INTRODUCTION
Hepatocellular carcinoma often develops against a background of chronic viral hepatitis including hepatitis B virus (HBV) and hepatitis C virus (HCV), autoimmune hepatic diseases including autoimmune hepatitis and primary biliary cirrhosis, alcoholic liver injury, and non-alcoholic steatohepatitis (NASH). We report a case of hepatic cancer in which no abnormality existed in liver function, hepatitis virus markers were negative, and the background liver was normal, and which was histopathologically clear-cell carcinoma, in which clear cells accounted for about 60% of the tissue. This patient presented with no background of carcinogenic risk factors, such as viral hepatitis, and showed rare histopathology.
CASE REPORT
A 36-year-old woman had a medical checkup on October 17, 2005. A tumor was found in S7 of the liver by abdominal US. She was referred and admitted to our department on November 28, 2005 for close examination and treatment. She had a history of alcohol consumption of 1 L beer per day for 15 years. Her family had no history of hepatic disease. On admission, her conjunctivas were not jaundiced, and heart and respiratory sounds were normal. The liver, spleen and tumor were not palpable.
Laboratory tests on admission showed no abnormality such as inflammation or abnormal liver function for peripheral blood and biochemical tests. Hepatitis B surface antigen (HBsAg), hepatitis B core antibody (HBcAb) and HCV antibody (HCVAb) were negative. All tumor markers tested showed normal values: specifically, 16 mAu/mL for PIVKA-II (criterion, < 40), 6.2 ng/mL for AFP (criterion, ≤ 10.0), 20.2 U/mL for CA19-9 (criterion, ≤ 37), and 0.9 ng/mL for CEA (criterion, ≤ 5.0). Anti-nuclear and anti-mitochondrial antibodies were negative.
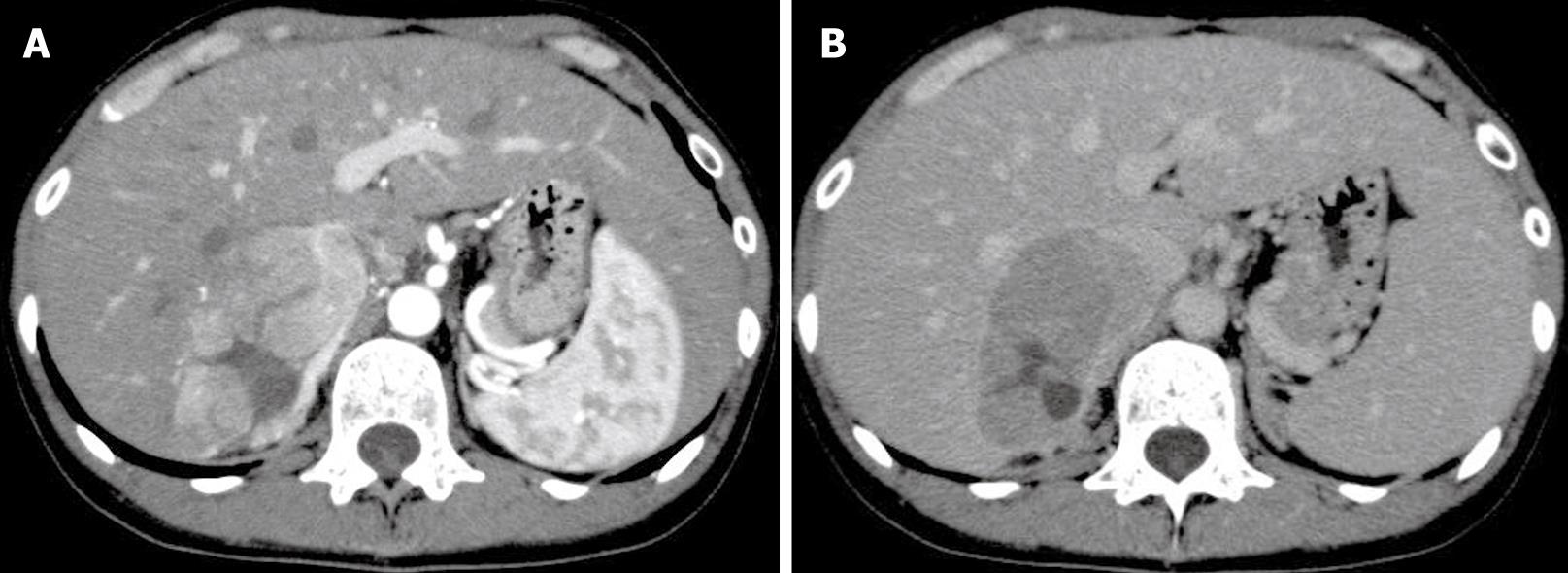
Abdominal US and CT showed a tumor of about 60 mm diameter in S7 of the liver. The lesion was visible on early phase imaging. However, there was also a part that was not visible in the image. It was apparent as a low-density area in the portal phase image (Figure 1A and B). Abdominal MRI showed that the T1-weighted image revealed a well-defined low-intensity area in S7, with a high-intensity part inside. The tumor was depicted as a high-intensity area in the T2-weighted image. Angiography showed that increased tumor vascularity was observed from the right hepatic artery and the inferior phrenic artery to the tumor area. A slight tumor stain was visible in the tumor area in S7 of the liver. Although the imaging results suggested hepatocellular carcinoma, all hepatitis virus and tumor markers were negative, and non-cancerous tissues had no signs of chronic disease.
