Published online Mar 14, 2008. doi: 10.3748/wjg.14.1549
Revised: January 24, 2008
Published online: March 14, 2008
AIM: To measure plasma D-dimer levels in cirrhotic patients with and without ascites, assessing the effect of ascites resolution in D-dimer concentration.
METHODS: Seventy consecutive cirrhotic patients (M = 44, F = 26, mean age 65 years, SD ± 13), observed from October 2005 to March 2006 were enrolled. Circulating D-dimer levels were measured using a latex-enhanced, immunoturbidimetric test. In patients with ascites (n = 42) the test was repeated after ascites resolution.
RESULTS: Ascites was present in 42 patients (group A) and absent in 28 (group B). Group A patients had more advanced liver disease. Hepatocellular carcinoma (HCC) was diagnosed in 14 patients and was more frequent in group B. Above normal range D-dimers were found in 45/70 patients. High D-dimers were more frequent in group A than in group B (P = 0.001). High D-dimers were associated with presence of HCC (P = 0.048) only in group B. After ascites resolution, obtained in all patients, mean D-dimer values decreased in those 34 patients with high basal levels (P = 0.007), returning to normal in 17.
CONCLUSION: In patients with liver cirrhosis, ascites and HCC are the main factors associated with increased fibrinolytic activity.
- Citation: Spadaro A, Tortorella V, Morace C, Fortiguerra A, Composto P, Bonfiglio C, Alibrandi A, Luigiano C, Caro GD, Ajello A, Ferraù O, Freni MA. High circulating D-dimers are associated with ascites and hepatocellular carcinoma in liver cirrhosis. World J Gastroenterol 2008; 14(10): 1549-1552
- URL: https://www.wjgnet.com/1007-9327/full/v14/i10/1549.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1549
Hemostasis is a dynamic process resulting from the balance between procoagulant and anticoagulant factors. The liver is the site of production of most proteins which favour and inhibit the process of coagulation and fibrinolysis. Patients with liver cirrhosis may develop a serious coagulopathy whose origin is commonly ascribed to a defective hepatic synthesis of clotting factors often in association with thrombocytopenia secondary to portal hypertension. In addition, patients with advanced liver disease have a hyperfibrinolytic state which contributes to the bleeding tendency causing a premature removal of the hemostatic plug. Plasma levels of fragment D-dimer represent an accurate marker of fibrinolytic activity. The finding of high D-dimer plasma concentration in patients with liver cirrhosis, decompensated by ascites, led Agarwal et al[1] to suggest a major role of ascites in the pathogenesis of hyperfibrinolytic state associated with liver failure. The aims of this study were: (1) to evaluate the relationship between the presence of ascites and hyperfibrinolytic state in liver cirrhosis measuring the circulating levels of D-dimer in patients with and without ascites and (2) to assess the effect of ascites resolution in the plasma concentration of D-dimers.
The study was designed to measure plasma D-dimer levels in all consecutive patients with cirrhosis of the liver referring to the Unit of Clinica Medica, Messina University, Italy, during a 6 mo period (October 2005-March 2006), with the exclusion of patients with deep venous or portal vein thrombosis. We included 70 patients and excluded 1 patient with portal vein thrombosis. Informed consents were obtained from all patients. The diagnosis of cirrhosis was either histological or made by clinical and/or imaging signs of portal hypertension. The etiology of liver disease was HCV infection in 31 patients, HBV infection in 4, past alcohol abuse in 15, autoimmune in 2, HBV + HCV coinfection in 1, primary biliary cirrhosis in 1, HCV + alcohol abuse in 2 and cryptogenic in 14. Hepatocellular carcinoma (HCC) was diagnosed in 14 patients. All patients underwent color-Doppler ultrasonographic study of the abdomen and the legs. Severity of liver disease was classified by Child-Pugh score. Patients were divided into 2 groups according to the presence of ascites. Patients with ascites were treated with fluid and salt restriction, diuretics and/or paracenthesis. The resolution of ascites was confirmed by ultrasonography. D-dimer levels were measured also after disappearance of abdominal fluid. The demographic and clinical features of the patients studied are shown in Table 1.
