Published online Jan 7, 2008. doi: 10.3748/wjg.14.76
Revised: September 16, 2007
Published online: January 7, 2008
AIM: To evaluate role of midkine secretion during Cadmium (Cd) exposure in the human hepatocyte cell line Hep3B cells.
METHODS: Different dosages of Cd (0.5-1-5-10 &mgr;g/mL) were applied to Hep3B cells and their effects to apoptosis, lactate dehydrogenase (LDH) leakage and midkine secretion were evaluated as time dependent manner. Same experiments were repeated with exogenously applied midkine (250-5000 pg/mL) and/or 5 &mgr;g/mL Cd.
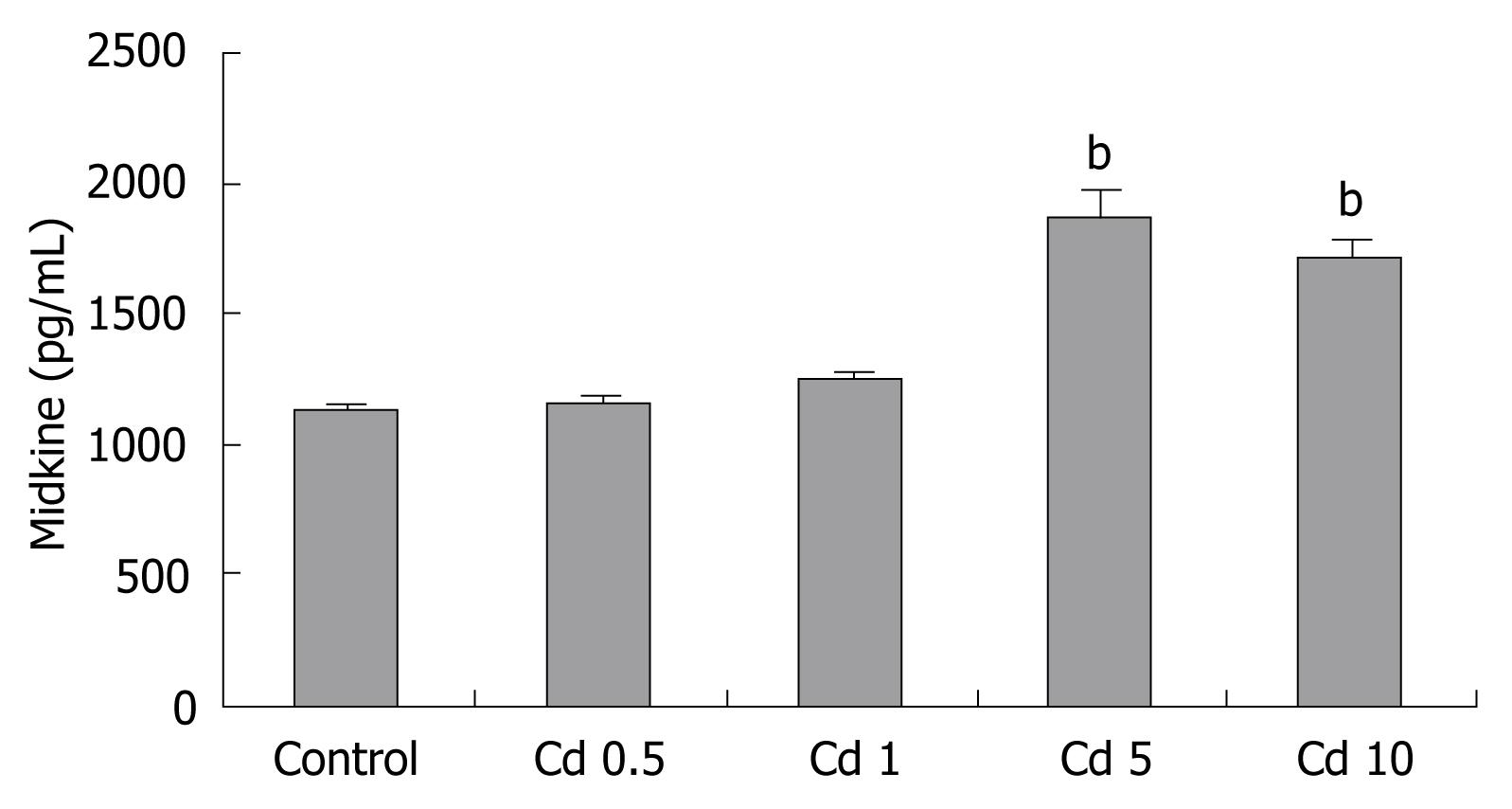
RESULTS: Cd exposure induced prominent apoptosis and LDH leakage beginning from lower dosages at the 48th h. Cd induced midkine secretion with higher dosages (P < 0.001), (control, Cd 0.5-1-5-10 &mgr;g/mL respectively: 1123 ± 73, 1157 ± 63, 1242 ± 90, 1886 ± 175, 1712 ± 166 pg/mL). Exogenous 500-5000 pg/mL midkine application during 5 &mgr;g/mL Cd toxicity prevented caspase-3 activation (control, Cd toxicity, 250, 500, 1000, 2500, 5000 pg/mL midkine+ Cd toxicity, respectively: 374 ± 64, 1786 ± 156, 1545 ± 179, 1203 ± 113, 974 ± 116, 646 ± 56, 556 ± 63 cfu) LDH leakage and cell death in Hep3B cells (P < 0.001).
CONCLUSION: Our results showed that midkine secretion from Hep3B cells during Cd exposure protects liver cells from Cd induced cellular damage. Midkine has anti-apoptotic and cytoprotective role during Cd toxicity. Further studies are needed to explain the mechanism of midkine secretion and cytoprotective role of midkine during Cd exposure. Midkine may be a promising therapeutic agent in different toxic hepatic diseases.