Figure 1 A: Dynamic enhanced CT showed a nodular lesion in the liver that was enhanced in the early image.
However, the interior of the mass showed a low-density area; B: In the subsequent late phase, the lesion revealed washout of contrast enhancement.
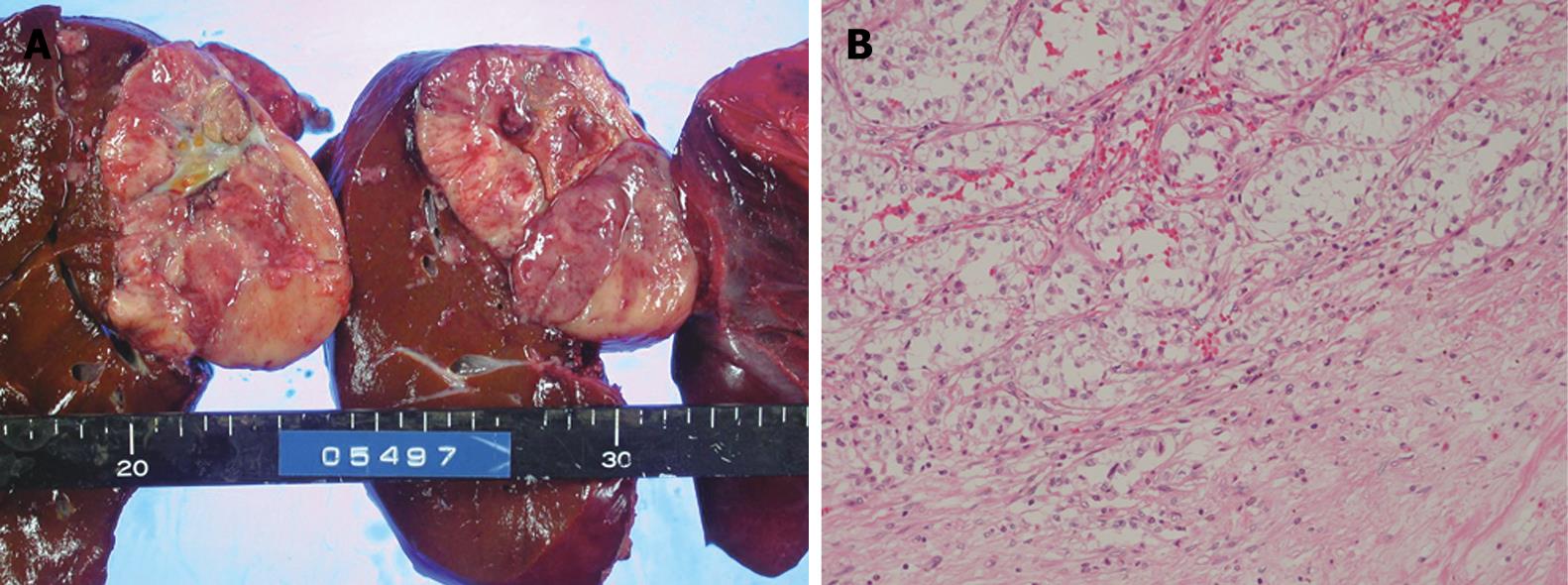
Consequently, aspiration biopsy of the liver was carried out for definitive diagnosis. Based on histopathology, the patient was diagnosed with moderately differentiated hepatocellular carcinoma, trabecular type, and right hepatectomy was performed on December 27, 2005. A 60-mm tumor was located in the posterior segment of the right hepatic lobe; the tumor was partly adherent to the caudal portion of the adrenal gland. The tumor had a fibrous capsule. Its cut surface was whitish, and tumorous tissue had a necrotic part at the center (Figure 2A). Histopathology showed the border between the tumor and the non-cancerous part formed a fibrous capsule. Approximately 60% of the tumor showed sheet-like growth of clear, atypical cells (Figure 2B). Other areas of the tumor were composed of moderately-differentiated HCC with a trabecular growth pattern. The tumor center was occupied by necrotic tissue.