| Ascites | No ascites | P value | |
| n = 42 | n = 28 | ||
| Gender M/F | 24/18 | 20/8 | 0.735 |
| Mean age ± SD (yr) | 67 ± 14 | 64 ± 14 | 0.068 |
| Child-Pugh class (%) | 0.004 | ||
| A | 8 (19.0) | 18 (64.3) | |
| B | 18 (42.9) | 8 (28.6) | |
| C | 16 (38.0) | 2 (7.1) | |
| Patients with HCC (%) | 2 (4.8) | 12 (42.8) | 0.000 |
Peripheral blood was collected into tubes containing sodium citrate solution. After centrifugation (10 min, 1500 ×g), the supernatant plasma was removed. Plasma D-dimer was measured by a latex-enhanced, immunoturbidimetric test using a commercially available kit (D-dimer PLUS, Dade Behring, Marburg, Germany). The D-dimer concentration was expressed in &mgr;g/L with a normal range of 125-350 &mgr;g/L.
Differences between groups were evaluated by non parametric permutation Test (NPC test) for numerical variables and by Anderson Darling Test for categorical variables[2]. Association between D-dimer values and gender of patients, Child-Pugh class and HCC presence was analyzed by Log-Likelihood ratio test[3]. Correlation between D-dimer values and age of patients was evaluated using biserial correlation coefficient[4]. Influence of the variables, gender, age, Child-Pugh class and HCC presence on D-dimer values was estimated by a logistic regression model[5]. Softwares SPPS, Windows 11.0 (2001) for Binary Logistic Regression, Microsoft Excel (2002) for G test, Methodologica S.R.L. (2001) for nonparametric analysis NPC test and Anderson Darling test were used.
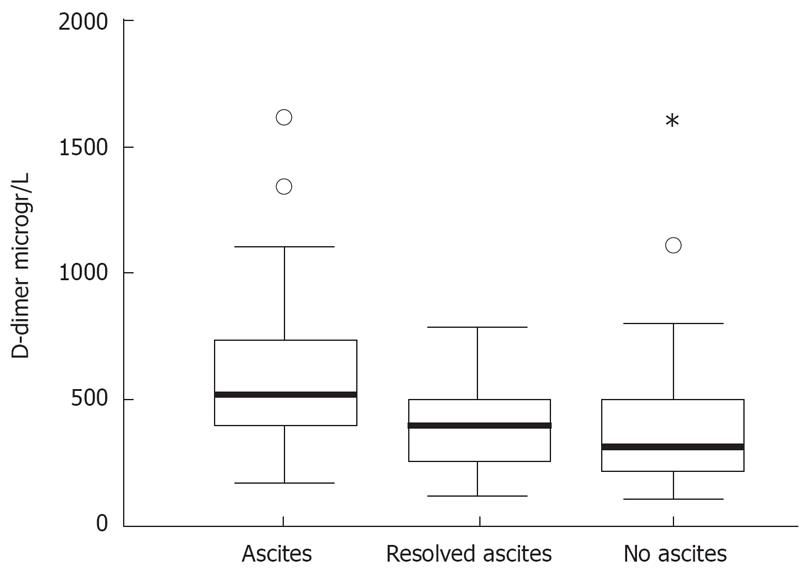
Ascites was present in 42 patients (group A) and absent in 28 (group B). No significant differences in age and gender were found between the two groups. Patients of group A had more advanced liver disease. HCC was more frequent in patients of group B (Table 1). Plasma D-dimer levels above the normal range were found in 45/70 patients (64.3%). D-dimer above normal values were more frequent (P = 0.001) in group A (34/42) than in group B (11/28). D-dimer mean values were higher (P = 0.001) in group A (649 ± 420 g/L) than in group B (359 ± 219 g/L, Figure 1). In all patients of group A resolution of ascites was obtained either by fluid and salt restriction and conventional diuretic treatment (in 36) or by paracenthesis (in 6). After disappearance of ascitic fluid, confirmed by ultrasonography, mean D-dimer values decreased in all 34 patients with high basal levels (P = 0.007), returning to normal range in 17. In these patients, D-dimer values after resolution of ascites (438 ± 279 g/L) were not significantly different from those found in patients without ascites, group B, (Figure 1).
