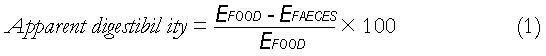Published online Mar 7, 2007. doi: 10.3748/wjg.v13.i9.1393
Revised: December 25, 2006
Accepted: January 17, 2007
Published online: March 7, 2007
After a meal the activity of the gut increases markedly as digestion takes place. Associated with this increase in activity is an increase in blood flow, which has been shown to be dependent on factors such as caloric content and constitution of the meal. Much qualitative work has been carried out regarding mechanisms for the presence of food in a section of gut producing increased blood flow to that section, but there are still many aspects of this process that are not fully understood. In this paper we briefly review current knowledge on several relevant areas relating to gut blood flow, focusing on quantitative data where available and highlighting areas where further research is needed. We then present new data on the effect of feeding on flow in the superior mesenteric artery. Finally, we describe a framework for combining this data to produce a single model describing the mechanisms involved in postprandial hyperaemia. For a section of the model, where appropriate data are available, preliminary results are presented.
- Citation: Jeays AD, Lawford PV, Gillott R, Spencer PA, Bardhan KD, Hose DR. A framework for the modeling of gut blood flow regulation and postprandial hyperaemia. World J Gastroenterol 2007; 13(9): 1393-1398
- URL: https://www.wjgnet.com/1007-9327/full/v13/i9/1393.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i9.1393
This study was motivated by our previous work on blood flow in the superior mesenteric artery (SMA). When we explored the literature on the vasculature downstream of the SMA in order to develop appropriate boundary conditions for our work on computational fluid dynamic modeling[1], we found little quantitative information on the smaller vessels of the mesentery. These vessels control the distribution of blood to the small intestine and as such are of great importance in understanding gut physiology and problems such as gut hypoperfusion following surgery[2].
This paper reviews current knowledge on blood flow regulation in the gut vasculature, presents new data on flow in the SMA and develops a framework for its integration with a structural model of the gut vasculature (to be published separately).
Following a meal, food arrives in the stomach, mixes with gastric juices to become chyme, and is slowly emptied into the small intestine. This rate of emptying is largely governed by the stomach and is dependent on the chemical and physical properties of the food. The chyme is digested as it travels through the small intestine and this propagating metabolic front is met by an increase in blood flow through the capillaries supplying the gut wall, which can be seen at a macroscopic level as an increase in flow through the superior mesenteric artery.
Except for the proximal part of the duodenum, the small intestine is wholly vascularised by vessels arising from the superior mesenteric artery, which originates from the aorta at the level of the L1 vertebra[3]. The vessels run through a convoluted sheet of tissue called the mesentery and then branch and anastomose (forming arcades) before reaching the vascular bed of the gut wall.
The direction of blood to the appropriate part of the gut is achieved by changes in vascular smooth muscle (VSM) tone, with the basal state being partial tone, thus allowing for both dilatation and contraction of the vessel wall. As well as affecting the diameter of the vessel lumen and hence its resistance to flow, VSM tone also mediates vessel wall stiffness[4].
There are two mechanisms likely to be of key importance in the control of gut blood flow following feeding. These are the response of vessels to food stimulus and the myogenic response.
The aim of modeling this system is to bring together the knowledge and understanding available in each of the specialist fields alluded to above, and to combine the information to allow greater appreciation of the organ as a whole. By definition, the model will not represent every facet of the system being studied, nor should it. Instead, the aim of the model is to capture the key factors that play an important part in determining gut blood flow and allow ‘what if’ questions to be asked. In order to be predictive and to allow a wide range of questions to be posed, the model must be flexible.
Gastric emptying rates show substantial inter- and intra-subject variability and are dependent on meal nutrient content and physical properties. Emptying of digestible solids is generally characterized by a lag phase followed by a period during which the emptying rate is approximately constant. Animal studies suggest that the rate-limiting factor leading to this constant rate of gastric emptying is trituration (grinding up of solid food). In contrast, isotonic liquid meals need no trituration and hence exhibit a different pattern of gastric emptying, emptying more quickly and following an exponential relationship[5]. Nutrient-containing liquids lie between these two extremes with the rate of emptying rising quickly to 1.5-3 kCal/min[5,6]. The presence of solid and liquid foods in the same meal leads to modification of the emptying profiles of both foods[5].