- Citation: Yazihan N, Ataoglu H, Akcil E, Yener B, Salman B, Aydin C. Midkine secretion protects Hep3B cells from cadmium induced cellular damage. World J Gastroenterol 2008; 14(1): 76-80
- URL: https://www.wjgnet.com/1007-9327/full/v14/i1/76.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.76
Cadmium (Cd) exposure occurs widely in the general population, especially low-level chronic exposure through smoking and dietary sources, but it is known as one of the most toxic environmental and industrial pollutants. Cd accumulates in the body because of slow excretion[1]. Cd causes toxicity in different organs. Acute and chronic Cd exposure mostly results in hepatotoxicity[2]. It seems that the level of damage depends on the dosage and duration of Cd application. Exposure of cells to toxic chemicals is known to up-regulate the expression of a number of stress proteins and results in activation of apoptotic pathways and consequently cellular damage. In vivo and in vitro studies showed that inflammation and oxidative damage are main mechanisms of Cd induced toxicity[3–6]. Cd induces mitochondria-dependent apoptotic pathways where caspase-3 and caspase-9 are activated[7].
Midkine is a heparin binding growth factor. It takes part in cancer and inflammation[8]. Although midkine is a mitogenic factor during carcinogenesis, it plays a critical role in ischemia induced inflammatory damage[9]. It was demonstrated that midkine acts as an antiapoptotic factor in HepG2 cells; furthermore, midkine suppressed the activity of caspase-3, which plays a significant role in the apoptotic pathway. Pretreatment with midkine prevents tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in the HepG2 cells[10]. TRAIL alone triggered massive apoptosis accompanied by caspase activation in tissue explants from patients with liver steatosis or hepatitis C viral infection[11]. TNF-alpha, released from nonparenchymal cells as well as associated cytokines, are responsible for clinical expression and tissue damage observed with cadmium-induced hepatotoxicity[12]. It was shown that midkine expression was upregulated in a marine gastropod limpet patella caerulea after they were exposed to sublethal doses of Cd[13]. But, whether midkine takes part in Cd induced mechanisms in human cells is still unknown. In this study we aimed to evaluate effects of Cd induced midkine secretion in the human hepatocyte cell line Hep3B cells, and its effects to cellular proliferation, apoptosis and biochemical parameter of cellular integrity during Cd exposure.
Human hepatoma cell line Hep3B cells were obtained from the ATCC. Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (PAA, Austuria), supplemented with fetal calf serum (FCS), (PAA, Austuria), L-glutamine (Sigma, USA), streptomycin (Sigma, USA) and penicillin (Sigma, USA). Cadmium (CdCl2, Sigma Chemical Co., St. Louis, MO, USA) was dissolved in water, sterilized by 0.22 &mgr;m pore size cellulose acetate membrane filters, and added to cultures at the indicated time and concentrations. CdCl2 toxicity was studied in Hep3B cell line. Human recombinant midkine was obtained from Peprotech (UK). Cell counts were tested by 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT, Sigma, USA). For evaluation of apoptosis caspase-3 levels were measured by a flourometric kit (Biotium, USA). Lactate Dehydrogenase (LDH) level was measured with a kit using an automatic multianalyzer (Roche; P800). Midkine levels were measured by an ELISA development kit (Peprotech,UK).
The human hepatoma cell line Hep3B was cultured in RPMI-1640 medium, supplemented with 10% v/v fetal calf serum, 2 mmol/L L-glutamine, streptomycin (100 &mgr;g/mL) and penicillin (100 IU/mL) in a humidified atmosphere containing 5% CO2 at 37°C. One day before the experiments, cells were seeded on 96-well microtitre plates (Nunc, Denmark) at 2 × 105 cells/mL.
Depending on the groups, different concentrations of cadmium (0.5-1-5-10 &mgr;g/mL) and midkine (250, 500, 1000, 2500, 5000 pg/mL) were added to medium for 48 h. For evaluation of the effects of midkine during cadmium toxicity; cadmium was used at a dosage of 5 &mgr;g/mL. n = 6, for every experimental group.
LDH and caspase-3 levels were evaluated from cadmium and/or midkine treated cells at the 48th h. MTT was measured at the 2nd, 24th and 48th h. Midkine was measured from supernatants. After supernatants were removed cell surface was washed with sterile phosphate buffered saline (PBS) and cells were harvested with lysis solution and caspase-3 levels of groups were measured from cell lysates. LDH measurement was done from both of the supernatant and cell lysates.
MTT, a colorimetric assay based upon the ability of living cells to reduce MTT into formazan, was used for evaluation of the effects of dose and time dependent effects of cadmium and midkine on cellular death or proliferation (2nd, 24th, 48th h). Cell number % was calculated as ratio of cell number of effected group vs control group × 100 at the determined hour.
Hep3B cells were plated in 96 multiwell cell culture plates as 3 × 105 cells/mL. LDH is normally present in the cytosol of hepatocytes. In response to cell damage LDH is released from the cells. Therefore, to determine cell death, we measured secreted and intracellular LDH levels and calculated % released LDH at the 48th h for each group. To do this, the medium was collected to measure enzyme activities. The adherent cells were lysed. Both medium and cell lysates were used for quantitative determination of LDH activity (IU/L) which was performed with an automatic multianalyzer (Roche) using a kit (Roche). Released enzyme fractions for each sample were calculated as the ratio of enzyme present in the medium vs the sum of the levels of same enzyme in the supernatant and in the cells.
Caspase-3 levels: The presence of apoptosis was determined by caspase-3 levels. Equal numbers of cells were used for caspase-3 level measurements. Cells were lysed with assay buffer (50 mmol/L HEPES, pH 7.4, 100 mmol/L NaCl, 0.1% CHAPS, 10 mmol/LM DTT, 2 mmol/L EDTA, 2 mmol/L EGTA, Triton X-100, 0.1%). Caspase-3 levels were measured by DEVD-R110 Fluorometric HTS Assay Kit from cell lysates. The fluorogenic substrate (Ac-DEVD)2-R110 was used for this assay. It is completely hydrolyzed by the enzyme in two successive steps. Cleavage of the first DEVD peptide results in the monopeptide Ac-DEVD-R110 intermediate, which has absorption and emission wavelengths similar to those of R110 (λabs/λabs = 496/520 nm), but has only about 10% the fluorescence of the latter. Hydrolysis of the second DEVD peptide releases the dye R110, leading to a substantial fluorescence increase.
Equal volumes of sample and caspase-3 detection buffer were added to assay plate, and then incubated at 37°C for 1 h in an incubator. Results were read with a fluorometer at 470 nm excitation filter and 520 nm emission filters. R110 was used for generating a standard curve to calculate amount of substrate conversion.
Results of the experiments were analyzed by One Way ANOVA, followed by a multiple comparison test using SPSS 10.0. P < 0.05 was accepted as statistically significant. Results were given as mean ± SEM.