In the whole population of patients or the separate groups A and B, high D-dimer levels were not associated with gender neither with Child-Pugh class (Anderson-Darling test) and did not correlate with anagraphic age. Only in patients without ascites, group B, high D-dimer levels were associated with presence of HCC (P = 0.048). When we inserted the variables age, gender, Child-Pugh class and presence of HCC in a logistic regression model (where the response variable was represented by normal or high D-dimer class), we found that high levels of D-dimer were significantly dependent on the presence of HCC (P = 0.030) in patients without ascites (group B) and negatively dependent on age (P = 0.019) in patients with ascites (Group A).
Patients with liver cirrhosis have a bleeding tendency that is often not evident from routine clotting studies. In such patients, the incidence of hyperfibrinolysis varies from 19% to 95%[6–8] and may contribute to serious bleeding complications. The pathogenesis of hyperfibrinolysis in liver cirrhosis is not yet clearly known. Having found high D-dimer levels in the blood and in the ascitic fluid, Agarwal et al suggested ascites among the possible causes of increased fibrinolysis in patients with liver cirrhosis[1].
The aim of our study was (1) to evaluate the relationship between the presence of ascites and hyperfibrinolytic state in cirrhotic patients measuring the circulating levels of D-dimer in those with and without ascites and (2) to assess the effect of ascites resolution in the concentration of D-dimers. High D-dimer levels in 64% of patients with liver cirrhosis were observed. This finding is in agreement with that of Agarwal et al who reported increased plasma D-dimers in 63% of patients with liver cirrhosis[1]. This percentage is higher than that reported in another study where abnormal D-dimers were found only in 17% of patients with chronic liver disease. However, 11 out of 86 patients included were not cirrhotic and the number of ascitic patients was not specified[9] . When we divided our patients into two groups, according to the presence of ascites, we found high D-dimers in 81% of patients with ascites and in 39% of patients without. This finding is also consistent with that of Agarwal et al who showed increased plasma D-dimer values in 93% and 33% of patients with and without ascites, respectively[1]. Furthermore, our patients with ascites had mean D-dimer values significantly higher than those without. After resolution of ascites, circulating D-dimers decreased significantly in all patients, returning to normal in half of them. Also, mean D-dimer levels in cirrhotic patients with resolved ascites were not significantly different from those in patients who entered the study without ascites. Therefore, our data confirm the association between circulating high D-dimer levels and the presence of ascites found in cirrhotic patients.
Another interesting finding of our study is the close association between presence of HCC and high D-dimer values in patients without ascites. HCC is often associated with thrombotic invasion of portal or hepatic vein. Although we excluded from the study all patients with clinical and imaging features of thrombosis, we cannot rule out the presence of microvascular invasion in patients with HCC. In fact, Kim et al[10] also found increased circulating D-dimers in patients with HCC, even in absence of tumor thrombosis in a major branch of the portal or the hepatic vein.
The underlying mechanism for high D-dimer plasmatic levels in cirrhotic patients with ascites remains to be clarified. Some authors suggest the exchange of some coagulation and fibrinolytic proteins between plasma and ascitic fluid[111]. Violi et al propose that hyperfibrinolysis in cirrhotic patients might represent a state of low grade disseminated intravascular coagulation secondary to the passage of gut absorbed bacterial material into the systemic circulation[12]. On the basis of this finding, Piscaglia et al argue that the association between high plasma D-dimers and ascites might be due only to more advanced liver disease with portal hypertension favouring bacterial translocation. In our study, however, we were not able to demonstrate any correlation between circulating D-dimers and severity of disease[13].
Although some of our patients with high D-dimers had no ascites, it must be underlined that 64% of them had HCC. In conclusion, our study shows that high D-dimers are associated either with presence of ascites or with HCC. In patients with liver cirrhosis, high D-dimer levels in absence of ascites require more careful monitoring for HCC. Depletion of ascitic fluid might prevent bleeding complications, especially if invasive procedures become necessary.
Patients with advanced liver disease have a hyperfibrinolytic state which contributes to the bleeding tendency causing a premature removal of the hemostatic plug. Plasma levels of fragment D-dimer represent an accurate marker of fibrinolytic activity.