A model where nutrient content is detected locally within the gut and to some extent influences blood flow requires information on the progression of chyme through the small intestine. Standard scintigraphic methods for investigating small bowel transit times give little direct data on the rate of transit of food through the small intestine due to the gut’s convoluted nature and the poor resolution of gamma cameras. Instead, short intestinal transit times are often inferred from gastric emptying and colonic filling[7,8]. A spectrum of transit times is often presented, reflecting the fact that different parts of the chyme progress through the gut at different rates (although solid and liquid markers have been shown to progress along the bowel at similar rates[7]). Motion of chyme through the small intestine is governed by irregular pressure waves moving along the gut[9] and it has been suggested that transit is fast initially, acting to spread chyme through the gut, and then slows as digestion takes place[10]. However, quantitative data is limited.
In a scintigraphic study of six subjects, Malagelada et al[7] reported median pylorus to caecum transit times in the range of 95 to 245 min. For 8 of 12 tests (considering progression of solid and liquid meals in each subject) a normal distribution was considered by the authors to be a good model for the transit time spectrum. No significant difference in mean transit times was observed after solid and liquid meals. The full-width half maximums (FWHM) of the normal distributions were in the range 33-261 min with mean FWHMs of 169 min (liquid) and 63 min (solid). Whilst there was no difference in mean transit rates, the liquid meal tended to become more spread out during its passage through the small intestine.
Math 1
Assuming that digestion is exclusively confined to the small intestine, small intestinal efficiency will be equal to the ‘apparent digestibility’ of the foodstuff, as given by equation 1 above[11].
Where EFOOD and EFAECES are the caloric contents of the food and faeces respectively. At the beginning of the twentieth century, Atwater published several papers[12-14] on the available energy in various foodstuffs, finding the E values in equation 1; his key results are reproduced in Table 1.
From the 1970s, Chou et al[15-17] have performed work on characterizing the response of the gut to various luminal contents. In descending order, the most potent inducers of increased blood flow to the gut are: lipids and fats (in combination with bile salts), glucose and other carbohydrates, proteins, peptides, amino acids. Chou et al[15] also experimented with the solid and fluid phases of the chyme and they found that the compounds responsible for postprandial hyperaemia exist in the hydrolytic products of food digestion.
The basic control mechanisms relating the arrival of chyme in an intestinal segment to an increase in local blood flow are not fully understood. Chou and Coatney[16] suggested five categories of potential influences on local postprandial hyperaemia. The understanding of these various effects has recently been reviewed by Matheson et al[18]. The five categories are: (1) Direct effects of absorbed nutrients: Intra-arterial injection of some nutrients (micellar solutions containing bile and oleic acid, capoic acid or taurocholate) has an effect on jejunal blood flow, while most amino acids and carbohydrates do not. Carbon dioxide and hydrogen ions present in the gut can diffuse into the blood supply and also have an effect; (2) Enteric nervous system effects and reflexes: It has been shown that extrinsic sympathetic and parasympathetic innervation is not required for post-prandial hyperaemia; however, the role of other (non-adrenergic, non-cholinergic) neurons cannot be ruled out; (3) Gastrointestinal hormones and peptides: While many of these can have vasoactive effects, levels measured experimentally have always been too low to produce vaso-dilatation; (4) Local non-metabolic vasoactive mediators; (5) Local metabolic vasoactive mediators.
As well as the factors mentioned above, the myogenic response may be of key importance. The myogenic response is an increase in vascular tone associated with an increase in transmural pressure, or a decrease in vascular tone associated with a decrease in transmural pressure. This has been reviewed from a biological perspective by Schubert and Mulvany[19] and by Davis and Hill[20]. The in vivo myogenic response is not well understood. Most experiments to date have been performed in vitro and often give conflicting results.