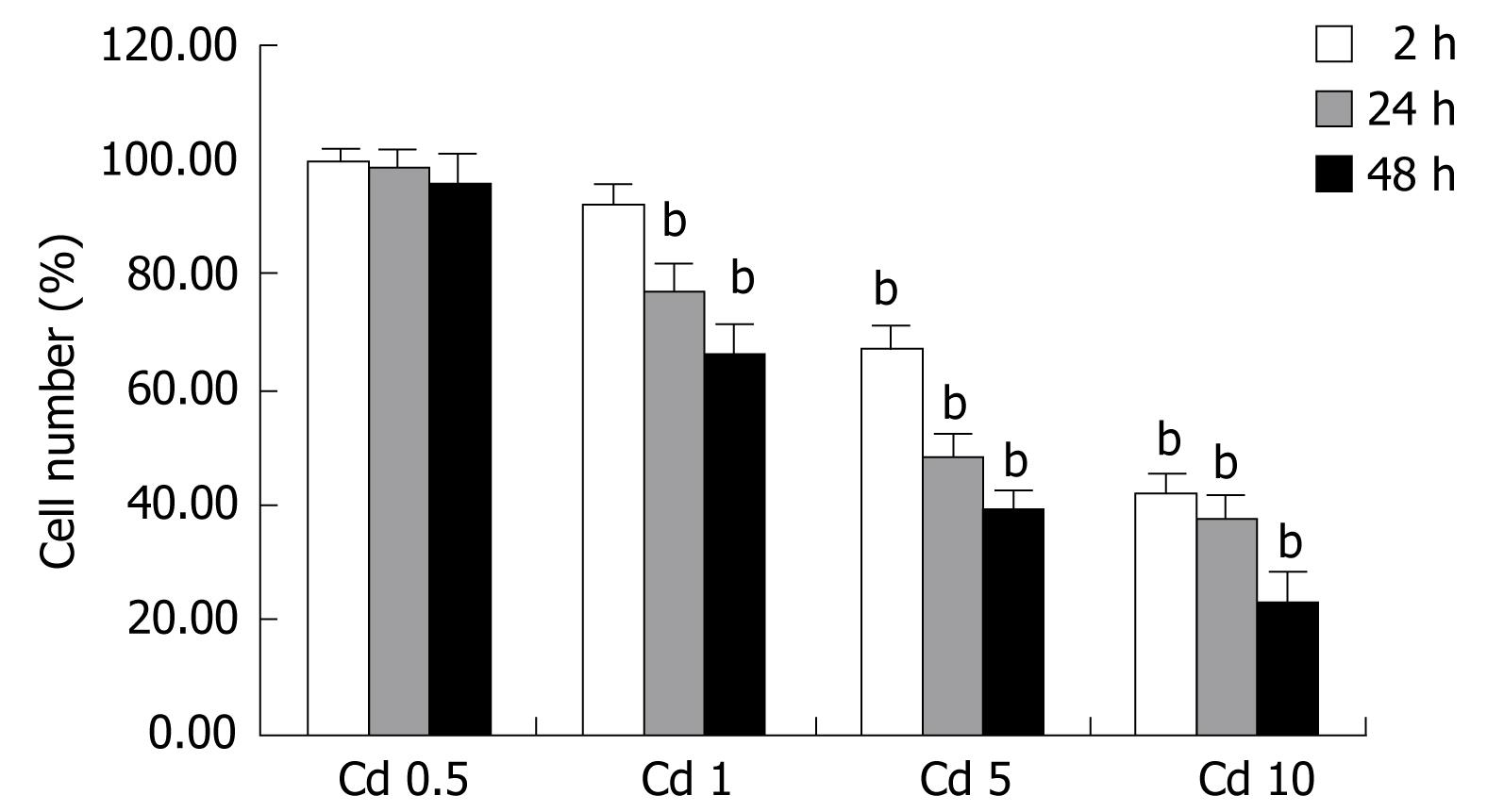
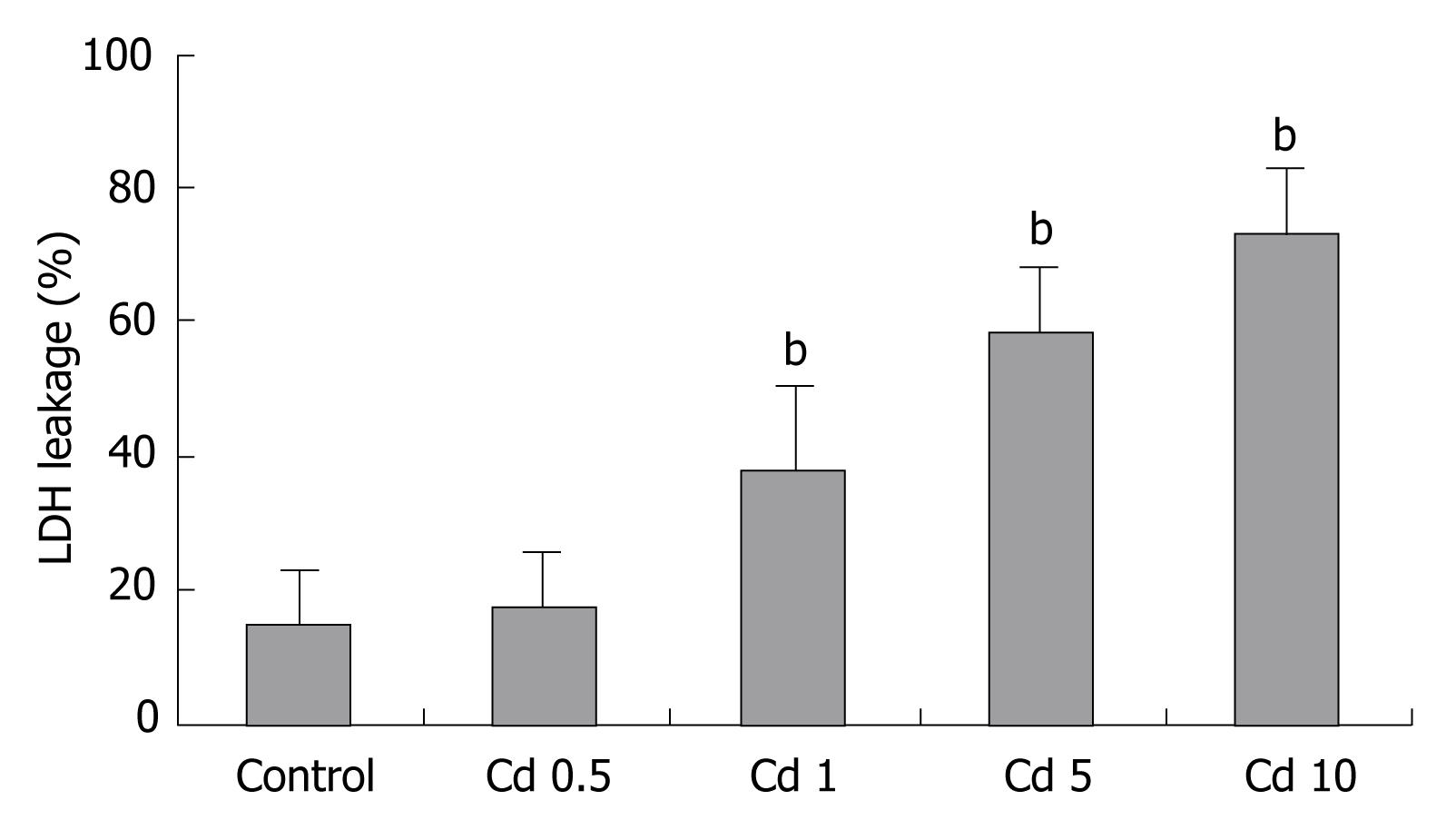
We characterized the concentration-dependent cytotoxic effect of Cd on human hepatocyte cell line as a function of time. Cadmium exposure decreased living cell number depending on the dosage. Minimal cytotoxic (< 5%) effect was seen at the 0.5 &mgr;g/mL dosage. As shown in Figure 1, cell exposure to 1 &mgr;g/mL Cd for up to 2 h only slightly affected cell viability as revealed by MTT measurements compared to control values estimated in untreated cells, but it becomes apparent at the 24th and 48th h. Cd exposure caused cellular damage in a dose and time dependent manner in the