Harrison GS, Frederick BA, Crawford ED, Glode LM. Binding and cytotoxicity of conjugated and recombinant fusion proteins targeted to the gonadotropin-releasing hormone receptor. Cancer Res. 2004;64:2090-2095. |
| 15. | Ball BA, Sabeur K, Nett T, Liu IK. Effects of a GnRH cytotoxin on reproductive function in peripubertal male dogs. Theriogenology. 2006;66:766-774. |
| 16. | Honjo E, Watanabe K. Expression of mature pokeweed antiviral protein with or without C-terminal extrapeptide in Escherichia coli as a fusion with maltose-binding protein. Biosci Biotechnol Biochem. 1999;63:1291-1294. |
| 17. | D’Cruz OJ, Uckun FM. Pokeweed antiviral protein: a potential nonspermicidal prophylactic antiviral agent. Fertil Steril. 2001;75:106-114. |
| 18. | Roday S, Saen-oon S, Schramm VL. Vinyldeoxyadenosine in a sarcin-ricin RNA loop and its binding to ricin toxin a-chain. Biochemistry. 2007;46:6169-6182. |
| 19. | Katayama DS, Cornell Manning M, Jarosz P. Solution behavior of a novel biopharmaceutical drug candidate: a gonadotropin-toxin conjugate. Drug Dev Ind Pharm. 2006;32:1175-1184. |
| 20. | Parikh BA, Baykal U, Di R, Tumer NE. Evidence for retro-translocation of pokeweed antiviral protein from endoplasmic reticulum into cytosol and separation of its activity on ribosomes from its activity on capped RNA. Biochemistry. 2005;44:2478-2490. |
| 21. | Baykal U, Tumer NE. The C-terminus of pokeweed antiviral protein has distinct roles in transport to the cytosol, ribosome depurination and cytotoxicity. Plant J. 2007;49:995-1007. |
| 22. | Picard D, Kao CC, Hudak KA. Pokeweed antiviral protein inhibits brome mosaic virus replication in plant cells. J Biol Chem. 2005;280:20069-20075. |
| 23. | Uckun FM, Rustamova L, Vassilev AO, Tibbles HE, Petkevich AS. CNS activity of Pokeweed anti-viral protein (PAP) in mice infected with lymphocytic choriomeningitis virus (LCMV). BMC Infect Dis. 2005;5:9. |
| 24. | Rajamohan F, Venkatachalam TK, Irvin JD, Uckun FM. Pokeweed antiviral protein isoforms PAP-I, PAP-II, and PAP-III depurinate RNA of human immunodeficiency virus (HIV)-1. Biochem Biophys Res Commun. 1999;260:453-458. |
| 25. | Aceto S, Di Maro A, Conforto B, Siniscalco GG, Parente A, Delli Bovi P, Gaudio L. Nicking activity on pBR322 DNA of ribosome inactivating proteins from Phytolacca dioica L. leaves. Biol Chem. 2005;386:307-317. |
| 26. | Wang M, Hudak KA. A novel interaction of pokeweed antiviral protein with translation initiation factors 4G and iso4G: a potential indirect mechanism to access viral RNAs. Nucleic Acids Res. 2006;34:1174-1181. |
| 27. | Iyer RP, Jin Y, Roland A, Morrey JD, Mounir S, Korba B. Phosphorothioate di- and trinucleotides as a novel class of anti-hepatitis B virus agents. Antimicrob Agents Chemother. 2004;48:2199-2205. |
| 28. | Brunelle MN, Jacquard AC, Pichoud C, Durantel D, Carrouee-Durantel S, Villeneuve JP, Trepo C, Zoulim F. Susceptibility to antivirals of a human HBV strain with mutations conferring resistance to both lamivudine and adefovir. Hepatology. 2005;41:1391-1398. |
| 29. | Uckun FM, Rajamohan F, Pendergrass S, Ozer Z, Waurzyniak B, Mao C. Structure-based design and engineering of a nontoxic recombinant pokeweed antiviral protein with potent anti-human immunodeficiency virus activity. Antimicrob Agents Chemother. 2003;47:1052-1061. |
| 30. | D’Cruz OJ, Waurzyniak B, Uckun FM. Mucosal toxicity studies of a gel formulation of native pokeweed antiviral protein. Toxicol Pathol. 2004;32:212-221. |