The myogenic response is seen primarily in small arteries and arterioles and varies between arterial beds. It is widely held to be dependent mainly on the reaction of vascular smooth muscle to changes in vessel wall tension (hoop stress). Osol et al[21] showed that in rats the response is weaker in small mesenteric vessels than in cerebral vessels that are equivalent. No similar studies have been carried out in human mesenteric vessels.
Whilst work continues in each of these areas the dominant mechanism controlling postprandial hyperaemia in humans has not yet been isolated.
In order to build the model, the mechanical and vasoactive properties that govern the resistance to blood flow in the mesenteric vessels must be characterized. These important parameters may be considered as two sets; those that change with VSM tone, and those that can be considered constant.
The key parameters that vary with vascular tone are vessel diameter and stiffness. Ideally, from a modeling perspective, these would be well-documented in terms of basal values and deviations from these values associated with each of the mechanisms described above. However, this is not the case. The complex, layered structure of the vessel wall combined with the difficulties associated with in vivo measurement, mean that the mechanical behavior of human arteries has yet to be characterized thoroughly[22].
The constant properties are the branching patterns and lengths of the mesenteric vessels. Whilst some contrast-enhanced x-ray images exist[23] from which a model of a segment of gut could be derived, no one has yet numerically recorded the branching patterns of an entire human mesentery.
Due to the invasive nature of most flow measurement techniques and poor spatial resolution of non-invasive techniques; accurate measurement of flow in the smaller arteries of the mesentery and gut is at present impossible in normal human subjects. Studies have however been carried out measuring flow in the parent vessel (the SMA)[24,25]. Such data can be used to validate models of the downstream vasculature. We added to this knowledge by using a flow-measuring phase contrast sequence on a 1.5T clinical MRI scanner to quantify the effect of a 600 kCal Scandishake® (registered trademark of Axcan Pharma) test meal on blood flow in the SMA. Twenty-one anatomical and flow images were acquired representing an average cardiac cycle over the 5 min acquisition time. For details of phase contrast MRI, the reader is referred to Edelman et al[26].
Eighteen healthy volunteers (11 male, 7 female, average age 36) fasted from midnight on the day of the scan and a base-line scan was taken between 9 and 10 am to give a measure of resting SMA flow. Each was then fed a 600 kCal Scandishake test meal containing 11.7 g of protein, 69.5 g of carbohydrates including 35.8 g of sugars, and 30.4 g of fat including 14.8 g of saturates. A second scan was taken 30 min postprandial (identified as the time of peak SMA blood flow in a previous ultrasound study[27]).
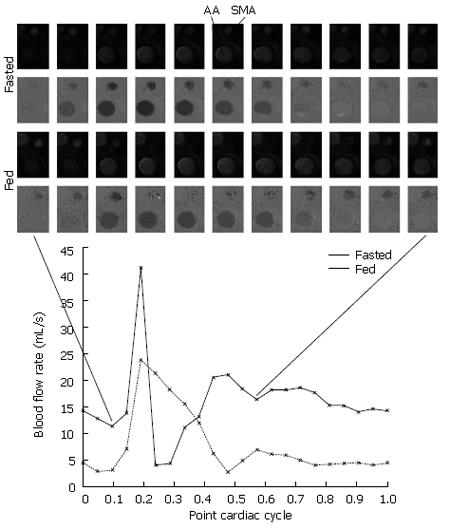
For the postprandial scan, in 15 of the 18 subjects the phase contrast signal was lost around peak systole as previously observed in some[24] but not all[25] previous similar experiments. In contrast to other authors[24], we believe that this is not due to a change in the shape of the flow waveform, but signal loss was due to disturbed flow causing intravoxel dephasing through the systolic period. Figure 1 shows postprandial PCMRA magnitude and phase images for one subject. The absence of the SMA at peak systole in both the magnitude and phase images supports the disturbed flow hypothesis.