Hep3B cell line. Cytotoxicity was more prominent with higher dosages at the 24th and 48th h (P < 0.001, Figure 1). Regarding to these data, 5 &mgr;g/mL CdCl2 concentration which with moderate-high toxic impact was chosen for subsequent experiments with different dosages of midkine.
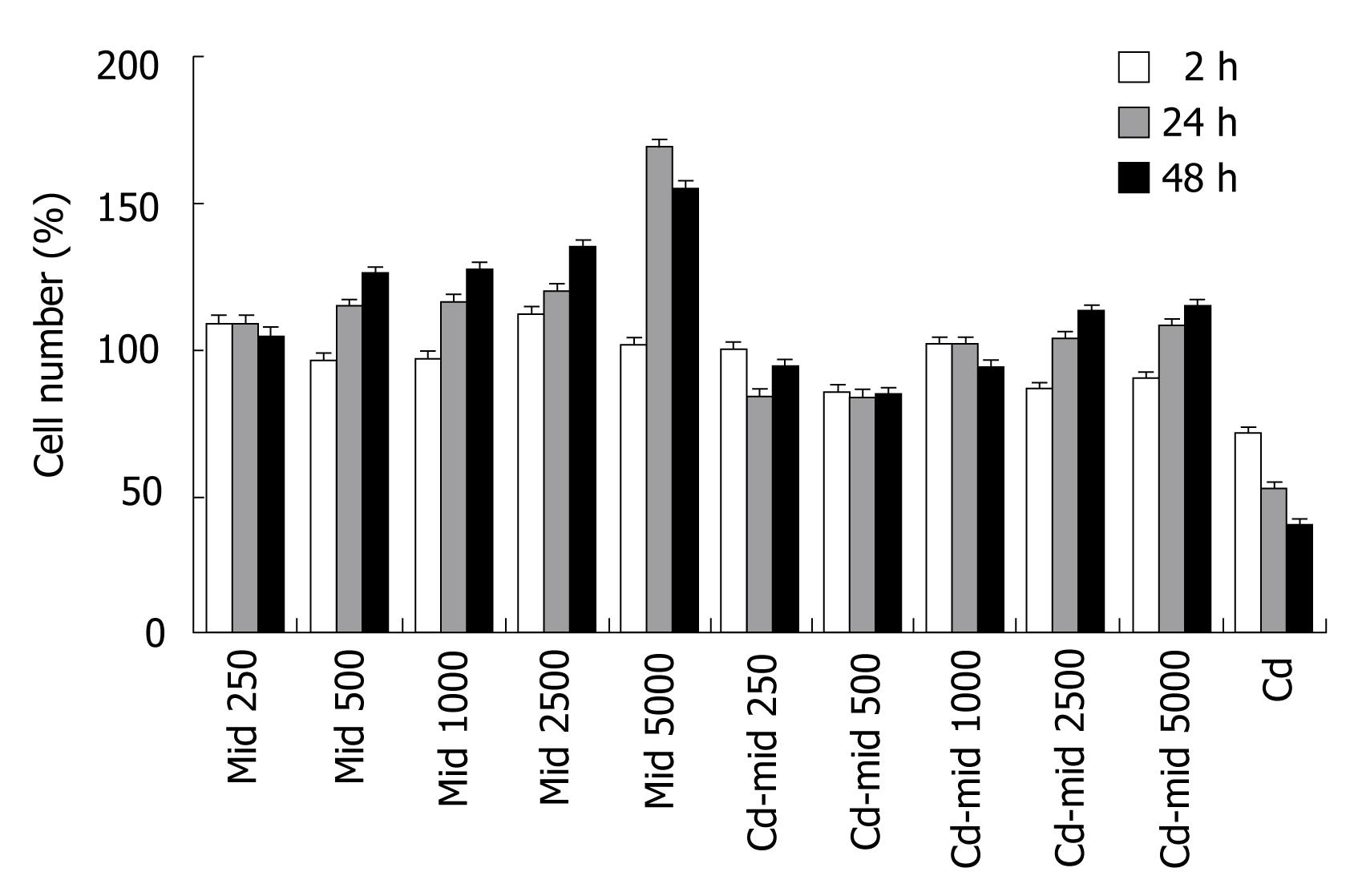
Midkine treatment caused proliferation of Hep3B cells in a dose and time dependent manner compared to control group. The highest increase in cell number was at the 48th h and 5000 pg/mL midkine concentration (P < 0.001, Figure 2). Midkine treatment during Cd toxicity prevents cell death, even with the lowest dosages (P < 0.001, Figure 2).