The finding of high D-dimer plasma concentration in patients with liver cirrhosis, decompensated by ascites, led Agarwal et al to suggest a major role of ascites in the pathogenesis of hyperfibrinolytic state associated with liver failure.
High D-dimer plasma levels in patients with liver cirrhosis are associated with presence of ascites and decline after its resolution. In absence of ascites, high D-dimer values are associated with presence of hepatocellular carcinoma (HCC).
In patients with liver cirrhosis depletion of ascitic fluid might prevent bleeding complications, especially if invasive procedures become necessary. In absence of ascites, high D-dimer levels rise suspect of HCC and require more careful monitoring.
Fragment D-dimer is a degradation product from a specific region of cross-linked fibrin. High plasma D-dimer levels represent a sensitive but not specific marker of fibrinolysis and thrombosis.
This was a modest but interesting paper with some potential clinic significance.
| 1. | Agarwal S, Joyner KA Jr, Swaim MW. Ascites fluid as a possible origin for hyperfibrinolysis in advanced liver disease. Am J Gastroenterol. 2000;95:3218-3224. |
| 2. | Pesarin F. Non Parametric Combination Methodology. Multivariate permutation tests: with application in biostatistics. Chichester, New York, Weinheim, Brisbane, Singapore, Toronto: John Wiley & Sons 2001; 133-163. |
| 3. | Fahrmeir L, Tutz G. Survival models. Multivariate statistical modelling based on generalized linear models. 2nd ed. New York: Springer 2001; 385-429. |
| 4. | Che PY, Popovich PM. Correlation: parametric and non-parametric measures. Newbury Park , CA: Sage University Paper 2002; 95-97. |
| 5. | Kleimbaum DG. Introduction to logistic regression. Logistic regression. New York: Springer-Verlag 1994; 2-37. |
| 6. | Kang Y, Lewis JH, Navalgund A, Russell MW, Bontempo FA, Niren LS, Starzl TE. Epsilon-aminocaproic acid for treatment of fibrinolysis during liver transplantation. Anesthesiology. 1987;66:766-773. |
| 7. | Porte RJ, Bontempo FA, Knot EA, Lewis JH, Kang YG, Starzl TE. Systemic effects of tissue plasminogen activator-associated fibrinolysis and its relation to thrombin generation in orthotopic liver transplantation. Transplantation. 1989;47:978-984. |
| 8. | Steib A, Gengenwin N, Freys G, Boudjema K, Levy S, Otteni JC. Predictive factors of hyperfibrinolytic activity during liver transplantation in cirrhotic patients. Br J Anaesth. 1994;73:645-648. |
| 9. | Hu KQ, Yu AS, Tiyyagura L, Redeker AG, Reynolds TB. Hyperfibrinolytic activity in hospitalized cirrhotic patients in a referral liver unit. Am J Gastroenterol. 2001;96:1581-1586. |
| 10. | Kim HK, Lee KR, Yang JH, Yoo SJ, Lee SW, Jang HJ, Park SJ, Moon YS, Park JW, Kim CM. Plasma levels of D-dimer and soluble fibrin polymer in patients with hepatocellular carcinoma: a possible predictor of tumor thrombosis. Thromb Res. 2003;109:125-129. |
| 11. | Toschi V, Rocchini GM, Motta A, Fiorini GF, Cimminiello C, Violi F, Castelli C, Sironi D, Gibelli A. The hyperfibrinolytic state of liver cirrhosis: possible pathogenetic role of ascites. Biomed Pharmacother. 1993;47:345-352. |
| 12. | Violi F, Ferro D, Basili S, Quintarelli C, Musca A, Cordova C, Balsano F. Hyperfibrinolysis resulting from clotting activation in patients with different degrees of cirrhosis. The CALC Group. Coagulation Abnormalities in Liver Cirrhosis. Hepatology. 1993;17:78-83. |
| 13. | Piscaglia F, Donati G, Giannini R, Bolondi L. Liver cirrhosis, ascites, and hyperfibrinolysis. Am J Gastroenterol. 2001;96:3222. |