In 2 of the 15 subjects, the same signal loss was also witnessed before feeding. For these reason we consider only the diastolic portion of the cardiac cycle. At 30 min post-prandial, mean diastolic flow was 2.8 times that of the fasted value (Table 2).
| Parameter | Mean | SD | Range | |
| Heart rate (BPM) | Fasted | 62.1 | 8.5 | 45-76 |
| Postprandial | 69.3 | 9.9 | 50-90 | |
| Change1 | 11.9% | 16.5% | 0%-33% | |
| Mean SMA diastolic flow (mL/s) | Fasted | 4.3 | 3.3 | 0.6-14.5 |
| Postprandial | 11.9 | 4.8 | 3.0-18.7 | |
| Change1 | 177% | 45.5% | 881%-18.6% | |
| Total SMA diastolic flow (mL/heart beat) | Fasted | 4.1 | 2.8 | 0.6-11.9 |
| Postprandial | 10.4 | 4.4 | 2.7-17.7 | |
| Change1 | 154% | 57.1% | 798%-20.8% |
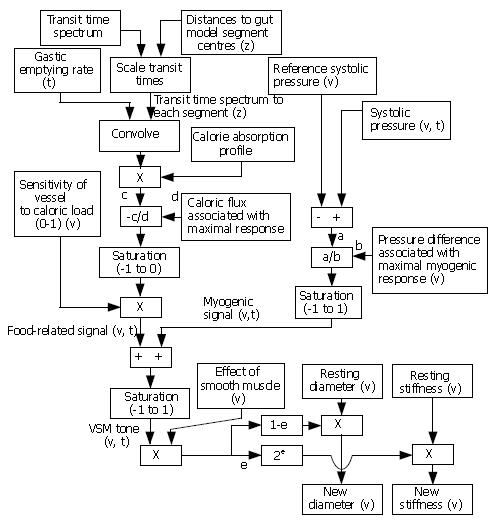
We propose an integrated model of the small intestine and the mesenteric arteries with arteries modeled as a branching tree structure and the gut as a series of connected segments. The arterial model is a digital implementation[28] of Westerhof’s branching tree model first published in 1969[29], with each artery having specified properties in terms of segment length, internal radius, wall thickness and wall stiffness. One key function of the intestinal model is to modulate the radius and stiffness of each vessel in accordance with food intake and other parameters. A suggested framework for this control is shown in Figure 2.
Due to the absence of quantitative human data in many areas, the model framework is designed to work logically within the limits of physiologically realistic values for the various inputs and to capture the key understood processes without recourse to the detailed underlying biology. For example, no caloric input, along with expected systolic pressure, will ensure that vessel stiffness and diameter remain at their basal levels, while saturation elements within the model reflect the fact that there are limits on the range of effect of the myogenic and calorie dependent response.
Data relating to sensitivity of vessels to caloric load, pressure difference associated with maximal myogenic response, and the effect of smooth muscle, is particularly difficult to obtain. For this reason, we now show results for the digestion part of the model alone (top left of figure 2 down to signal ‘c’), based on the consumption of a 600 kCal milkshake as used in our MRI study. Gastric emptying-rate data, a small intestinal transit time spectrum and a calorie absorption profile are the inputs to this part of the model. The model assumes a single food traveling along the gut with the three inputs inferred from the composition of the food being modeled.
The osmolarity of the milkshake was 880 mOsm/L putting it firmly in the “nutrient-containing liquid” category (normal osmolarity of blood 300 mOsm/L), therefore a gastric emptying rate of 3 kCal/minute was assumed.
The intestinal transit model consisted of a normally distributed transit time spectrum with mean transit time of 172 min and FWHM 169 min. It was assumed that each particle of chyme traveled along the gut at a constant rate.