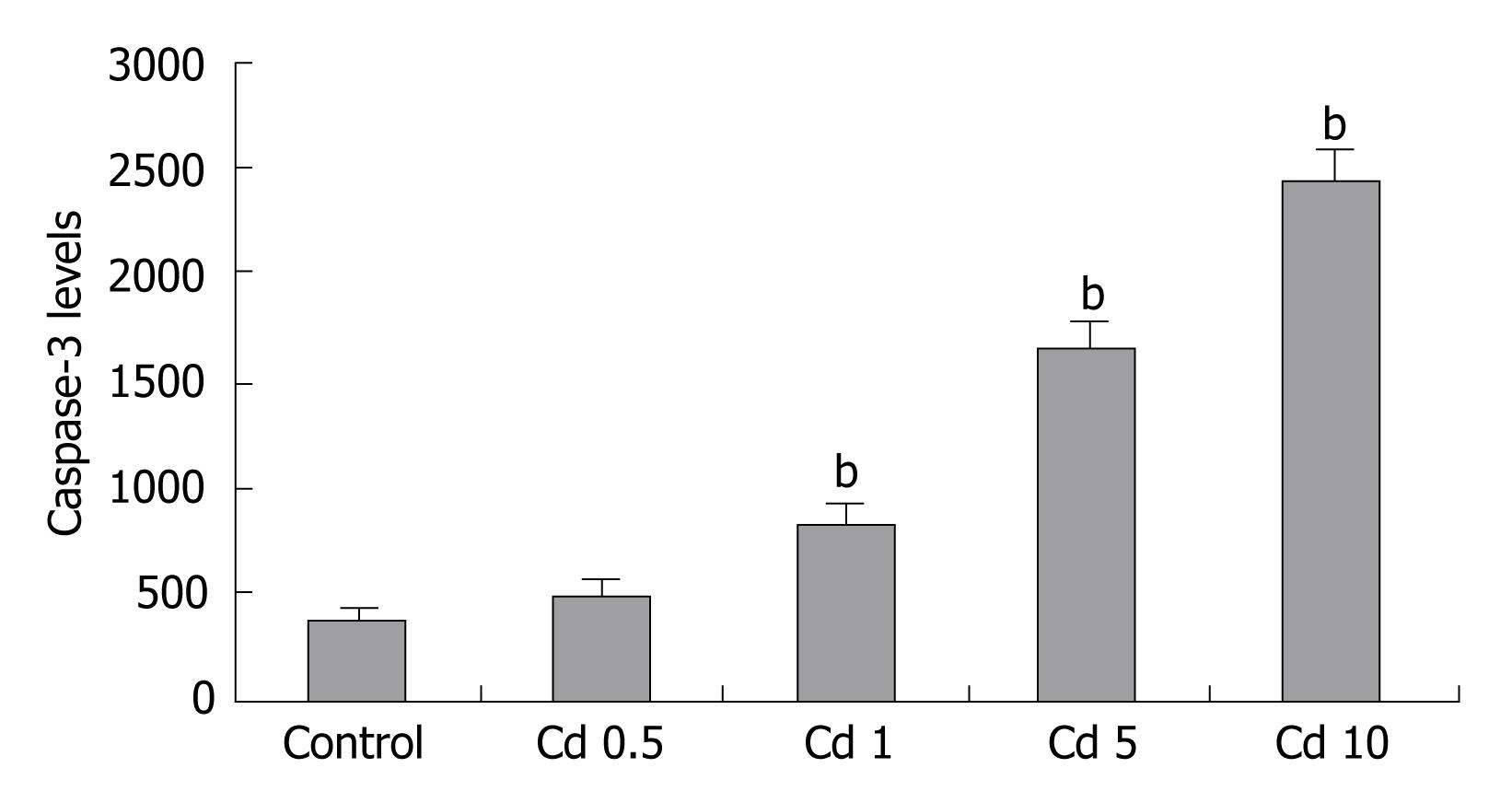
Increased apoptosis was seen in Cd treated cells, which was confirmed with increased caspase-3 levels. Lowest dosage of Cd application did not increase caspase-3 levels compared to untreated cells. Activation of caspase-3 started at the 1 &mgr;g/mL Cd dosage (P < 0.001, Figure 3).