Figure 2 A: The tumor had a fibrous capsule.
Its cut surface was whitish, and tumorous tissue had a necrotic part at the center; B: Histological examination showed sheet-like growth of clear, atypical cells (HE, × 40).
DISCUSSION
Hepatocellular carcinoma often develops against a background of chronic liver disease. Specifically, 77.4% and 62.6% of cases in Japan are associated with chronic hepatitis and liver cirrhosis, respectively. Among cases with histopathological diagnosis, only 7.6% have normal liver as the background, as in this present case[1]. Furthermore, HBV- or HCV-antigen-positive liver cirrhosis accounts for about 80% of cases of liver cirrhosis; other causes include primary biliary cirrhosis, autoimmune hepatitis, alcoholic cirrhosis, NASH, and Budd-Chiari syndrome[2]. In the present case, although the patient had a history of alcohol consumption, there was no sign of alcoholic fibrosis in non-cancerous tissues. For that reason, it was presumed pathologically that the hepatocellular carcinoma developed from the normal liver. Liver cancer is considered to be comparatively rare in young persons, and patients < 35 years old account for 0.6% of the cases diagnosed with clinical hepatocellular carcinoma[1]. Moreover, according to data from 1994 to 1995 by the Follow-up Committee, Liver Cancer Study Group of Japan in 1998, 60 cases of liver cancer in young patients aged < 35 years old consisted of 12 HCVAb-positive (20%), 34 HBsAg-positive (57%), three positive cases each (5%) for HCVAb and HBsAg, and 11 virus-marker-negative cases (18%), which indicates that HBsAg is often positive among younger patients with liver cancer[3]. Although virus markers were negative in our case, reports also exist of occult HBV infection, in which serum HBV marker was negative, but HBV existed in the serum and liver tissue[4], and comparatively frequent incorporation of HBV in hepatocellular carcinoma developed in normal liver[5–7]. Therefore, involvement of viruses in this case is not implausible.
Clear-cell hepatocellular carcinoma is not frequent and has been reported to account for 7.5%-12.5% of all liver cancer cases[89]. The existence of clear cells, as well as fatty changes, is characteristic of well-differentiated hepatocellular carcinoma in the early stage; its frequency is presumed to be decreased along with enlargement of the cancer[10]. In a large hepatocellular carcinoma, as that presented in this case, it has been reported that clear-cell hepatocellular carcinoma occurs at a frequency of 0.9%-8.8%[11]. The mechanism of development of clear cells is presumed to involve metabolic disorders and abnormalities of sugar metabolism for reasons including decreased portal blood flow and underdeveloped tumor arteries in the early stage of cancer[1012]. Histopa-thologically, it is important to distinguish clear-cell hepatocellular carcinoma from liver metastases from other organs, especially renal cell carcinoma, and it can be distinguished by immunostaining[13]. As for the present case, the possibility of malignant tumors derived from other organs was clinically eliminated, and immunostaining of excised samples excluded liver metastasis from renal cancer. On the other hand, fibrolamellar hepatocellular carcinoma and epithelioid hemangioendothelioma are malignant liver tumors that are often seen in young persons without background liver diseases. However, the present case did not have the same histopathology as those diseases. Prognosis of clear-cell hepatocellular carcinoma has been reported to be better than[814], the same as, or worse than[912] that of common hepatocellular carcinoma. Further careful follow-up observations are needed in the future.
S- Editor Liu Y L- Editor Kerr C E- Editor Lu W