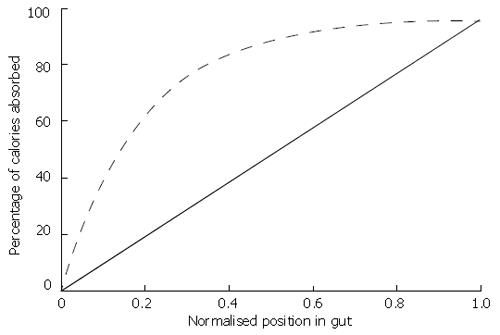
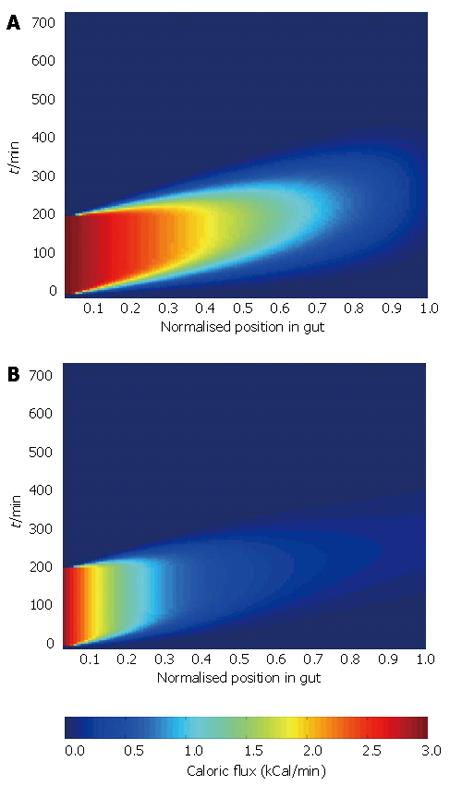
The calorie absorption profile relates the percentage of calories that have been absorbed from the food at a given distance along the gut. Applying the nutritional data above to table one and weighting by caloric load, we calculate a coefficient of apparent digestibility for the milkshake of 96%. This becomes the final value in the calorie absorption profile. We consider two calorie absorption profiles; one is a linear profile assuming that caloric absorption is spread evenly along the gut, the other an exponentially-governed profile assuming that just over half of the calories are absorbed in the proximal 20% of the gut. Both profiles are shown in Figure 3.
Combining the three inputs in the manner defined in Figure 2 allows us to generate a surface showing caloric flux past each point in the gut with time. Surface plots for the two absorption profiles are shown in Figure 4.
The analysis illustrates the dependence of the pattern of caloric flux on the absorption profile, which is likely to result in altered arterial demand. This highlights the need for good quality data to drive the model, and demonstrates the ability of the model to show the effect of changes of individual inputs on the wider system.
This paper introduces the potential utility of modeling techniques to aid understanding of the complex interactions within the gut and its supporting vasculature.
We have reviewed the available data on diverse subjects that play an important role in the regulation of gut blood flow and postprandial hyperaemia, developed a modeling framework for the simulation of these processes, and presented preliminary results from the part of the model where the required data is available. We have also presented new data on blood flow in the SMA following feeding.
The development of this type of model brings together concepts and data from diverse fields and aids understanding of the physiology at organ and system levels.
S- Editor Liu Y L- Editor Lutze M E- Editor Lu W
| 1. | Jeays AD, Lawford PV, Gillott R, Spencer P, Barber DC, Bardhan KD, Hose DR. Characterisation of the haemodynamics of the superior mesenteric artery. J Biomech. 2007;40:1916-1926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Wakeling HG, McFall MR, Jenkins CS, Woods WG, Miles WF, Barclay GR, Fleming SC. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth. 2005;95:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 417] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 3. | Gray H. Gray's Anatomy. 38th ed. Philadelphia: Churchill Livingston 1995; . |
| 4. | Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864-2869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 837] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 5. | Horowitz M, Dent J, Fraser R, Sun W, Hebbard G. Role and integration of mechanisms controlling gastric emptying. Dig Dis Sci. 1994;39:7S-13S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 80] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Brener W, Hendrix TR, McHugh PR. Regulation of the gastric emptying of glucose. Gastroenterology. 1983;85:76-82. [PubMed] |
| 7. | Malagelada JR, Robertson JS, Brown ML, Remington M, Duenes JA, Thomforde GM, Carryer PW. Intestinal transit of solid and liquid components of a meal in health. Gastroenterology. 1984;87:1255-1263. [PubMed] |
| 8. | Camilleri M, Colemont LJ, Phillips SF, Brown ML, Thomforde GM, Chapman N, Zinsmeister AR. Human gastric emptying and colonic filling of solids characterized by a new method. Am J Physiol. 1989;257:G284-G290. [PubMed] |