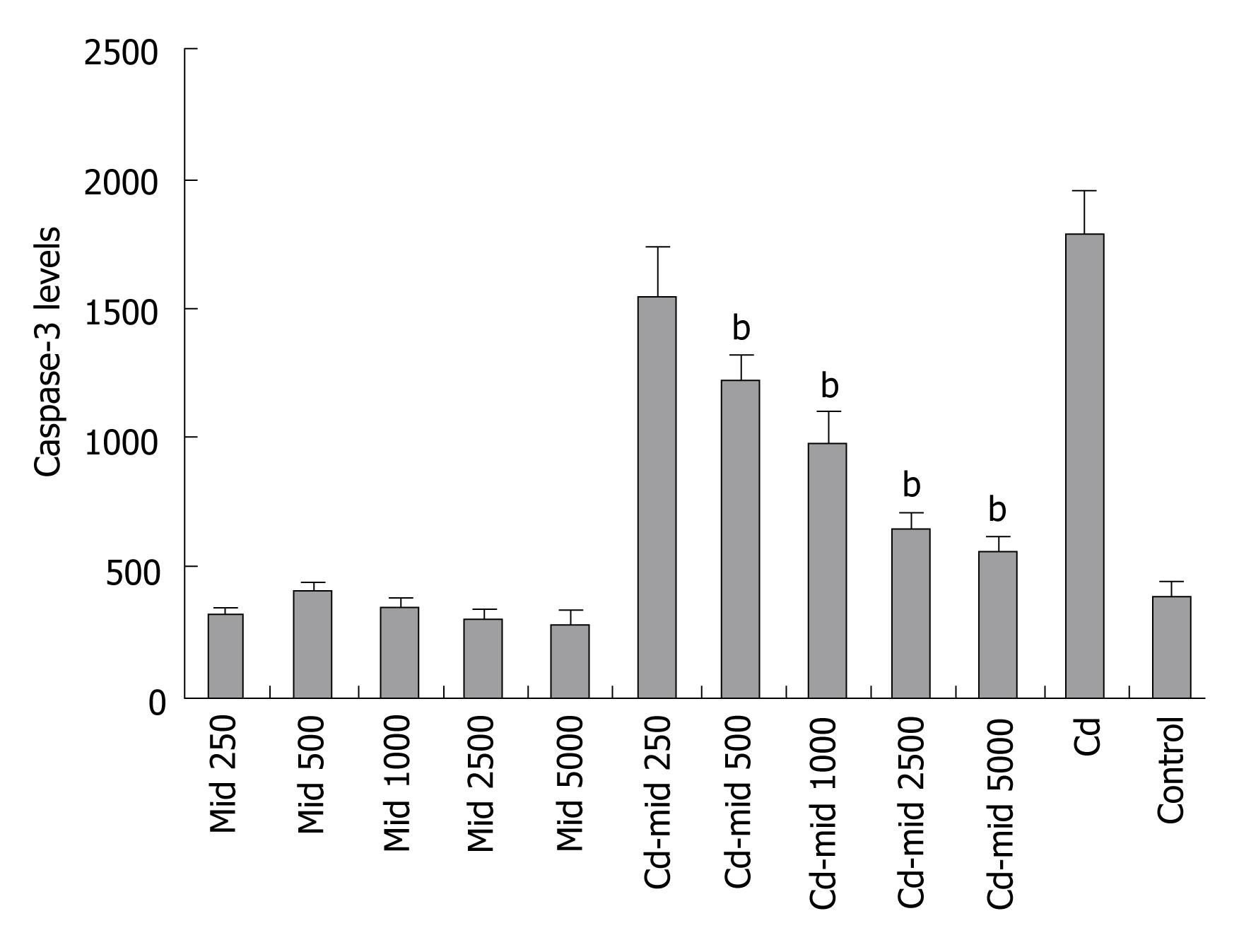
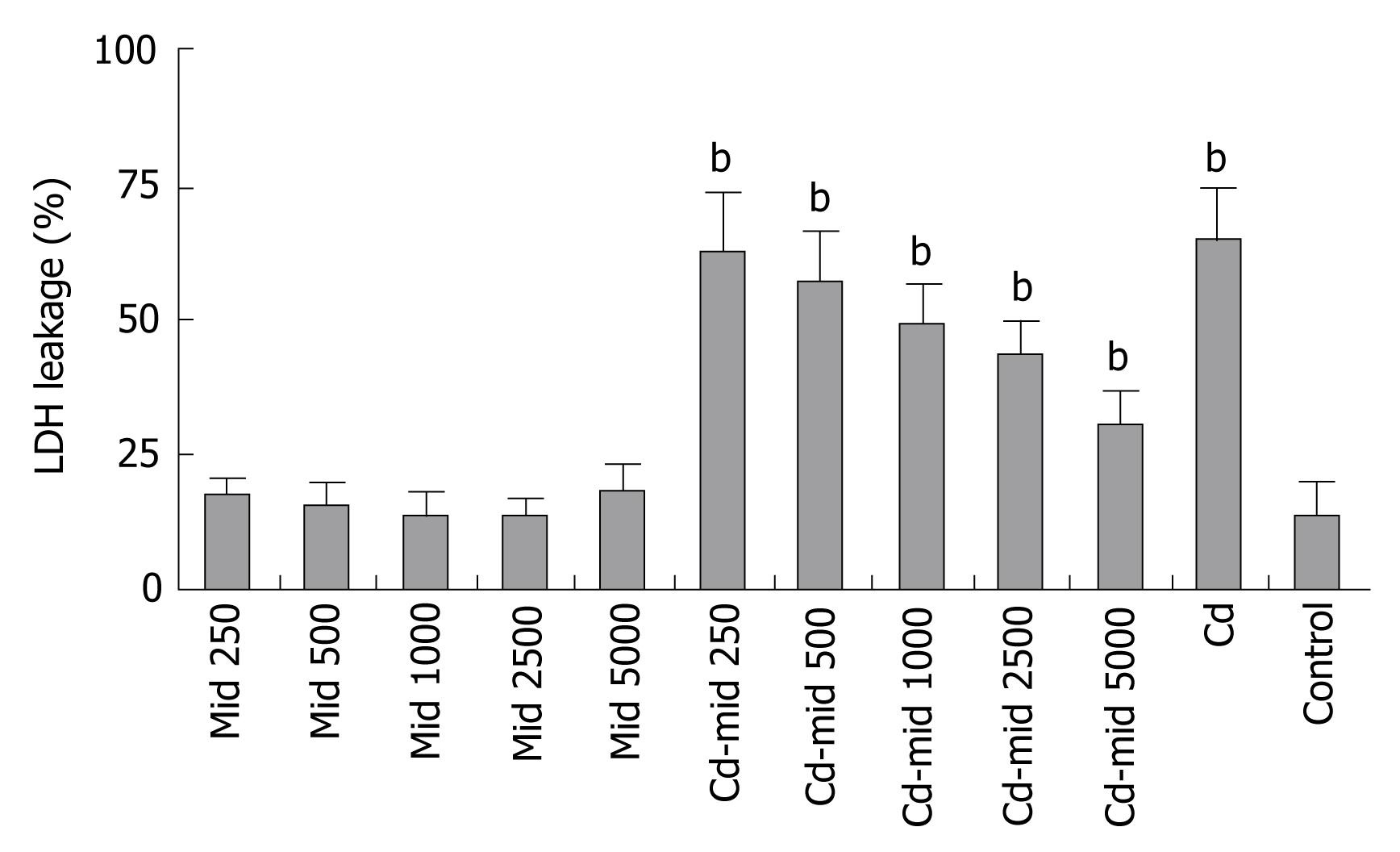
Midkine treatment decreased caspase-3 levels in the Hep3B cells. It prevents Cd induced apoptosis prominently starting from 500 pg/mL concentration of midkine application (P < 0.001, Figure 4).
Incubation of Hep3B cells with Cd resulted in cytotoxicity as assessed by LDH released into the incubation media. LDH release in the Hep3B cells to media started at the 1 &mgr;g/mL Cd dosage (Figure 5).
Midkine treatment at the same time with 5 &mgr;g/mL Cd exposure decreased LDH release in the Hep3B cells (Figure 6).
Measurable basal midkine secretion was found in the Hep3B cells under normal conditions. Cd treatment induced midkine secretion in the Hep3B cells in a dose dependent manner (Figure 7).
Acute/chronic Cd exposure mostly results in hepatotoxicity, where it is a good model to study toxic substance-induced liver damage[2]. Midkine family has strong anti-apoptotic function so they are obviously considered non specific (i.e., for Cd) mechanisms of defence[10]. Intense midkine expression has also been found in increased various human tumors and level of midkine expression correlates negatively with the patients’ prognosis[1415]. Furthermore, midkine accumulation is noted in senile plaques in the brains of Alzheimer’s disease patients[16]. Midkine is expressed around the damaged neuronal site after cerebral infarction[17], suggesting a role for midkine in tissue repair.
The results of the present study supports the idea that Cd exposure causes cytotoxicity and apoptosis in the Hep3B cells. In both time and dose-response studies, LDH leakage, which is very important parameter to detect hepatocellular integrity, was greater in Cd treated cells. These effects were more prominent at the 48th h. During Cd exposure, activation of caspase-3 was detected in Hep3B cells, suggesting a caspase-dependent pathway is involved in Cd toxicity. Cd can upregulate the expression of a number of genes that produce products that can detoxify Cd and/or repair Cd induced lesions. Our studies showed that midkine is one of them. The induction pathways or receptors of midkine expressed by Cd exposure is still unclear. Midkine is multifunctional heparin-binding growth factor and cytokine and has anti-apoptotic and cell-protecting activities[8]. Untreated Hep3B cells have also a basal midkine secretion. In our study, midkine treatment decreased apoptosis and increased cellular proliferation in a dose and time dependent manner. It has cytoprotective, anti-apoptotic effects against Cd toxicity in Hep3B cells. It seems that midkine is produced endogenously and released in to medium as a defense mechanism of Hep3B cells against Cd toxicity. Among midkine receptors, receptor-type protein tyrosine phophatase z (PTP z) has been studied extensively. Midkine stimulates phosphorylation of specific members of the JAK/STAT pathway, namely JAK1, JAK2, and STAT1α [1819]. In addition, low density lipoprotein receptor-related protein (LRP) has also been identified as a receptor[20]. The midkine receptor is considered to be a molecular complex containing these proteins. The downstream signaling systems of these receptors include ERK, which participates in the reduction of necrotic and apoptotic cell death[21]. Internalization of midkine in to cell and nuclear targeting is important for its antiapoptotic function[22]. Activation of these receptors and intracellular pathways might take part in cytoprotective effects of midkine during Cd toxicity. Human and experimental studies have shown that apoptosis plays a role in hepatocyte death in alcoholic liver disease and nonalcoholic steatohepatitis and apoptosis levels correlate with the severity of the liver disease[23–26]. LDL receptor-related protein (LRP) is another midkine receptor. LRP is important for lipid and lipoprotein uptake to cells[27]. Lipid profiles of steatohepatitis patients were found disturbed[28]. It was shown that midkine takes part in the inflammatory and repair processes after partial hepatectomy. They suggested that midkine is beneficial for liver regeneration[29]. ERK, JNK signal pathways disturbed during Cd toxicity are activated by midkine[18192230]. Beneficial effects of exogenous midkine to minimize Cd induced damage would provide a new perspective for innovation in the treatment of Cd intoxications and in NonAlcoholic Fatty Liver Disease (disease near exclusively characterized by apoptotic process), in Drug Induced Liver Injury and in the combined form, illnesses far long more evident than Cd intoxication in the every day practice of gastroenterologists and hospitals. But further acute and chronic in vitro and in vivo studies are needed to evaluate which intracellular pathway(s) is activated during these processes.
Acute/chronic Cadmium (Cd) exposure mostly results in hepatotoxicity, where it is a good model to study toxic substance-induced liver damage. Midkine is expressed around the damaged tissues after ischemic damage and suggesting a role for midkine in tissue repair. Its expression has been found in tumors and level of midkine expression correlates negatively with the patients’ prognosis. Midkine is cytoprotective and has anti-apoptotic effect.
Beneficial effects of exogenous midkine to minimize Cd induced damage would provide a new perspective for innovation in the treatment of Cd intoxications and other liver diseases.
Cd is well known environmental toxic substance which mainly damages liver. In this study we showed that Cd treatment induces midkine secretion from hepatocytes. Midkine might have protective role during Cd toxicity.
These finding may be used in the different liver diseases such as alcoholic, toxic and non- alcoholic liver disease models.
In this experimental in vitro study, the authors showed that midkine secretion from Hep3B cells during Cd exposure protects liver cells from Cd induced cellular damage. Midkine has anti-apoptotic and cytoprotective role during Cd toxicity. Midkine may be a promising therapeutic agent in different toxic hepatic diseases.
| 1. | Nishijo M, Nakagawa H, Morikawa Y, Tabata M, Senma M, Miura K, Takahara H, Kawano S, Nishi M, Mizukoshi K. Mortality of inhabitants in an area polluted by cadmium: 15 year follow up. Occup Environ Med. 1995;52:181-184. |
| 2. | Gubrelay U, Mehta A, Singh M, Flora SJ. Comparative hepatic and renal toxicity of cadmium in male and female rats. J Environ Biol. 2004;25:65-73. |
| 3. | Coutant A, Lebeau J, Bidon-Wagner N, Levalois C, Lectard B, Chevillard S. Cadmium-induced apoptosis in lymphoblastoid cell line: involvement of caspase-dependent and -independent pathways. Biochimie. 2006;88:1815-1822. |
| 4. | Trinchella F, Riggio M, Filosa S, Volpe MG, Parisi E, Scudiero R. Cadmium distribution and metallothionein expression in lizard tissues following acute and chronic cadmium intoxication. Comp Biochem Physiol C Toxicol Pharmacol. 2006;144:272-278. |
| 5. | Amara S, Abdelmelek H, Garrel C, Guiraud P, Douki T, Ravanat JL, Favier A, Sakly M, Ben Rhouma K. Influence of static magnetic field on cadmium toxicity: study of oxidative stress and DNA damage in rat tissues. J Trace Elem Med Biol. 2006;20:263-269. |
| 6. | Ramirez DC, Gimenez MS. Induction of redox changes, inducible nitric oxide synthase and cyclooxygenase-2 by chronic cadmium exposure in mouse peritoneal macrophages. Toxicol Lett. 2003;145:121-132. |
| 7. | Lasfer M, Vadrot N, Aoudjehane L, Conti F, Bringuier AF, Feldmann G, Reyl-Desmars F. Cadmium induces mitochondria-dependent apoptosis of normal human hepatocytes. Cell Biol Toxicol. 2008;24:55-62. |
| 8. | Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem (Tokyo). 2002;132:359-371. |
| 9. | Sato W, Kadomatsu K, Yuzawa Y, Muramatsu H, Hotta N, Matsuo S, Muramatsu T. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J Immunol. 2001;167:3463-3469. |
| 10. | Ohuchida T, Okamoto K, Akahane K, Higure A, Todoroki H, Abe Y, Kikuchi M, Ikematsu S, Muramatsu T, Itoh H. Midkine protects hepatocellular carcinoma cells against TRAIL-mediated apoptosis through down-regulation of caspase-3 activity. Cancer. 2004;100:2430-2436. |
| 11. | Volkmann X, Fischer U, Bahr MJ, Ott M, Lehner F, Macfarlane M, Cohen GM, Manns MP, Schulze-Osthoff K, Bantel H. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 2007;46:1498-508. |
| 12. | Kayama F, Yoshida T, Elwell MR, Luster MI. Role of tumor necrosis factor-alpha in cadmium-induced hepatotoxicity. Toxicol Appl Pharmacol. 1995;131:224-234. |
| 13. | Vanucci S, Minerdi D, Kadomatsu K, Mengoni A, Bazzicalupo M. Putative midkine family protein up-regulation in Patella caerulea (Mollusca, Gastropoda) exposed to sublethal concentrations of cadmium. Aquat Toxicol. 2005;75:374-379. |
| 14. | Tong Y, Mentlein R, Buhl R, Hugo HH, Krause J, Mehdorn HM, Held-Feindt J. Overexpression of midkine contributes to anti-apoptotic effects in human meningiomas. J Neurochem. 2007;100:1097-1107. |
| 15. | Dai LC, Wang X, Yao X, Lu YL, Ping JL, He JF. Enhanced therapeutic effects of combined chemotherapeutic drugs and midkine antisense oligonucleotides for hepatocellular carcinoma. World J Gastroenterol. 2007;13:1989-1994. |
| 16. | Yasuhara O, Muramatsu H, Kim SU, Muramatsu T, Maruta H, McGeer PL. Midkine, a novel neurotrophic factor, is present in senile plaques of Alzheimer disease. Biochem Biophys Res Commun. 1993;192:246-251. |
| 17. | Yoshida Y, Goto M, Tsutsui J, Ozawa M, Sato E, Osame M, Muramatsu T. Midkine is present in the early stage of cerebral infarct. Brain Res Dev Brain Res. 1995;85:25-30. |
| 18. | Sakaguchi N, Muramatsu H, Ichihara-Tanaka K, Maeda N, Noda M, Yamamoto T, Michikawa M, Ikematsu S, Sakuma S, Muramatsu T. Receptor-type protein tyrosine phosphatase zeta as a component of the signaling receptor complex for midkine-dependent survival of embryonic neurons. Neurosci Res. 2003;45:219-224. |
| 19. | Ratovitski EA, Kotzbauer PT, Milbrandt J, Lowenstein CJ, Burrow CR. Midkine induces tumor cell proliferation and binds to a high affinity signaling receptor associated with JAK tyrosine kinases. J Biol Chem. 1998;273:3654-3660. |
| 20. | Muramatsu H, Zou K, Sakaguchi N, Ikematsu S, Sakuma S, Muramatsu T. LDL receptor-related protein as a component of the midkine receptor. Biochem Biophys Res Commun. 2000;270:936-941. |
| 21. | Owada K, Sanjyo N, Kobayashi T, Kamata T, Mizusawa H, Muramatsu H, Muramatsu T, Michikawa M. Midkine inhibits apoptosis via extracellular signal regulated kinase (ERK) activation in PC12 cells. J Med Dent Sci. 1999;46:45-51. |
| 22. | Shibata Y, Muramatsu T, Hirai M, Inui T, Kimura T, Saito H, McCormick LM, Bu G, Kadomatsu K. Nuclear targeting by the growth factor midkine. Mol Cell Biol. 2002;22:6788-6796. |
| 23. | Ziol M, Tepper M, Lohez M, Arcangeli G, Ganne N, Christidis C, Trinchet JC, Beaugrand M, Guillet JG, Guettier C. Clinical and biological relevance of hepatocyte apoptosis in alcoholic hepatitis. J Hepatol. 2001;34:254-260. |
| 24. | Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437-443. |
| 25. | Colmenero J, Bataller R, Sancho-Bru P, Bellot P, Miquel R, Moreno M, Jares P, Bosch J, Arroyo V, Caballeria J. Hepatic expression of candidate genes in patients with alcoholic hepatitis: correlation with disease severity. Gastroenterology. 2007;132:687-697. |
| 26. | Tarantino G, Conca P, Basile V, Gentile A, Capone D, Polichetti G, Leo E. A prospective study of acute drug-induced liver injury in patients suffering from non-alcoholic fatty liver disease. Hepatol Res. 2007;37:410-415. |
| 27. | Yu KC, Chen W, Cooper AD. LDL receptor-related protein mediates cell-surface clustering and hepatic sequestration of chylomicron remnants in LDLR-deficient mice. J Clin Invest. 2001;107:1387-1394. |
| 28. | de Almeida IT, Cortez-Pinto H, Fidalgo G, Rodrigues D, Camilo ME. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin Nutr. 2002;21:219-223. |
| 29. | Ochiai K, Muramatsu H, Yamamoto S, Ando H, Muramatsu T. The role of midkine and pleiotrophin in liver regeneration. Liver Int. 2004;24:484-491. |
| 30. | Qu W, Fuquay R, Sakurai T, Waalkes MP. Acquisition of apoptotic resistance in cadmium-induced malignant transfor-mation: specific perturbation of JNK signal transduction pathway and associated metallothionein overexpression. Mol Carcinog. 2006;45:561-571. |