| 9. | Husebye E. The patterns of small bowel motility: physiology and implications in organic disease and functional disorders. Neurogastroenterol Motil. 1999;11:141-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 175] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Johansson C, Ekelund K. Relation between body weight and the gastric and intestinal handling of an oral caloric load. Gut. 1976;17:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Merrill AL, Bernice KW. Energy Value of Foods: basis and derivation (Agriculture Handbook No. 74).. Washington: US government printing office 1973; . |
| 12. | Atwater WO, Bryant AP. The availability and fuel values of food materials. Conn Agric Exp Stn. 12th ed. Middletown, CT: Storrs Agricultural Experiment Station Storrs 1900; 73. |
| 13. | Atwater WO, Bryant AP. The availability and fuel values of food materials. Conn Agric Exp Stn. 12th ed. Middletown, CT: Storrs Agricultural Experiment Station Storrs 1900; 69. |
| 14. | Atwater WO. The demands of the body for nourishment and dietary standards. Middletown, CT: Storrs Agricultural Experiment Station 1903; 123–146. |
| 15. | Chou CC, Kvietys P, Post J, Sit SP. Constituents of chyme responsible for postprandial intestinal hyperemia. Am J Physiol. 1978;235:H677-H682. [PubMed] |
| 16. | Chou CC, Coatney RW. Nutrient-induced changes in intestinal blood flow in the dog. Br Vet J. 1994;150:423-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Chou CC, Hsieh CP, Yu YM, Kvietys P, Yu LC, Pittman R, Dabney JM. Localization of mesenteric hyperemia during digestion in dogs. Am J Physiol. 1976;230:583-589. [PubMed] |
| 18. | Matheson PJ, Wilson MA, Garrison RN. Regulation of intestinal blood flow. J Surg Res. 2000;93:182-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 208] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Schubert R, Mulvany MJ. The myogenic response: established facts and attractive hypotheses. Clin Sci (Lond). 1999;96:313-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387-423. [PubMed] |
| 21. | Osol G, Laher I, Cipolla M. Protein kinase C modulates basal myogenic tone in resistance arteries from the cerebral circulation. Circ Res. 1991;68:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Schulze-Bauer CA, Holzapfel GA. Determination of constitutive equations for human arteries from clinical data. J Biomech. 2003;36:165-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Bergeron L, Tang M, Morris SF. A review of vascular injection techniques for the study of perforator flaps. Plast Reconstr Surg. 2006;117:2050-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Chan FP, Li KCP, Nayak KS, Hilfiker PR, Pauly JM. Efficient Characterization of Mesenteric Blood Flow Using Color-Flow Real-Time Interactive MRI. Philadelphia: Proceedings ISMRM Seventh Scientific Session 1999; 1638. |
| 25. | Masui T, Isoda H, Mochizuki T, Takahashi M, Kaneko M, Shirakawa T, Ota A. Effects of meal intake on the flow velocity in the superior mesenteric artery: evaluation with 2D phase mapping MRI. J Comput Assist Tomogr. 1994;18:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Edelman RR, Hesselink JR, Zlatkin MB. Clinical Magnetic Resonance Imaging. 2nd ed. Philadelphia: W.B.Sauders Company 1996; . |
| 27. | Spencer PA, Pramanik A, Chaudhuri A, Hill E, Berry C, Pryce WI, Willemse PJA, Bardhan KD. Small Intestinal Blood Flow in Man: The Effects of Age and Caloric Loading. Gut. 2000;46:A67. |
| 28. | Jones DM, Hose DR, Lawford PV, Hill DLG, Razavi RS, Barber DC. Creation of patient-specific CFD models by morphing a previously-meshed reference geometry using image registration. In: Rueckert D, Hajnal J, Yang GZ, editors. Medical Image Understanding and Analysis 2004; Proceedings of the eighth annual conference; September 23-24; London: England, 2004: 173-176. |
| 29. | Westerhof N, Bosman F, De Vries CJ, Noordergraaf A. Analog studies of the human systemic arterial tree. J Biomech. 1969;2:121-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |